Quantitative and Qualitative Platelet Derangements in Cardiac Surgery and Extracorporeal Life Support
Abstract
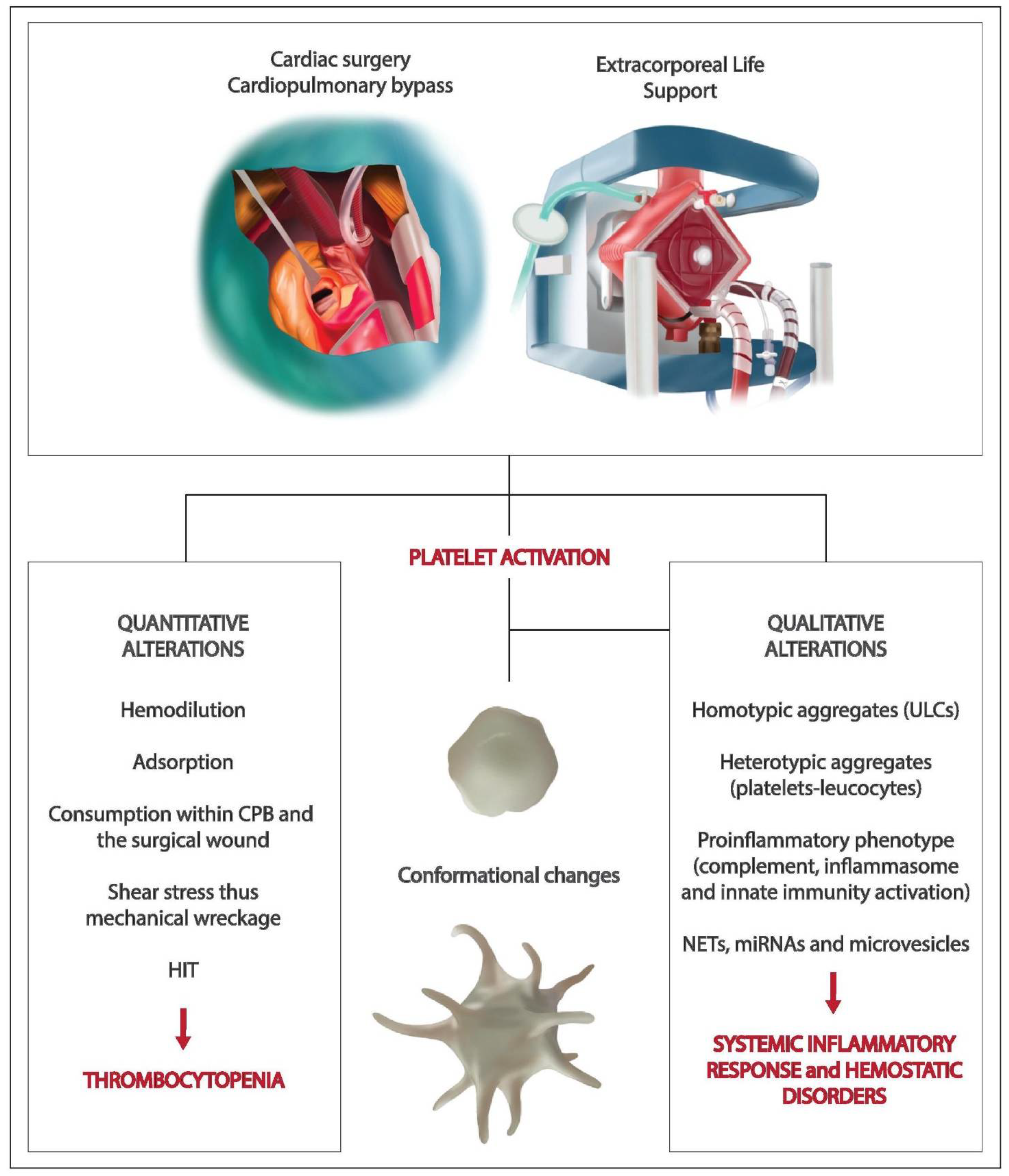
1. Introduction
2. Platelets, Cardiac Surgery and Extracorporeal Circulation: The Axis of Hemostasis, Inflammation and Innate Immunity
3. Platelets and Extracorporeal Membrane Oxygenation
4. Platelets and Aortic Biological Prosthesis
5. Heparin-Induced Thrombocytopenia
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Der Meijden, P.E.J.; Heemskerk, J.W.M. Platelet biology and functions: New concepts and clinical perspectives. Nat. Rev. Cardiol. 2019, 16, 166–179. [Google Scholar] [CrossRef]
- Gawaz, M.; Langer, H.; May, A.E. Platelets in inflammation and atherogenesis. J. Clin. Investig. 2005, 115, 3378–3384. [Google Scholar] [CrossRef]
- Mezger, M.; Gobel, K.; Kraft, P.; Meuth, S.G.; Kleinschnitz, C.; Langer, H.F. Platelets and vascular inflammation of the brain. Hämostaseologie 2015, 35, 244–251. [Google Scholar] [CrossRef]
- Engelmann, B.; Massberg, S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 2012, 13, 34–45. [Google Scholar] [CrossRef]
- Carestia, A.; Mena, H.A.; Olexen, C.M.; Wilczyñski, J.M.O.; Negrotto, S.; Errasti, A.E.; Gómez, R.M.; Jenne, C.N.; Silva, E.A.C.; Schattner, M. Platelets Promote Macrophage Polarization toward Pro-inflammatory Phenotype and Increase Survival of Septic Mice. Cell Rep. 2019, 28, 896–908.e5. [Google Scholar] [CrossRef]
- Garraud, O.; Ecognasse, F. Are Platelets Cells? And if Yes, are They Immune Cells? Front. Immunol. 2015, 6, 70. [Google Scholar] [CrossRef]
- Griffin, B.R.; Bronsert, M.; Reece, T.B.; Pal, J.D.; Cleveland, J.C.; Fullerton, D.A.; Gist, K.M.; Jovanovich, A.; Jalal, D.; Faubel, S.; et al. Thrombocytopenia After Cardiopulmonary Bypass Is Associated With Increased Morbidity and Mortality. Ann. Thorac. Surg. 2020, 110, 50–57. [Google Scholar] [CrossRef]
- Kertai, M.D.; Zhou, S.; Karhausen, J.A.; Cooter, M.; Jooste, E.; Li, Y.-J.; White, W.D.; Aronson, S.; Podgoreanu, M.V.; Gaca, J.G.; et al. Platelet Counts, Acute Kidney Injury, and Mortality after Coronary Artery Bypass Grafting Surgery. Anesthesiology 2016, 124, 339–352. [Google Scholar] [CrossRef]
- Jiritano, F.; Lorusso, R.; Santarpino, G. Causes of Thrombocytopenia in Cardiac Surgery: Looking for the Holy Grail? Ann. Thorac. Surg. 2020, 110, 751–752. [Google Scholar] [CrossRef]
- Squiccimarro, E.; Labriola, C.; Malvindi, P.G.; Margari, V.; Guida, P.; Visicchio, G.; Kounakis, G.; Favale, A.; Dambruoso, P.; Mastrototaro, G.; et al. Prevalence and Clinical Impact of Systemic Inflammatory Reaction After Cardiac Surgery. J. Cardiothorac. Vasc. Anesthesia 2019, 33, 1682–1690. [Google Scholar] [CrossRef]
- Deptula, J.; Glogowski, K.; Merrigan, K.; Hanson, K.; Felix, D.; Hammel, J.; Duncan, K. Evaluation of biocompatible cardiopulmonary bypass circuit use during pediatric open heart surgery. J. Extra Corporeal Technol. 2006, 38, 22–26. [Google Scholar]
- Tabuchi, N.; Shibamiya, A.; Koyama, T.; Fukuda, T.; Van Oeveren, W.; Sunamori, M. Activated Leukocytes Adsorbed on the Surface of an Extracorporeal Circuit. Artif. Organs 2003, 27, 591–594. [Google Scholar] [CrossRef]
- Vardon-Bounes, F.; Ruiz, S.; Gratacap, M.-P.; Garcia, C.; Payrastre, B.; Minville, V. Platelets Are Critical Key Players in Sepsis. Int. J. Mol. Sci. 2019, 20, 3494. [Google Scholar] [CrossRef]
- Mezger, M.; Nording, H.; Sauter, R.; Graf, T.; Heim, C.; Von Bubnoff, N.; Ensminger, S.M.; Langer, H.F. Platelets and Immune Responses During Thromboinflammation. Front. Immunol. 2019, 10, 1731. [Google Scholar] [CrossRef]
- Rossaint, J.; Kühne, K.; Skupski, J.; Van Aken, H.; Looney, M.R.; Hidalgo, A.; Zarbock, A. Directed transport of neutrophil-derived extracellular vesicles enables platelet-mediated innate immune response. Nat. Commun. 2016, 7, 13464. [Google Scholar] [CrossRef]
- Zahler, S.; Massoudy, P.; Hartl, H.; Hähnel, C.; Meisner, H.; Becker, B.F. Acute cardiac inflammatory responses to postischemic reperfusion during cardiopulmonary bypass. Cardiovasc. Res. 1999, 41, 722–730. [Google Scholar] [CrossRef]
- Warltier, D.C.; Laffey, J.G.; Boylan, J.F.; Cheng, D.C.H. The Systemic Inflammatory Response to Cardiac Surgery. Anesthesiology 2002, 97, 215–252. [Google Scholar] [CrossRef]
- Clark, S.R.; Ma, A.C.; A Tavener, S.; McDonald, B.; Goodarzi, Z.; Kelly, M.M.; Patel, K.D.; Chakrabarti, S.; McAvoy, E.; Sinclair, G.D.; et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 2007, 13, 463–469. [Google Scholar] [CrossRef]
- Perdomo, J.; Leung, H.H.L.; Ahmadi, Z.; Yan, F.; Chong, J.J.H.; Passam, F.H.; Chong, B.H. Neutrophil activation and NETosis are the major drivers of thrombosis in heparin-induced thrombocytopenia. Nat. Commun. 2019, 10, 1322. [Google Scholar] [CrossRef]
