Markers of Cellular Proliferation, Apoptosis, Estrogen/Progesterone Receptor Expression and Fibrosis in Selective Progesterone Receptor Modulator (Ulipristal Acetate)-Treated Uterine Fibroids
Abstract
1. Introduction
2. Methodology
2.1. Study Groups and Inclusion/Exclusion Criteria
2.2. Histological Analysis
2.3. Immunohistochemistry
2.4. TUNEL Assay
2.5. Quantitative Computer Image Analysis of Mallory’s Trichrome Staining and Immunoexpression of ER, PR, Ki67 and PCNA
2.6. Statistical Analysis
3. Results
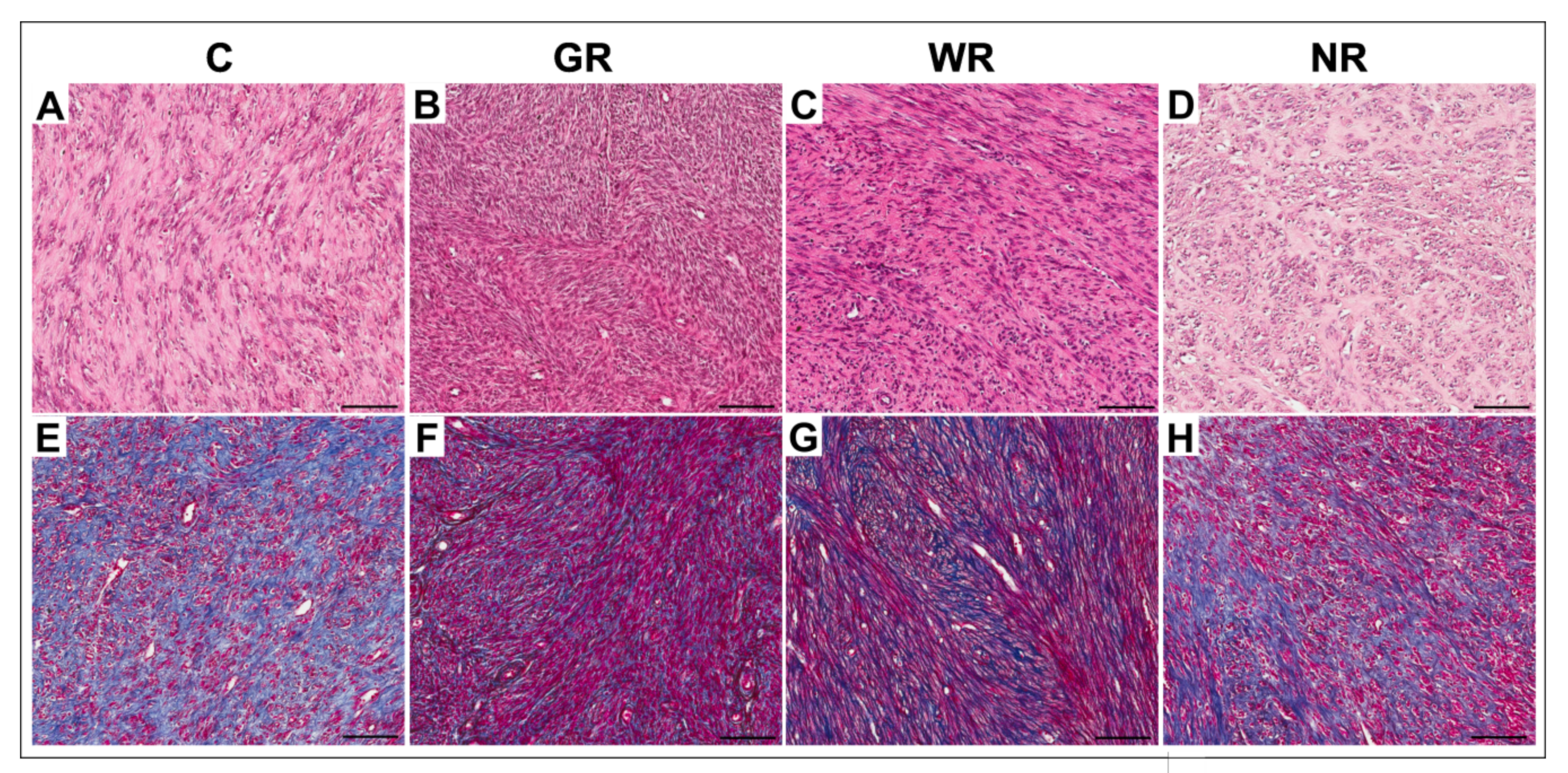
3.1. Morphological Studies
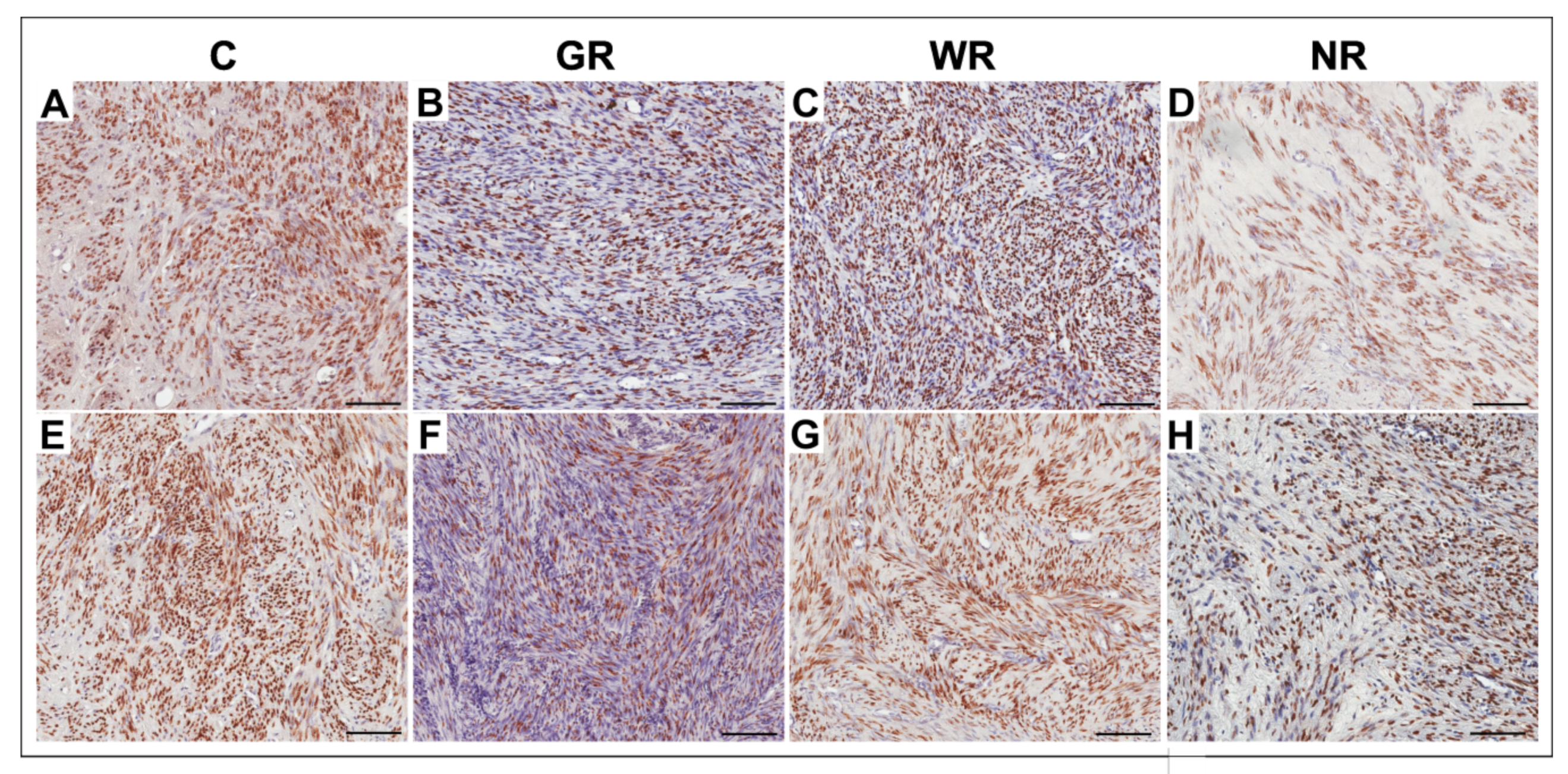
3.2. Quantitative Analysis of ER- and PR-Positive Cells
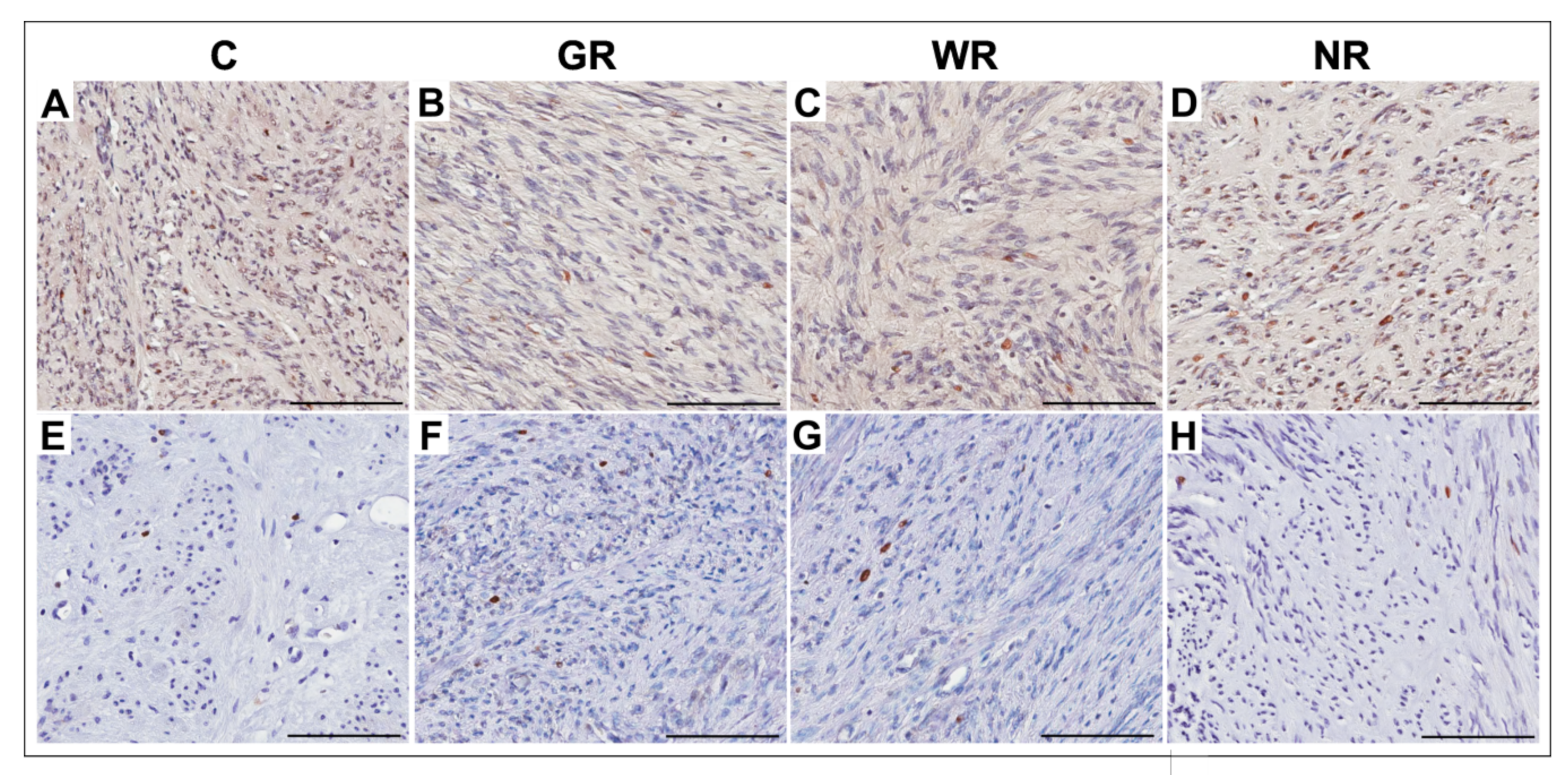
3.3. Quantitative Analysis of PCNA- and Ki67-Positive Cells
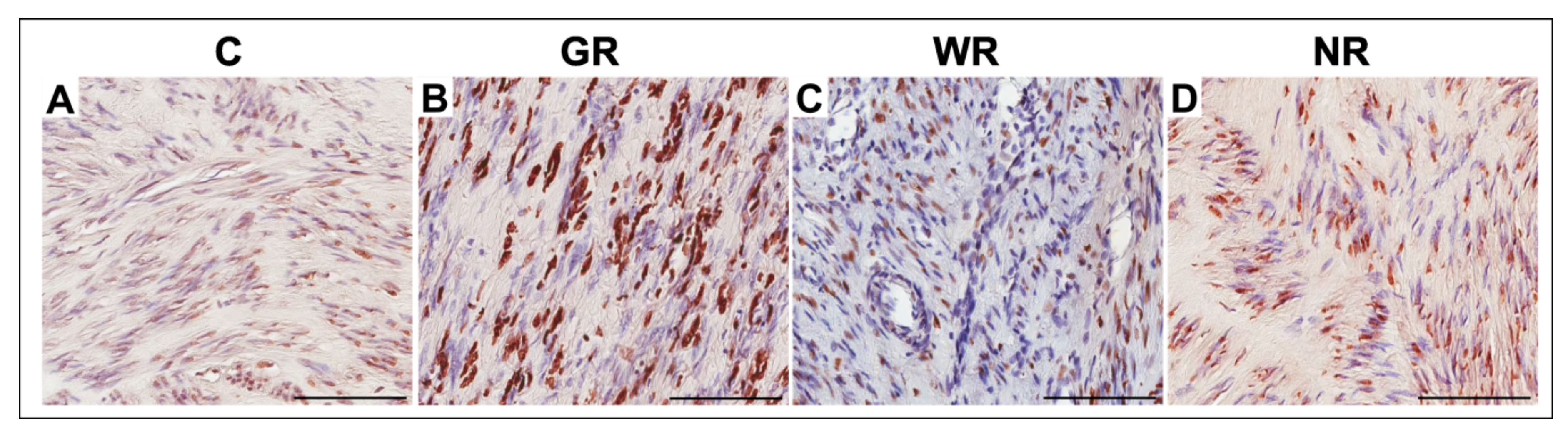
3.4. Quantitative Analysis of TUNEL-Positive Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wise, L.A.; Laughlin-Tommaso, S.K. Epidemiology of Uterine Fibroids: From Menarche to Menopause. Clin. Obstet. Gynecol. 2016, 59, 2–24. [Google Scholar] [CrossRef]
- Giuliani, E.; As-Sanie, S.; Marsh, E.E. Epidemiology and management of uterine fibroids. Int. J. Gynaecol. Obstet. 2020, 149, 3–9. [Google Scholar] [CrossRef]
- Botía, C.P.; Camarasa, S.C.; Baixauli, F.R.; Sanchez, A.C. Uterine Fibroids: Understanding their Origins to Better Understand their Future Treatments. J. Tumor Res. 2017, 3, 130. [Google Scholar]
