Healing of Chronic Wounds with Platelet-Derived Growth Factors from Single Donor Platelet-Rich Plasma following One Freeze-Thaw Cycle. A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
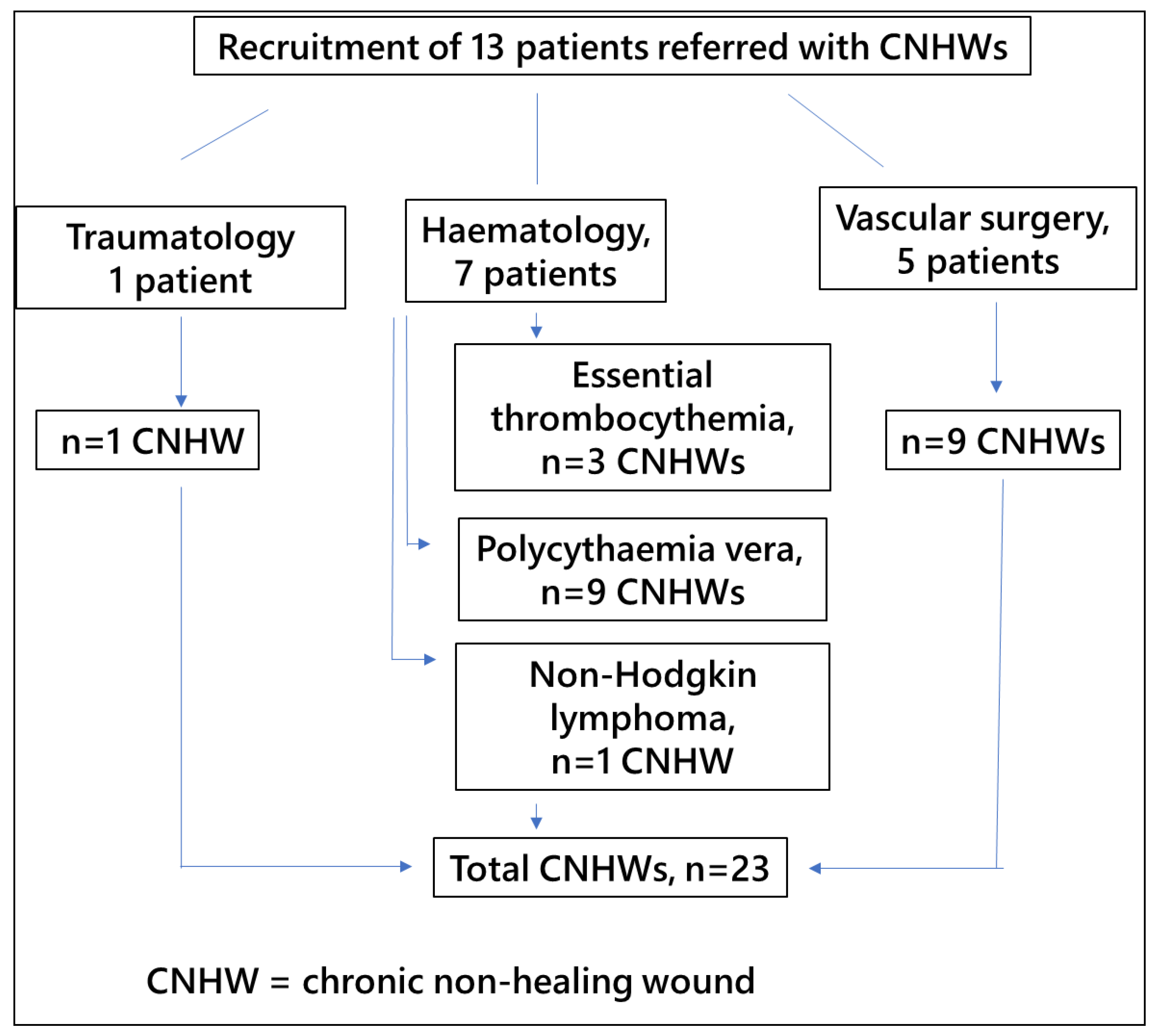