- Rossaint, J.; Herter, J.M.; Van Aken, H.; Napirei, M.; Döring, Y.; Weber, C.; Soehnlein, O.; Zarbock, A. Synchronized integrin engagement and chemokine activation is crucial in neutrophil extracellular trap–mediated sterile inflammation. Blood 2014, 123, 2573–2584. [Google Scholar] [CrossRef]
- Orlova, V.V.; Choi, E.Y.; Xie, C.; Chavakis, E.; Bierhaus, A.; Ihanus, E.; Ballantyne, C.M.; Gahmberg, C.G.; E Bianchi, M.; Nawroth, P.P.; et al. A novel pathway of HMGB1-mediated inflammatory cell recruitment that requires Mac-1-integrin. EMBO J. 2007, 26, 1129–1139. [Google Scholar] [CrossRef]
- Maugeri, N.; Campana, L.; Gavina, M.; Covino, C.; De Metrio, M.; Panciroli, C.; Maiuri, L.; Maseri, A.; D’Angelo, A.; Bianchi, M.E.; et al. Activated platelets present high mobility group box 1 to neutrophils, inducing autophagy and promoting the extrusion of neutrophil extracellular traps. J. Thromb. Haemost. 2014, 12, 2074–2088. [Google Scholar] [CrossRef]
- Cognasse, F.; Nguyen, K.A.; Damien, P.; McNicol, A.; Pozzetto, B.; Hamzeh-Cognasse, H.; Garraud, O. The inflammatory role of platelets via their TLRs and Siglec receptors. Front. Immunol. 2015, 6, 83. [Google Scholar] [CrossRef]
- Weyrich, A.S.; Elstad, M.R.; McEver, R.P.; McIntyre, T.M.; Moore, K.L.; Morrissey, J.H.; Prescott, S.M.; A Zimmerman, G. Activated platelets signal chemokine synthesis by human monocytes. J. Clin. Investig. 1996, 97, 1525–1534. [Google Scholar] [CrossRef] [PubMed]
- Blair, P.; Flaumenhaft, R. Platelet α-granules: Basic biology and clinical correlates. Blood Rev. 2009, 23, 177–189. [Google Scholar] [CrossRef]
- Rolfes, V.; Ribeiro, L.S.; Hawwari, I.; Böttcher, L.; Rosero, N.; Maasewerd, S.; Santos, M.L.S.; Próchnicki, T.; Silva, C.M.D.S.; Wanderley, C.W.D.S.; et al. Platelets Fuel the Inflammasome Activation of Innate Immune Cells. Cell Rep. 2020, 31, 107615. [Google Scholar] [CrossRef]
- Peerschke, E.I.B.; Yin, W.; Ghebrehiwet, B. Complement activation on platelets: Implications for vascular inflammation and thrombosis. Mol. Immunol. 2010, 47, 2170–2175. [Google Scholar] [CrossRef]
- Del Conde, I.; Crúz, M.A.; Zhang, H.; López, J.A.; Afshar-Kharghan, V. Platelet activation leads to activation and propagation of the complement system. J. Exp. Med. 2005, 201, 871–879. [Google Scholar] [CrossRef]
- Hamad, O.A.; Ekdahl, K.N.; Nilsson, P.H.; Andersson, J.; Magotti, P.; Lambris, J.D.; Nilsson, B. Complement activation triggered by chondroitin sulfate released by thrombin receptor-activated platelets. J. Thromb. Haemost. 2008, 6, 1413–1421. [Google Scholar] [CrossRef]
- Yin, W.; Ghebrehiwet, B.; Peerschke, E.I.B. Expression of complement components and inhibitors on platelet microparticles. Platelets 2008, 19, 225–233. [Google Scholar] [CrossRef]
- Morrell, C.N.; Aggrey, A.A.; Chapman, L.M.; Modjeski, K.L. Emerging roles for platelets as immune and inflammatory cells. Blood 2014, 123, 2759–2767. [Google Scholar] [CrossRef]
- Mause, S.F.; Von Hundelshausen, P.; Zernecke, A.; Koenen, R.R.; Weber, C. Platelet Microparticles. Arter. Thromb. Vasc. Biol. 2005, 25, 1512–1518. [Google Scholar] [CrossRef]
- Rousseau, M.; Duchez, A.-C.; Lee, C.H.C.; Boilard, E.; Laffont, B.; Corduan, A.; Provost, P. Platelet microparticles reprogram macrophage gene expression and function. Thromb. Haemost. 2016, 115, 311–323. [Google Scholar] [CrossRef]
- Gidlöf, O.; Van Der Brug, M.; Öhman, J.; Gilje, P.; Olde, B.; Wahlestedt, C.; Erlinge, D. Platelets activated during myocardial infarction release functional miRNA, which can be taken up by endothelial cells and regulate ICAM1 expression. Blood 2013, 121, 3908–3917. [Google Scholar] [CrossRef] [PubMed]
- Laffont, B.; Corduan, A.; Plé, H.; Duchez, A.-C.; Cloutier, N.; Boilard, E.; Provost, P. Activated platelets can deliver mRNA regulatory Ago2•microRNA complexes to endothelial cells via microparticles. Blood 2013, 122, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Poon, K.-S.; Palanisamy, K.; Chang, S.-S.; Sun, K.-T.; Chen, K.-B.; Li, P.-C.; Lin, T.-C.; Li, C.-Y. Plasma exosomal miR-223 expression regulates inflammatory responses during cardiac surgery with cardiopulmonary bypass. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Müller, I.; Klocke, A.; Alex, M.; Kotzsch, M.; Luther, T.; Morgenstern, E.; Zieseniss, S.; Zahler, S.; Preissner, K.; Engelmann, B. Intravascular tissue factor initiates coagulation via circulating microvesicles and platelets. FASEB J. 2003, 17, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Mukai, N.; Nakayama, Y.; Ishi, S.; Ogawa, S.; Maeda, S.; Anada, N.; Murakami, S.; Mizobe, T.; Sawa, T.; Nakajima, Y. Changes in MicroRNA Expression Level of Circulating Platelets Contribute to Platelet Defect After Cardiopulmonary Bypass. Crit. Care Med. 2018, 46, e761–e767. [Google Scholar] [CrossRef]