- Ying, Z.; Weiyuan, Z. Dual actions of progesterone on uterine leiomyoma correlate with the ratio of progesterone receptor A: B. Gynecol. Endocrinol. 2009, 25, 520–523. [Google Scholar] [CrossRef]
- Rabe, T.; Saenger, N.; Ebert, A.D. Selective Progesterone Receptor Modulators for the Medical Treatment of Uterine Fibroids with a Focus on Ulipristal Acetate. BioMed Res. Int. 2018, 2018, 1374821. [Google Scholar]
- Donnez, J.; Donnez, O.; Courtoy, G.E. The place of selective progesterone receptor modulators in myoma therapy. Minerva Ginecol. 2016, 68, 313–320. [Google Scholar]
- Powell, M.; Dutta, D. Esmya® and the PEARL studies: A review. Women’s Health 2016, 12, 544–548. [Google Scholar] [CrossRef]
- Benagiano, G.; Bastianelli, C.; Farris, M.; Brosens, I. Selective progesterone receptor modulators: An update. Expert Opin. Pharmacother. 2014, 15, 1403–1415. [Google Scholar] [CrossRef]
- Courtoy, G.E.; Donnez, J.; Marbaix, E.; Dolmans, M.M. In vivo mechanisms of uterine myoma volume reduction with ulipristal acetate treatment. Fertil. Steril. 2015, 104, 426–434.e1. [Google Scholar] [CrossRef]
- Shin, S.J.; Kim, J.; Lee, S. Ulipristal acetate induces cell cycle delay and remodeling of extracellular matrix. Int. J. Mol. Med. 2018, 42, 1857–1864. [Google Scholar] [CrossRef]
- Shimomura, Y.; Matsuo, H.; Samoto, T.; Maruo, T. Up-regulation by progesterone of proliferating cell nuclear antigen and epidermal growth factor expression in human uterine leiomyoma. J. Clin. Endocrinol. Metab. 1998, 83, 2192–2198. [Google Scholar] [CrossRef]