- Murase, M.; Nakayama, Y.; Sessler, D.; Mukai, N.; Ogawa, S.; Nakajima, Y. Changes in platelet Bax levels contribute to impaired platelet response to thrombin after cardiopulmonary bypass: Prospective observational clinical and laboratory investigations. Br. J. Anaesth. 2017, 119, 1118–1126. [Google Scholar] [CrossRef]
- De Backer, D.; Dubois, M.-J.; Schmartz, D.; Koch, M.; Ducart, A.; Barvais, L.; Vincent, J.-L. Microcirculatory Alterations in Cardiac Surgery: Effects of Cardiopulmonary Bypass and Anesthesia. Ann. Thorac. Surg. 2009, 88, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Koning, N.J.; Atasever, B.; Vonk, A.B.; Boer, C. Changes in Microcirculatory Perfusion and Oxygenation During Cardiac Surgery With or Without Cardiopulmonary Bypass. J. Cardiothorac. Vasc. Anesthesia 2014, 28, 1331–1340. [Google Scholar] [CrossRef]
- Di Dedda, U.; Ranucci, M.; Porta, A.; Bari, V.; Ascari, A.; Fantinato, A.; Baryshnikova, E.; Cotza, M. The combined effects of the microcirculatory status and cardiopulmonary bypass on platelet count and function during cardiac surgery. Clin. Hemorheol. Microcirc. 2018, 70, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Karhausen, J.; Choi, H.W.; Maddipati, K.R.; Mathew, J.P.; Ma, Q.; Boulaftali, Y.; Lee, R.H.; Bergmeier, W.; Abraham, S.N. Platelets trigger perivascular mast cell degranulation to cause inflammatory responses and tissue injury. Sci. Adv. 2020, 6, eaay6314. [Google Scholar] [CrossRef]
- Hill, J.D.; O’Brien, T.G.; Murray, J.J.; Dontigny, L.; Bramson, M.L.; Osborn, J.J.; Gerbode, F. Prolonged Extracorporeal Oxygenation for Acute Post-Traumatic Respiratory Failure (Shock-Lung Syndrome). N. Engl. J. Med. 1972, 286, 629–634. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Lin, J.-W.; Yu, H.-Y.; Ko, W.-J.; Jerng, J.-S.; Chang, W.-T.; Chen, W.-J.; Huang, S.-C.; Chi, N.-H.; Wang, C.-H.; et al. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: An observational study and propensity analysis. Lancet 2008, 372, 554–561. [Google Scholar] [CrossRef]
- Sy, E.; Sklar, M.C.; Lequier, L.; Fan, E.; Kanji, H.D. Anticoagulation practices and the prevalence of major bleeding, thromboembolic events, and mortality in venoarterial extracorporeal membrane oxygenation: A systematic review and meta-analysis. J. Crit. Care 2017, 39, 87–96. [Google Scholar] [CrossRef]
- Yoshimoto, Y.; Hasebe, T.; Takahashi, K.; Amari, M.; Nagashima, S.; Kamijo, A.; Hotta, A.; Takahashi, K.; Suzuki, T. Ultrastructural characterization of surface-induced platelet activation on artificial materials by transmission electron microscopy. Microsc. Res. Tech. 2013, 76, 342–349. [Google Scholar] [CrossRef]
- Chen, Z.; Mondal, N.K.; Zheng, S.; Koenig, S.C.; Slaughter, M.S.; Griffith, B.P.; Wu, Z.J. High shear induces platelet dysfunction leading to enhanced thrombotic propensity and diminished hemostatic capacity. Platelets 2017, 30, 112–119. [Google Scholar] [CrossRef]
- Sun, W.; Wang, S.; Chen, Z.; Zhang, J.; Li, T.; Arias, K.; Griffith, B.P.; Wu, Z.J. Impact of high mechanical shear stress and oxygenator membrane surface on blood damage relevant to thrombosis and bleeding in a pediatric ECMO circuit. Artif. Organs 2020, 44, 717–726. [Google Scholar] [CrossRef]
- Lukito, P.; Wong, A.; Jing, J.; Arthur, J.F.; Marasco, S.F.; Murphy, D.A.; Bergin, P.J.; Shaw, J.A.; Collecutt, M.; Andrews, R.K.; et al. Mechanical circulatory support is associated with loss of platelet receptors glycoprotein Ibα and glycoprotein VI. J. Thromb. Haemost. 2016, 14, 2253–2260. [Google Scholar] [CrossRef]
- Chung, J.H.; Yeo, H.J.; Kim, D.; Lee, S.M.; Han, J.; Kim, M.; Cho, W.H. Changes in the Levels of Beta-thromboglobulin and Inflammatory Mediators during Extracorporeal Membrane Oxygenation Support. Int. J. Artif. Organs 2017, 40, 575–580. [Google Scholar] [CrossRef]
- Kalbhenn, J.; Schlagenhauf, A.; Rosenfelder, S.; Schmutz, A.; Zieger, B. Acquired von Willebrand syndrome and impaired platelet function during venovenous extracorporeal membrane oxygenation: Rapid onset and fast recovery. J. Hear. Lung Transplant. 2018, 37, 985–991. [Google Scholar] [CrossRef]
- Fuchs, G.; Berg, N.; Broman, L.M.; Wittberg, L.P. Flow-induced platelet activation in components of the extracorporeal membrane oxygenation circuit. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Arachchillage, D.J.; Laffan, M.; Khanna, S.; Vandenbriele, C.; Kamani, F.; Passariello, M.; Rosenberg, A.; Aw, T.C.; Banya, W.; Ledot, S.; et al. Frequency of Thrombocytopenia and Heparin-Induced Thrombocytopenia in Patients Receiving Extracorporeal Membrane Oxygenation Compared With Cardiopulmonary Bypass and the Limited Sensitivity of Pretest Probability Score. Crit. Care Med. 2020, 48, e371–e379. [Google Scholar] [CrossRef]
- The Extracorporeal Life Support Organization (ELSO). Extracorporeal Life Support Organization (ELSO) General Guidelines for All ECLS Cases. Available online: https://www.elso.org/Portals/0/Files/elsoanticoagulationguideline8-2014-table-contents.pdf (accessed on 26 July 2020).
- Jiritano, F.; Serraino, G.F.; Cate, H.T.; Fina, D.; Matteucci, M.; Mastroroberto, P.; Lorusso, R. Platelets and extra-corporeal membrane oxygenation in adult patients: A systematic review and meta-analysis. Intensiv. Care Med. 2020, 46, 1154–1169. [Google Scholar] [CrossRef]
- Abrams, D.; Baldwin, M.R.; Champion, M.; Agerstrand, C.; Eisenberger, A.; Bacchetta, M.; Brodie, D. Thrombocytopenia and extracorporeal membrane oxygenation in adults with acute respiratory failure: A cohort study. Intensiv. Care Med. 2016, 42, 844–852. [Google Scholar] [CrossRef]
- Panigada, M.; Artoni, A.; Passamonti, S.M.; Maino, A.; Mietto, C.; L’Acqua, C.; Cressoni, M.; Boscolo, M.; Tripodi, A.; Bucciarelli, P.; et al. Hemostasis changes during veno-venous extracorporeal membrane oxy-genation for respiratory support in adults. Minerva Anestesiol. 2016, 82, 170–179. [Google Scholar]
- Ang, A.L.; Teo, D.; Lim, C.H.; Leou, K.K.; Tien, S.L.; Koh, M.B.C. Blood transfusion requirements and independent predictors of increased transfusion requirements among adult patients on extracorporeal membrane oxygenation - a single centre experience. Vox Sang. 2009, 96, 34–43. [Google Scholar] [CrossRef]
- Rauova, L.; Poncz, M.; McKenzie, S.E.; Reilly, M.P.; Arepally, G.; Weisel, J.W.; Nagaswami, C.; Cines, D.B.; Sachais, B.S.; Herre, J.; et al. Ultralarge complexes of PF4 and heparin are central to the pathogenesis of heparin-induced thrombocytopenia. Blood 2005, 105, 131–138. [Google Scholar] [CrossRef]
- Ontaneda, A.; Annich, G.M. Novel Surfaces in Extracorporeal Membrane Oxygenation Circuits. Front. Med. 2018, 5, 321. [Google Scholar] [CrossRef]
- Klein, S.; Hesselmann, F.; Djeljadini, S.; Berger, T.; Thiebes, A.L.; Schmitz-Rode, T.; Jockenhoevel, S.; Cornelissen, C.G. EndOxy: Dynamic Long-Term Evaluation of Endothelialized Gas Exchange Membranes for a Biohybrid Lung. Ann. Biomed. Eng. 2020, 48, 747–756. [Google Scholar] [CrossRef]
- Jiritano, F.; Santarpino, G.; Serraino, G.F.; Cate, H.T.; Matteucci, M.; Fina, D.; Mastroroberto, P.; Lorusso, R. Peri-procedural thrombocytopenia after aortic bioprosthesis implant: A systematic review and meta-analysis comparison among conventional, stentless, rapid-deployment, and transcatheter valves. Int. J. Cardiol. 2019, 296, 43–50. [Google Scholar] [CrossRef]
- Steinlechner, B.; Dworschak, M.; Birkenberg, B.; Duris, M.; Zeidler, P.; Fischer, H.; Milosevic, L.; Wieselthaler, G.; Wolner, E.; Quehenberger, P.; et al. Platelet Dysfunction in Outpatients With Left Ventricular Assist Devices. Ann. Thorac. Surg. 2009, 87, 131–137. [Google Scholar] [CrossRef]
- Chen, Z.; Mondal, N.K.; Ding, J.; Koenig, S.C.; Slaughter, M.S.; Wu, Z.J. Paradoxical Effect of Nonphysiological Shear Stress on Platelets and von Willebrand Factor. Artif. Organs 2016, 40, 659–668. [Google Scholar] [CrossRef]
- Vogt, F.; Moscarelli, M.; Pollari, F.; Kalisnik, J.M.; Pfeiffer, S.; Fittkau, M.; Sirch, J.; Pförringer, D.; Jessl, J.; Eckner, D.; et al. Two approaches—one phenomenon—thrombocytopenia after surgical and transcatheter aortic valve replacement. J. Card. Surg. 2020, 35, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Le Guyader, A.; Watanabe, R.; Berbé, J.; Boumediene, A.; Cogné, M.; Laskar, M. Platelet activation after aortic prosthetic valve surgery. Interact. Cardiovasc. Thorac. Surg. 2005, 5, 60–64. [Google Scholar] [CrossRef]
- Ravenni, G.; Celiento, M.; Ferrari, G.; Milano, A.; Scioti, G.; Pratali, S.; Bortolotti, U. Reduction in platelet count after aortic valve replacement: Comparison of three bioprostheses. J. Hear. Valve Dis. 2012, 21, 655–661. [Google Scholar]
- Stanger, O.; Grabherr, M.; Gahl, B.; Longnus, S.; Meinitzer, A.; Fiedler, M.; Tevaearai, H.; Carrel, T. Thrombocytopaenia after aortic valve replacement with stented, stentless and sutureless bioprostheses. Eur. J. Cardio-Thoracic Surg. 2016, 51, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Yerebakan, C.; Kaminski, A.; Westphal, B.; Kundt, G.; Ugurlucan, M.; Steinhoff, G.; Liebold, A. Thrombocytopenia after aortic valve replacement with the Freedom Solo stentless bioprosthesis. Interact. Cardiovasc. Thorac. Surg. 2008, 7, 616–620. [Google Scholar] [CrossRef][Green Version]
- Hilker, L.; Wodny, M.; Ginesta, M.; Wollert, H.-G.; Eckel, L. Differences in the recovery of platelet counts after biological aortic valve replacement. Interact. Cardiovasc. Thorac. Surg. 2008, 8, 70–73. [Google Scholar] [CrossRef]