- Cox, J.; Malik, M.; Britten, J.; Lewis, T.; Catherino, W.H. Ulipristal acetate and extracellular matrix production in human leiomyomas in vivo: A laboratory analysis of a randomized placebo controlled trial. Reprod. Sci. 2018, 25, 198–206. [Google Scholar] [CrossRef]
- Reis, F.M.; Coutinho, L.M.; Vannuccini, S.; Batteux, F.; Chapron, C.; Petraglia, F. Progesterone receptor ligands for the treatment of endometriosis: The mechanisms behind therapeutic success and failure. Hum. Reprod. Update 2020, 26, 565–585. [Google Scholar] [CrossRef]
- Munro, M.G.; Critchley, H.O.; Fraser, I.S.; FIGO Menstrual Disorders Working Group. The FIGO classification of causes of abnormal uterine bleeding in the reproductive years. Fertil. Steril. 2011, 95, 2204–2208.e3. [Google Scholar] [CrossRef]
- Lewis, T.D.; Malik, M.; Britten, J. A Comprehensive Review of the Pharmacologic Management of Uterine Leiomyoma. BioMed Res. Int. 2018, 2018, 2414609. [Google Scholar] [CrossRef]
- Mozzachio, K.; Moore, A.B.; Kissling, G.E.; Dixon, D. Immunoexpression of Steroid Hormone Receptors and Proliferation Markers in Uterine Leiomyoma and Normal Myometrial Tissues from the Miniature Pig, Sus scrofa. Toxicol. Pathol. 2016, 44, 450–457. [Google Scholar] [CrossRef]