- Miceli, A.; Gilmanov, D.; Murzi, M.; Parri, M.S.; Cerillo, A.; Bevilacqua, S.; Farneti, P.A.; Glauber, M. Evaluation of platelet count after isolated biological aortic valve replacement with Freedom Solo bioprosthesis. Eur. J. Cardio-Thoracic Surg. 2011, 41, 69–73. [Google Scholar] [CrossRef]
- Piccardo, A.; Rusinaru, D.; Petitprez, B.; Marticho, P.; Vaida, I.; Tribouilloy, C.; Caus, T. Thrombocytopenia After Aortic Valve Replacement With Freedom Solo Bioprosthesis: A Propensity Study. Ann. Thorac. Surg. 2010, 89, 1425–1430. [Google Scholar] [CrossRef]
- Sánchez, E.; Corrales, J.-A.; Fantidis, P.; Tarhini, I.S.; Khan, I.; Pineda, T.; González, J.-R. Thrombocytopenia after Aortic Valve Replacement with Perceval S Sutureless Bioprosthesis. J. Hear. Valve Dis. 2016, 25, 75–81. [Google Scholar]
- Mujtaba, S.S.; Ledingham, S.; Shah, A.R.; Schueler, S.; Clark, S.; Pillay, T. Thrombocytopenia After Aortic Valve Replacement: Comparison Between Sutureless Perceval S Valve and Perimount Magna Ease Bioprosthesis. Braz. J. Cardiovasc. Surg. 2018, 33, 169–175. [Google Scholar] [CrossRef]
- Andreas, M.; Wallner, S.; Habertheuer, A.; Rath, C.; Schauperl, M.; Binder, T.; Beitzke, D.; Rosenhek, R.; Loewe, C.; Wiedemann, D.; et al. Conventional versus rapid-deployment aortic valve replacement: A single-centre comparison between the Edwards Magna valve and its rapid-deployment successor. Interact. Cardiovasc. Thorac. Surg. 2016, 22, 799–805. [Google Scholar] [CrossRef]
- Jiritano, F.; Cristodoro, L.; Malta, E.; Mastroroberto, P. Thrombocytopenia after sutureless aortic valve implantation: Comparison between Intuity and Perceval bioprostheses. J. Thorac. Cardiovasc. Surg. 2016, 152, 1631–1633. [Google Scholar] [CrossRef] [PubMed]
- Gallet, R.; Seemann, A.; Yamamoto, M.; Hayat, D.; Mouillet, G.; Monin, J.-L.; Gueret, P.; Couetil, J.-P.; Dubois-Randé, J.-L.; Teiger, E.; et al. Effect of Transcatheter (via Femoral Artery) Aortic Valve Implantation on the Platelet Count and Its Consequences. Am. J. Cardiol. 2013, 111, 1619–1624. [Google Scholar] [CrossRef]
- Dvir, D.; Généreux, P.; Barbash, I.M.; Kodali, S.; Ben-Dor, I.; Williams, M.; Torguson, R.; Kirtane, A.J.; Minha, S.; Badr, S.; et al. Acquired thrombocytopenia after transcatheter aortic valve replacement: Clinical correlates and association with outcomes. Eur. Hear. J. 2014, 35, 2663–2671. [Google Scholar] [CrossRef] [PubMed]
- McCabe, J.M.; Huang, P.-H.; Riedl, L.A.; Ba, S.R.D.; Rn, J.G.; Rn, A.C.C.; Davidson, M.J.; Eisenhauer, A.C.; Welt, F.G. Incidence and implications of idiopathic thrombocytopenia following transcatheter aortic valve replacement with the Edwards Sapien©valves: A single center experience. Catheter. Cardiovasc. Interv. 2013, 83, 633–641. [Google Scholar] [CrossRef]
- Gul, M.; Uyarel, H.; Akgul, O.; Uslu, N.; Yildirim, A.; Eksik, A.; Aksu, H.U.; Ozal, E.; Pusuroglu, H.; Erol, M.K.; et al. Hematologic and Clinical Parameters After Transcatheter Aortic Valve Implantation (TAVI) in Patients With Severe Aortic Stenosis. Clin. Appl. Thromb. 2012, 20, 304–310. [Google Scholar] [CrossRef]
- Flaherty, M.P.; Mohsen, A.; Moore, J.B.; Bartoli, C.R.; Schneibel, E.; Rawasia, W.; Williams, M.L.; Grubb, K.J.; Hirsch, G.A. Predictors and clinical impact of pre-existing and acquired thrombocytopenia following transcatheter aortic valve replacement. Catheter. Cardiovasc. Interv. 2014, 85, 118–129. [Google Scholar] [CrossRef]
- Sedaghat, A.; Falkenberg, N.; Sinning, J.-M.; Kulka, H.; Hammerstingl, C.; Nickenig, G.; Oldenburg, J.; Pötzsch, B.; Werner, N. TAVI induces an elevation of hemostasis-related biomarkers, which is not causative for post-TAVI thrombocytopenia. Int. J. Cardiol. 2016, 221, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Enríquez, M.; Chollet, T.; Bataille, V.; Campelo-Parada, F.; Boudou, N.; Bouisset, F.; Grunenwald, E.; Porterie, J.; Freixa, X.; Regueiro, A.; et al. Comparison of the Frequency of Thrombocytopenia After Transfemoral Transcatheter Aortic Valve Implantation Between Balloon-Expandable and Self-Expanding Valves. Am. J. Cardiol. 2019, 123, 1120–1126. [Google Scholar] [CrossRef]
- Jilaihawi, H.; Doctor, N.; Chakravarty, T.; Kashif, M.; Mirocha, J.; Cheng, W.; Lill, M.; Nakamura, M.; Gheorghiu, M.; Makkar, R. Major thrombocytopenia after balloon-expandable transcatheter aortic valve replacement: Prognostic implications and comparison to surgical aortic valve replacement. Catheter. Cardiovasc. Interv. 2015, 85, 130–137. [Google Scholar] [CrossRef]
- Abu Saleh, W.K.; Tang, G.H.L.; Ahmad, H.; Cohen, M.; Undemir, C.; Lansman, S.L.S.L.; Reyes, M.; Barker, C.M.; Kleiman, N.S.; Reardon, M.J.; et al. Vascular complication can be minimized with a balloon-expandable, re-collapsible sheath in TAVR with a self-expanding bioprosthesis. Catheter. Cardiovasc. Interv. 2015, 88, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Mitrosz, M.; Kazimierczyk, R.; Sobkowicz, B.; Waszkiewicz, E.; Kralisz, P.; Frank, M.; Piszcz, J.; Galar, M.; Dobrzycki, S.; Musial, W.J.; et al. The causes of thrombocytopenia after transcatheter aortic valve implantation. Thromb. Res. 2017, 156, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Mitrosz, M.; Kazimierczyk, R.; Chlabicz, M.; Sobkowicz, B.; Waszkiewicz, E.; Lisowska, A.; Dobrzycki, S.; Musial, W.J.; Hirnle, T.; Kaminski, K.A.; et al. Perioperative thrombocytopenia predicts poor outcome in patients undergoing transcatheter aortic valve implantation. Adv. Med Sci. 2018, 63, 179–184. [Google Scholar] [CrossRef]
- Takagi, H.; Hari, Y.; Nakashima, K.; Ueyama, H.; Kuno, T.; Ando, T. Impact of postprocedural thrombocytopenia on mortality after transcatheter aortic valve implantation. J. Cardiovasc. Med. 2020, 21, 318–324. [Google Scholar] [CrossRef]
- Takahashi, S.; Yokoyama, N.; Watanabe, Y.; Katayama, T.; Hioki, H.; Yamamoto, H.; Kawasugi, K.; Kozuma, K. Predictor and Mid-Term Outcome of Clinically Significant Thrombocytopenia After Transcatheter Aortic Valve Selection. Circ. J. 2020, 84, 1020–1027. [Google Scholar] [CrossRef]
- Mullen, M.P.; Wessel, D.L.; Thomas, K.C.; Gauvreau, K.; Neufeld, E.J.; McGowan, F.X.; Dinardo, J.A. The Incidence and Implications of Anti-Heparin-Platelet Factor 4 Antibody Formation in a Pediatric Cardiac Surgical Population. Anesthesia Analg. 2008, 107, 371–378. [Google Scholar] [CrossRef]
- Dhakal, B.; Kreuziger, L.B.; Rein, L.; Kleman, A.; Fraser, R.; Aster, R.H.; Hari, P.; Padmanabhan, A. Disease burden, complication rates, and health-care costs of heparin-induced thrombocytopenia in the USA: A population-based study. Lancet Haematol. 2018, 5, e220–e231. [Google Scholar] [CrossRef]
- Dhakal, P.; Giri, S.; Pathak, R.; Bhatt, V.R. Heparin Reexposure in Patients With a History of Heparin-Induced Thrombocytopenia. Clin. Appl. Thromb. 2013, 21, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E.; Sheppard, J.-A.I.; Sigouin, C.S.; Kohlmann, T.; Eichler, P.; Greinacher, A. Gender imbalance and risk factor interactions in heparin-induced thrombocytopenia. Blood 2006, 108, 2937–2941. [Google Scholar] [CrossRef]
- Stein, P.D.; Hull, R.D.; Matta, F.; Yaekoub, A.Y.; Liang, J. Incidence of Thrombocytopenia in Hospitalized Patients with Venous Thromboembolism. Am. J. Med. 2009, 122, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Karnes, J.H.; Cronin, R.M.; Rollin, J.; Teumer, A.; Pouplard, C.; Shaffer, C.M.; Blanquicett, C.; Bowton, E.A.; Cowan, J.D.; Mosley, J.D.; et al. A genome-wide association study of heparin-induced thrombocyto - penia using an electronic medical record. Thromb. Haemost. 2015, 113, 772–781. [Google Scholar] [CrossRef]
- Karnes, J.H.; Shaffer, C.M.; Cronin, R.; Bastarache, L.; Gaudieri, S.; James, I.; Pavlos, R.; Steiner, H.E.; Mosley, J.D.; Mallal, S.; et al. Influence of Human Leukocyte Antigen (HLA) Alleles and Killer Cell Immunoglobulin-Like Receptors (KIR) Types on Heparin-Induced Thrombocytopenia (HIT). Pharmacother. J. Hum. Pharmacol. Drug Ther. 2017, 37, 1164–1171. [Google Scholar] [CrossRef]
- Witten, A.; Bolbrinker, J.; Barysenka, A.; Huber, M.; Rühle, F.; Nowak-Göttl, U.; Garbe, E.; Kreutz, R.; Stoll, M. Targeted resequencing of a locus for heparin-induced thrombocytopenia on chromosome 5 identified in a genome-wide association study. J. Mol. Med. 2018, 96, 765–775. [Google Scholar] [CrossRef]
- Smythe, M.A.; Koerber, J.M.; Mattson, J.C. The Incidence of Recognized Heparin-Induced Thrombocytopenia in a Large, Tertiary Care Teaching Hospital. Chest 2007, 131, 1644–1649. [Google Scholar] [CrossRef] [PubMed]
- Amiral, J.; Peynaud-Debayle, E.; Wolf, M.; Bridey, F.; Vissac, A.M.; Meyer, D. Generation of antibodies to heparin-PF4 com-plexes without thrombocytopenia in patients treated with unfractionated or low-molecular-weight heparin. Am. J. Hematol. 1996, 52, 90–95. [Google Scholar] [CrossRef]
- Warkentin, T.E.; Sheppard, J.A.; Horsewood, P.; Simpson, P.J.; Moore, J.C.; Kelton, J.G. Impact of the patient population on the risk for heparin-induced thrombocytopenia. Blood 2000, 96, 1703–1708. [Google Scholar] [CrossRef] [PubMed]
- Cuker, A.; Cines, U.B. How I treat heparin-induced thrombocytopenia. Blood 2012, 119, 2209–2218. [Google Scholar] [CrossRef]
- McGowan, K.E.; Makari, J.; Diamantouros, A.; Bucci, C.; Rempel, P.; Selby, R.; Geerts, W. Reducing the hospital burden of heparin-induced thrombocytopenia: Impact of an avoid-heparin program. Blood 2016, 127, 1954–1959. [Google Scholar] [CrossRef]