- Borahay, M.A.; Asoglu, M.R.; Mas, A.; Adam, S.; Kilic, G.S.; Al-Hendy, A. Estrogen Receptors and Signaling in Fibroids: Role in Pathobiology and Therapeutic Implications. Reprod. Sci. 2017, 24, 1235–1244. [Google Scholar] [CrossRef]
- Burns, K.A.; Korach, K.S. Estrogen receptors and human disease: An update. Arch. Toxicol. 2012, 86, 1491–1504. [Google Scholar] [CrossRef]
- Grings, A.O.; Lora, V.; Ferreira, G.D.; Brum, I.S.; von Eye Corleta, H.; Capp, E. Protein expression of estrogen receptors α and β and aromatase in myometrium and uterine leiomyoma. Gynecol. Obstet. Investig. 2012, 73, 113–117. [Google Scholar] [CrossRef]
- Jakimiuk, A.J.; Bogusiewicz, M.; Tarkowski, R. Estrogen receptor α and β expression in uterine leiomyomas from premenopausal women. Fertil. Steril. 2004, 82, 1244–1249. [Google Scholar] [CrossRef]
- Plewka, D.; Marczyński, J.; Morek, M.; Bogunia, E.; Plewka, A. Receptors of hypothalamic-pituitary-ovarian-axis hormone in uterine myomas. BioMed Res. Int. 2014, 2014, 521313. [Google Scholar] [CrossRef]
- Khan, K.N.; Fujishita, A.; Koshiba, A.; Ogawa, K.; Mori, T.; Ogi, H.; Itoh, K.; Teramukai, S.; Kitawaki, J. Expression profiles of E/P receptors and fibrosis in GnRHa-treated and -untreated women with different uterine leiomyomas. PLoS ONE 2020, 15, e0242246. [Google Scholar] [CrossRef]
- Demura, T.A.; Revazova, Z.V.; Kogan, E.A.; Adamyan, L.V. The molecular mechanisms and morphological manifestations of leiomyoma reduction induced by selective progesterone receptor modulators. Arkh. Patol. 2017, 79, 19–26. (In Russian) [Google Scholar] [CrossRef]
- Tinelli, A.; Kosmas, I.P.; Mynbaev, O.A.; Malvasi, A.; Sparic, R.; Vergara, D. The Biological Impact of Ulipristal Acetate on Cellular Networks Regulating Uterine Leiomyoma Growth. Curr. Pharm. Des. 2020, 26, 310–317. [Google Scholar] [CrossRef]
- Kim, J.J.; Kurita, T.; Bulun, S.E. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr. Rev. 2013, 34, 130–162. [Google Scholar] [CrossRef]
- Lewis, T.D.; Malik, M.; Britten, J.; Parikh, T.; Cox, J.; Catherino, W.H. Ulipristal acetate decreases active TGF-β3 and its canonical signaling in uterine leiomyoma via two novel mechanisms. Fertil. Steril. 2019, 111, 806–815.e1. [Google Scholar] [CrossRef]
- Chung, Y.J.; Chae, B.; Kwak, S.H. Comparison of the inhibitory effect of gonadotropin releasing hormone (GnRH) agonist, selective estrogen receptor modulator (SERM), antiprogesterone on myoma cell proliferation in vitro. Int. J. Med. Sci. 2014, 11, 276–281. [Google Scholar] [CrossRef]
- Khan, K.N.; Kitajima, M.; Hiraki, K.; Fujishita, A.; Sekine, I.; Ishimaru, T.; Masuzaki, H. Changes in tissue inflammation, angiogenesis and apoptosis in endometriosis, adenomyosis and uterine myoma after GnRH agonist therapy. Hum. Reprod. 2010, 25, 642–653. [Google Scholar] [CrossRef]