- Suvarna, S.; Espinasse, B.; Qi, R.; Lubica, R.; Poncz, M.; Cines, U.B.; Wiesner, M.R.; Arepally, G.M. Determinants of PF4/heparin immunogenicity. Blood 2007, 110, 4253–4260. [Google Scholar] [CrossRef]
- Arepally, G.M.; Cines, D.B. Pathogenesis of heparin-induced thrombocytopenia. Transl. Res. 2020, 225, 131–140. [Google Scholar] [CrossRef]
- Francis, J.L.; Palmer, G.J.; Moroose, R.; Drexler, A. Comparison of bovine and porcine heparin in heparin antibody formation after cardiac surgery. Ann. Thorac. Surg. 2003, 75, 17–22. [Google Scholar] [CrossRef]
- Ahmad, S. Heparin-induced thrombocytopenia: Impact of bovine versus porcine heparin in HIT pathogenesis. Front. Biosci. 2007, 12, 3312–3320. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Greinacher, A.; Warkentin, T.E.; Chong, B.H. Heparin-induced thrombocytopenia. In Platelets, 4th ed.; Michelson, A.D., Ed.; Academic Press: New York, NY, USA, 2019; pp. 741–767. [Google Scholar]
- Hayes, V.; Johnston, I.; Arepally, G.M.; McKenzie, S.E.; Cines, D.B.; Rauova, L.; Poncz, M. Endothelial antigen assembly leads to thrombotic complications in heparin-induced thrombocytopenia. J. Clin. Investig. 2017, 127, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Gollomp, K.; Kim, M.; Johnston, I.; Hayes, V.; Welsh, J.; Arepally, G.M.; Kahn, M.; Lambert, M.P.; Cuker, A.; Cines, D.B.; et al. Neutrophil accumulation and NET release contribute to thrombosis in HIT. JCI Insight 2018, 3, 99445. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.L.; Arepally, G.; Konkle, B.A.; Mestichelli, B.; Shapiro, S.S.; Cines, D.B.; Poncz, M.; McNulty, S.; Amiral, J.; Hauck, W.W.; et al. Prevalence of Heparin-Associated Antibodies Without Thrombosis in Patients Undergoing Cardiopulmonary Bypass Surgery. Circ. 1997, 95, 1242–1246. [Google Scholar] [CrossRef]
- Pouplard, C.; May, M.-A.; Iochmann, S.; Amiral, J.; Vissac, A.-M.; Marchand, M.; Gruel, Y. Antibodies to Platelet Factor 4–Heparin After Cardiopulmonary Bypass in Patients Anticoagulated With Unfractionated Heparin or a Low-Molecular-Weight Heparin. Circ. 1999, 99, 2530–2536. [Google Scholar] [CrossRef]
- Koster, A.; Sänger, S.; Hansen, R.; Sodian, R.; Mertzlufft, F.; Harke, C.; Kuppe, H.; Hetzer, R.; Loebe, M. Prevalence and Persistence of Heparin/Platelet Factor 4 Antibodies in Patients with Heparin Coated and Noncoated Ventricular Assist Devices. ASAIO J. 2000, 46, 319–322. [Google Scholar] [CrossRef]
- Schenk, S.; El-Banayosy, A.; Prohaska, W.; Arusoglu, L.; Morshuis, M.; Koester-Eiserfunke, W.; Kizner, L.; Murray, E.; Eichler, P.; Koerfer, R.; et al. Heparin-induced thrombocytopenia in patients receiving mechanical circulatory support. J. Thorac. Cardiovasc. Surg. 2006, 131, 1373–1381.e4. [Google Scholar] [CrossRef]
- Warkentin, T.E.; Sheppard, J.I.; Sun, J.C.J.; Jung, H.; Eikelboom, J.W. Anti-PF4/heparin antibodies and venous graft occlusion in postcoronary artery bypass surgery patients randomized to postoperative unfractionated heparin or fondaparinux thromboprophylaxis. J. Thromb. Haemost. 2013, 11, 253–260. [Google Scholar] [CrossRef]
- Demma, L.J.; Winkler, A.M.; Levy, J.H. A Diagnosis of Heparin-Induced Thrombocytopenia with Combined Clinical and Laboratory Methods in Cardiothoracic Surgical Intensive Care Unit Patients. Anesthesia Analg. 2011, 113, 697–702. [Google Scholar] [CrossRef]
- Warkentin, T.E.; Arsenault, K.A.; Whitlock, R.; Eikelboom, J.; Yusuf, A.M. Prognostic importance of preoperative anti-PF4/heparin antibodies in patients undergoing cardiac surgery. Thromb. Haemost. 2012, 107, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Bennett-Guerrero, E.; Slaughter, T.F.; White, W.D.; Welsby, I.J.; Greenberg, C.S.; El-Moalem, H.; Ortel, T.L. Preoperative anti-PF4/heparin antibody level predicts adverse outcome after cardiac surgery. J. Thorac. Cardiovasc. Surg. 2005, 130, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Everett, B.M.; Yeh, R.; Foo, S.Y.; Criss, D.; Van Cott, E.M.; Laposata, M.; Avery, E.G.; Hoffman, W.D.; Walker, J.; Torchiana, D.; et al. Prevalence of Heparin/Platelet Factor 4 Antibodies Before and After Cardiac Surgery. Ann. Thorac. Surg. 2007, 83, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Selleng, S.; Malowsky, B.; Itterman, T.; Bagemühl, J.; Wessel, A.; Wollert, H.-G.; Warkentin, T.E.; Greinacher, A. Incidence and clinical relevance of anti–platelet factor 4/heparin antibodies before cardiac surgery. Am. Hear. J. 2010, 160, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, A.V.; Bonetti, L.; Zennaro, M.; Ambrosio, G.; Mattioli, G. Heparin/PF4 antibodies formation after heparin treatment: Temporal aspects and long-term follow-up. Am. Hear. J. 2009, 157, 589–595. [Google Scholar] [CrossRef]