- Ken Vu Greenspan, D.L.; Wu, T.-C.; Zacur, H.A.; Kurman, R.J. Cellular proliferation, estrogen receptor, progesterone receptor, and bcl-2 expression in GnRH agonist-treated uterine leiomyomas. Hum. Pathol. 1998, 29, 359–363. [Google Scholar] [CrossRef]
- Yun, B.S.; Seong, S.J.; Cha, D.H.; Kim, J.Y.; Kim, M.L.; Shim, J.Y.; Park, J.E. Changes in proliferating and apoptotic markers of leiomyoma following treatment with a selective progesterone receptor modulator or gonadotropin-releasing hormone agonist. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 191, 62–67. [Google Scholar] [CrossRef]
- Islam, M.S.; Ciavattini, A.; Petraglia, F.; Castellucci, M.; Ciarmela, P. Extracellular matrix in uterine leiomyoma pathogenesis: A potential target for future therapeutics. Hum. Reprod. Update 2018, 24, 59–85. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Akhtar, M.M.; Segars, J.H.; Castellucci, M.; Ciarmela, P. Molecular targets of dietary phytochemicals for possible prevention and therapy of uterine fibroids: Focus on fibrosis. Crit. Rev. Food Sci. Nutr. 2017, 57, 3583–3600. [Google Scholar] [CrossRef] [PubMed]
- Press Release 13/03/2020. Available online: https://www.ema.europa.eu/en/news/suspension-ulipristal-acetate-uterine-fibroids-during-ongoing-ema-review-liver-injury-risk (accessed on 28 January 2020).
- Last Updated 14/01/2021. Available online: https://www.ema.europa.eu/documents/referral/ulipristal-acetate-5mg-medicinal-products-article-31-referral-ulipristal-acetate-uterine-fibroids_en.pdf (accessed on 28 January 2020).
- Bradley, L.D.; Singh, S.S.; Simon, J.; Gemzell-Danielsson, K.; Petersdorf, K.; Groettrup-Wolfers, E.; Ren, X.; Zvolanek, M.; Seitz, C. Vilaprisan in women with uterine fibroids: The randomized phase 2b ASTEROID 1 study. Fertil. Steril. 2019, 111, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Ciebiera, M.; Vitale, S.G.; Ferrero, S.; Vilos, G.A.; Barra, F.; Caruso, S.; Laganà, A.S.; Sierant, A.; Cianci, A.; Jakiel, G. Vilaprisan, a New Selective Progesterone Receptor Modulator in Uterine Fibroid Pharmacotherapy-Will it Really be a Breakthrough? Curr. Pharm. Des. 2020, 26, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.; Sullivan, M.E.; Xu, Y.; Rogers, C.; Muzzio, M.; Helenowski, I.; Shidfar, A.; Zeng, Z.; Singhal, H.; Jovanovic, B.; et al. Selective Progesterone Receptor Modulators in Early-Stage Breast Cancer: A Randomized, Placebo-Controlled Phase II Window-of-Opportunity Trial Using Telapristone Acetate. Clin. Cancer Res. 2020, 26, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Davaadelger, B.; Murphy, A.R.; Clare, S.E.; Lee, O.; Khan, S.A.; Kim, J.J. Mechanism of Telapristone Acetate (CDB4124) on Progesterone Receptor Action in Breast Cancer Cells. Endocrinology 2018, 159, 3581–3595. [Google Scholar] [CrossRef]
| Group | Control (n = 20) | Good Reaction (n = 20) | Weak Reaction (n = 10) | No Reaction (n = 4) | |
|---|---|---|---|---|---|
| Age | X ± SD | 41.6 ± 4.6 | 42.1 ± 5.3 | 43.3 ± 5.4 | 43.0 ± 1.6 |
| Median (range) | 41.5 (33–51) | 42.0 (33–55) | 46.0 (33–47) | 43.0 (41–45) | |
| BMI | X ± SD | 24.6 ± 2.3 | 24.7 ± 2.1 | 26.0 ± 2.1 | 26.2 ± 2.1 |
| Median (range) | 24.2 (21.3–30.1) | 24.2 (22.2–29.7) | 26.1 (22.9–28.7) | 25.8 (24.0–29.0) | |
| Gravidity | X ± SD | 1.6 ± 0.9 | 1.4 ± 0.9 | 1.4 ± 1.0 | 1.0 ± 0.8 |
| Median (range) | 2.0 (0–3) | 1.0 (0–3) | 1.5 (0–3) | 1.0 (0–2) | |
| Parity | X ± SD | 1.2 ± 0.8 | 1.2 ± 0.7 | 1.3 ± 0.9 | 1.0 ± 0.8 |
| Median (range) | 1.0 (0–3) | 1.0 (0–2) | 1.0 (0–3) | 1.0 (0–2) | |
| Number of fibroids | X ± SD | 1.5 ± 0.9 | 1.8 ± 0.8 | 1.7 ± 0.9 | 1.5 ± 0.6 |
| Median (range) | 1.0 (1–4) | 2.0 (1–4) | 1.0 (1–3) | 1.5 (1–2) | |
| The volume of fibroids before treatment (cm3) | X ± SD | 74.0 ± 72.4 | 81.5 ± 71.7 | 38.0 ± 61.3 | 45.9 ± 20.8 |
| Median (range) | 44.9 (5.5–267.8) | 49.3 (16.0–297.8) | 14.8 (2.9–199.9) | 48.5 (18.1–68.4) | |
| The volume of fibroids after treatment (cm3) | X ± SD | - | 46.3 * ± 41.8 | 33.7 ± 59.0 | 67.3 ± 29.4 |
| Median (range) | - | 33.7 (0.0–124.2) | 9.1 (1.2–191.8) | 74.7 (25.9–94.1) | |
| The diameter of fibroids before treatment (cm) | X ± SD | 4.1 ± 1.4 | 5.1 ± 1.7 | 4.2 ± 1.8 | 4.2 ± 1.0 |
| Median (range) | 4.1 (1.7–6.9) | 5.2 (1.8–8.2) | 3.7 (2.0–7.2) | 4.6 (3.1–4.8) | |
| The diameter of fibroids after treatment (cm) | X ± SD | - | 4.1 ± 1.8 | 3.9 ± 2.0 | 5.0 ± 1.2 |
| Median (range) | - | 4.3 (1.3–6.9) | 3.5 (1.4–7.2) | 5.4 (3.7–6.0) | |
| Type of surgery | M/ASH/LSH | M/ASH/LSH | M/ASH/LSH | M/LSH | |
| Group | Collagen Volume Fraction (%) | |
|---|---|---|
| Median (Range) | X ± SD | |