- Dyke, C.M.; Smedira, N.G.; Koster, A.; Aronson, S.; McCarthy, H.L.; Kirshner, R.; Lincoff, A.M.; Spiess, B.D. A comparison of bivalirudin to heparin with protamine reversal in patients undergoing cardiac surgery with cardiopulmonary bypass: The EVOLUTION-ON study. J. Thorac. Cardiovasc. Surg. 2006, 131, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Koster, A.; Dyke, C.M.; Aldea, G.; Smedira, N.G.; Ii, H.L.M.; Aronson, S.; Hetzer, R.; Avery, E.; Spiess, B.; Lincoff, A.M. Bivalirudin During Cardiopulmonary Bypass in Patients With Previous or Acute Heparin-Induced Thrombocytopenia and Heparin Antibodies: Results of the CHOOSE-ON Trial. Ann. Thorac. Surg. 2007, 83, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Follis, F.; Filippone, G.; Montalbano, G.; Floriano, M.; Lobianco, E.; D’Ancona, G.; Follis, M. Argatroban as a substitute of heparin during cardiopulmonary bypass: A safe alternative? Interact. Cardiovasc. Thorac. Surg. 2010, 10, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Ullom, B.; Al-Baghdadi, Y.; Okumura, M. Challenges encountered with argatroban anticoagulation during cardiopulmonary bypass. Journal of Anaesthesiology, Clinical Pharmacology 2012, 28, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, T.; Kapetanakis, E.I.; Theodoraki, K.; Rellia, P.; Thanopoulos, A.; Kotiou, M.; Zarkalis, D.; Alivizatos, P. Cardiac surgery in patients with heparin-induced thrombocytopenia using preoperatively determined dosages of iloprost. Hear. Surg. Forum 2002, 5, 354–357. [Google Scholar]
- Voeller, R.K.; Melby, S.J.; Grizzell, B.E.; Moazami, N. Novel use of plasmapheresis in a patient with heparin-induced thrombocytopenia requiring urgent insertion of a left ventricular assist device under cardiopulmonary bypass. J. Thorac. Cardiovasc. Surg. 2010, 140, e56–e58. [Google Scholar] [CrossRef]
- Jaben, E.A.; Torloni, A.S.; Pruthi, R.K.; Winters, J.L. Use of plasma exchange in patients with heparin-induced thrombocytopenia: A report of two cases and a review of the literature. J. Clin. Apher. 2011, 26, 219–224. [Google Scholar] [CrossRef]
- A Cannon, M.; Butterworth, J.; Riley, R.D.; Hyland, J.M. Failure of argatroban anticoagulation during off-pump coronary artery bypass surgery. Ann. Thorac. Surg. 2004, 77, 711–713. [Google Scholar] [CrossRef] [PubMed]
- Dyke, C.M.; Aldea, G.; Koster, A.; Smedira, N.; Avery, E.; Aronson, S.; Spiess, B.D.; Lincoff, A.M. Off-Pump Coronary Artery Bypass with Bivalirudin for Patients with Heparin-Induced Thrombocytopenia or Antiplatelet Factor Four/Heparin Antibodies. Ann. Thorac. Surg. 2007, 84, 836–839. [Google Scholar] [CrossRef] [PubMed]
| “4 Ts” Category | 2 Points | 1 Point | 0 Points |
|---|---|---|---|
| Thrombocytopenia | Platelet count fall > 50% AND platelet nadir ≥ 20 × 109/L | Platelet count fall 30–50% OR platelet nadir 10–19 × 109/L | Platelet count fall < 30% OR platelet nadir < 10 × 109/L |
| Timing of platelet count fall | Clear onset between days 5–10 OR platelet fall ≤ 1 day (prior heparin exposure within 30 days) | Consistent with days 5–10 fall but not clear; onset after day 10 OR fall ≤ 1 day (prior heparin exposure 30–100 days ago) | Platelet count fall < 4 days without recent exposure |
| Thrombosis or other sequelae | New thrombosis OR skin necrosis; acute systemic reaction postintravenous heparin bolus | Progressive or recurrent thrombosis or non-necrotizing (erythematous) skin lesions or suspected thrombosis (not proven) | None |
| Other causes for thrombocytopenia | None apparent | Possible | Definite |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Squiccimarro, E.; Jiritano, F.; Serraino, G.F.; ten Cate, H.; Paparella, D.; Lorusso, R. Quantitative and Qualitative Platelet Derangements in Cardiac Surgery and Extracorporeal Life Support. J. Clin. Med. 2021, 10, 615. https://doi.org/10.3390/jcm10040615
Squiccimarro E, Jiritano F, Serraino GF, ten Cate H, Paparella D, Lorusso R. Quantitative and Qualitative Platelet Derangements in Cardiac Surgery and Extracorporeal Life Support. Journal of Clinical Medicine. 2021; 10(4):615. https://doi.org/10.3390/jcm10040615
Chicago/Turabian StyleSquiccimarro, Enrico, Federica Jiritano, Giuseppe Filiberto Serraino, Hugo ten Cate, Domenico Paparella, and Roberto Lorusso. 2021. "Quantitative and Qualitative Platelet Derangements in Cardiac Surgery and Extracorporeal Life Support" Journal of Clinical Medicine 10, no. 4: 615. https://doi.org/10.3390/jcm10040615
APA StyleSquiccimarro, E., Jiritano, F., Serraino, G. F., ten Cate, H., Paparella, D., & Lorusso, R. (2021). Quantitative and Qualitative Platelet Derangements in Cardiac Surgery and Extracorporeal Life Support. Journal of Clinical Medicine, 10(4), 615. https://doi.org/10.3390/jcm10040615