| Control (n = 20) | 43.9 (20.4−78.3) | 47.7 ± 16.4 |
| Good reaction (n = 20) | 32.5 (5.6−77.8) | 37.5 * ± 23.6 |
| Weak reaction (n = 10) | 46.3 (26.1−71.4) | 44.4 ± 13.4 |
| No reaction (n = 4) | 43.1 (22.7−83.3) | 46.8 ± 17.7 |
| Group | Estrogen Receptor | Progesterone Receptor | ||
|---|---|---|---|---|
| Median (Range) | X ± SD | Median (Range) | X ± SD | |
| Control (n = 20) | 53.2 (33.2−78.8) | 54.1 ± 9.6 | 54.4 (41.3−63.4) | 53.8 ± 5.2 |
| Good reaction (n = 20) | 14.4 (2.5−77.2) | 25.4 * ± 20.7 | 24.9 (2.7−64.1) | 28.8 * ± 14.9 |
| Weak reaction (n = 10) | 47.5 (41.7−63.1) | 50.5 ± 7.1 | 52.5 (40.5−63.9) | 52.8 ± 7.5 |
| No reaction (n = 4) | 35.3 (24.0−81.9) | 51.7 ± 25.9 | 54.3 (33.4−68.2) | 53.1 ± 9.2 |
| Group | Ki67 | PCNA | ||
|---|---|---|---|---|
| Median (Range) | X ± SD | Median (Range) | X ± SD | |
| Control (n = 20) | 2.0 (0.6−3.0) | 2.0 ± 0.5 | 18.0 (10.2−28.7) | 18.4 ± 4.4 |
| Good reaction (n = 20) | 0.4 (0.1−1.9) | 0.6 * ± 0.4 | 10.8 (6.9−17.1) | 11.3 * ± 2.6 |
| Weak reaction (n = 10) | 1.4 (0.6−2.8) | 1.5 ** ± 0.5 | 12.2 (7.0−29.3) | 16.2 ** ± 7.0 |
| No reaction (n = 4) | 1.7 (1.1−2.4) | 1.7 ± 0.3 | 18.0 (13.6−19.6) | 17.8 ± 1.9 |
| Group | Apoptosis | |
|---|---|---|
| Median (Range) | X ± SD | |
| Control (n = 20) | 12.0 (1.4−32.2) | 12.9 ± 7.1 |
| Good reaction (n = 20) | 25.1 (7.0−40.4) | 24.7 * ± 8.5 |
| Weak reaction (n = 10) | 14.5 (4.9−31.6) | 17.5 ± 9.3 |
| No reaction (n = 4) | 15.0 (3.4−31.3) | 16.6 ± 8.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szydłowska, I.; Grabowska, M.; Nawrocka-Rutkowska, J.; Piasecka, M.; Starczewski, A. Markers of Cellular Proliferation, Apoptosis, Estrogen/Progesterone Receptor Expression and Fibrosis in Selective Progesterone Receptor Modulator (Ulipristal Acetate)-Treated Uterine Fibroids. J. Clin. Med. 2021, 10, 562. https://doi.org/10.3390/jcm10040562
Szydłowska I, Grabowska M, Nawrocka-Rutkowska J, Piasecka M, Starczewski A. Markers of Cellular Proliferation, Apoptosis, Estrogen/Progesterone Receptor Expression and Fibrosis in Selective Progesterone Receptor Modulator (Ulipristal Acetate)-Treated Uterine Fibroids. Journal of Clinical Medicine. 2021; 10(4):562. https://doi.org/10.3390/jcm10040562
Chicago/Turabian StyleSzydłowska, Iwona, Marta Grabowska, Jolanta Nawrocka-Rutkowska, Małgorzata Piasecka, and Andrzej Starczewski. 2021. "Markers of Cellular Proliferation, Apoptosis, Estrogen/Progesterone Receptor Expression and Fibrosis in Selective Progesterone Receptor Modulator (Ulipristal Acetate)-Treated Uterine Fibroids" Journal of Clinical Medicine 10, no. 4: 562. https://doi.org/10.3390/jcm10040562
APA StyleSzydłowska, I., Grabowska, M., Nawrocka-Rutkowska, J., Piasecka, M., & Starczewski, A. (2021). Markers of Cellular Proliferation, Apoptosis, Estrogen/Progesterone Receptor Expression and Fibrosis in Selective Progesterone Receptor Modulator (Ulipristal Acetate)-Treated Uterine Fibroids. Journal of Clinical Medicine, 10(4), 562. https://doi.org/10.3390/jcm10040562