2.1. Study Design and Participants
Inclusion and Exclusion Criteria
2.2. Procedure
2.2.1. Plateletpheresis
2.2.2. Aliquoting
2.2.3. Cryopreservation
2.2.4. Application Procedure
2.3. Statistical Procedure
2.4. Ethical Considerations and Participant Involvement
2.5. Patient and Public Involvement
3. Results
3.1. Healing-Related Factors
3.2. Medical History
3.3. Blood Test Result-Related Factors
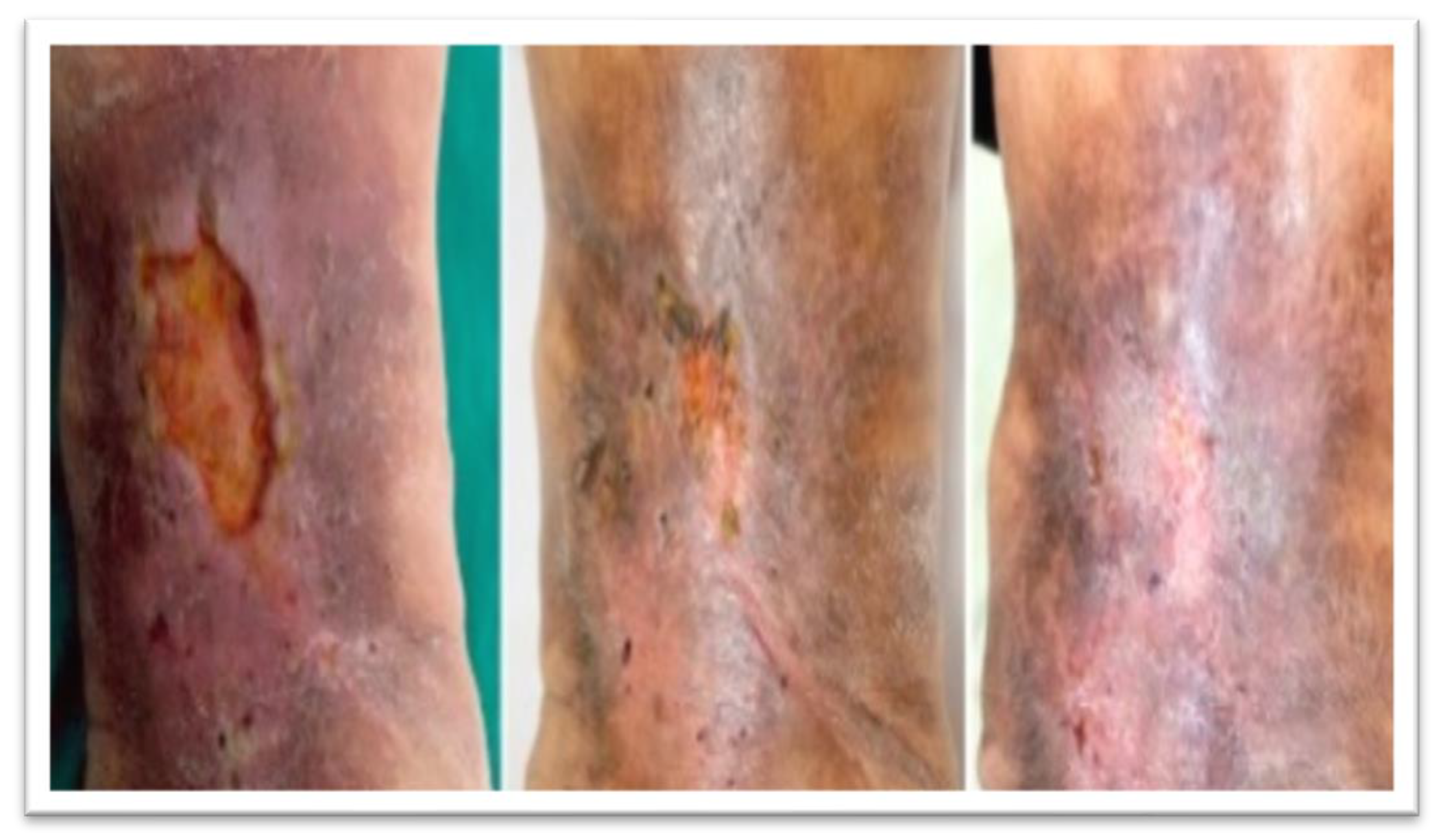
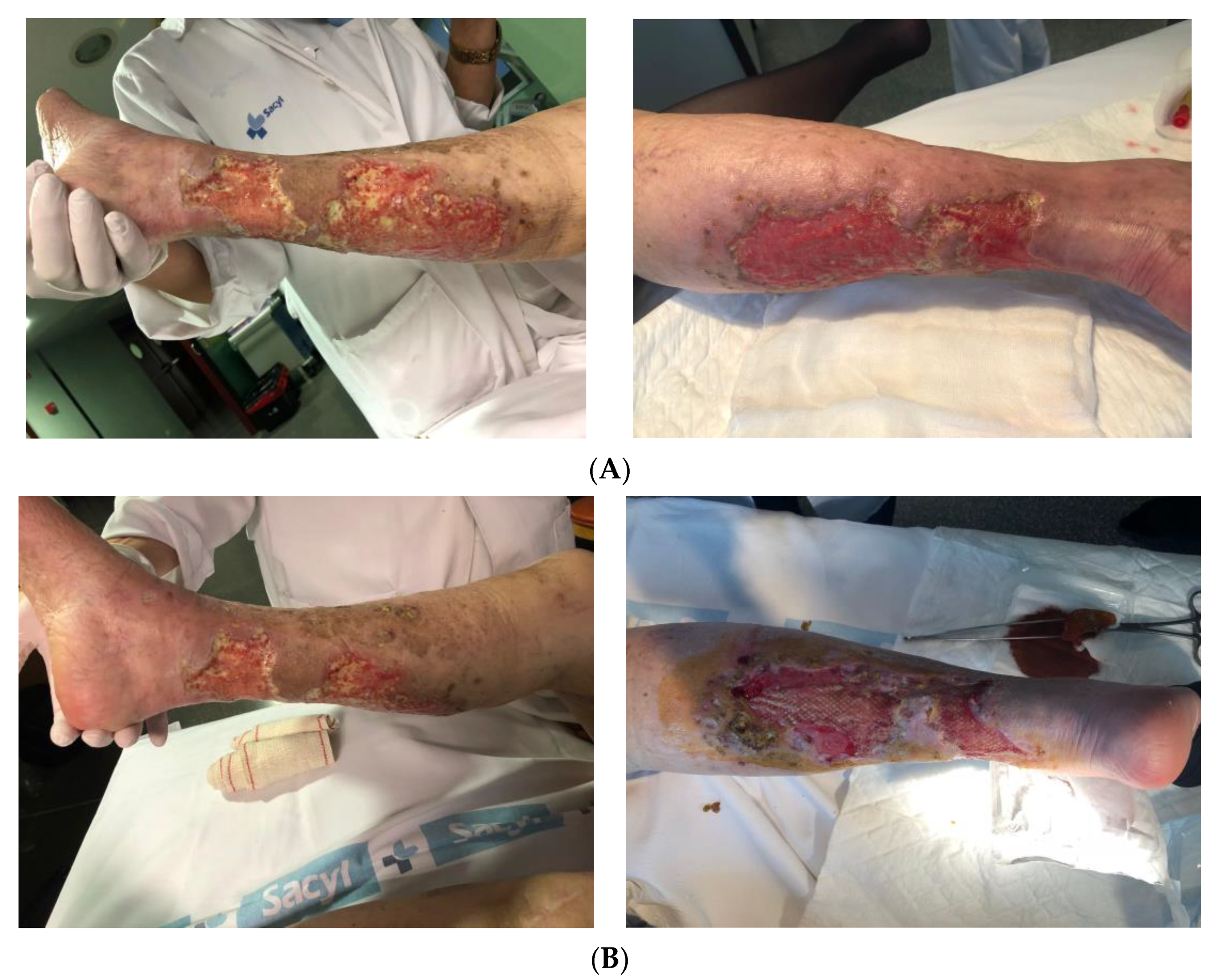
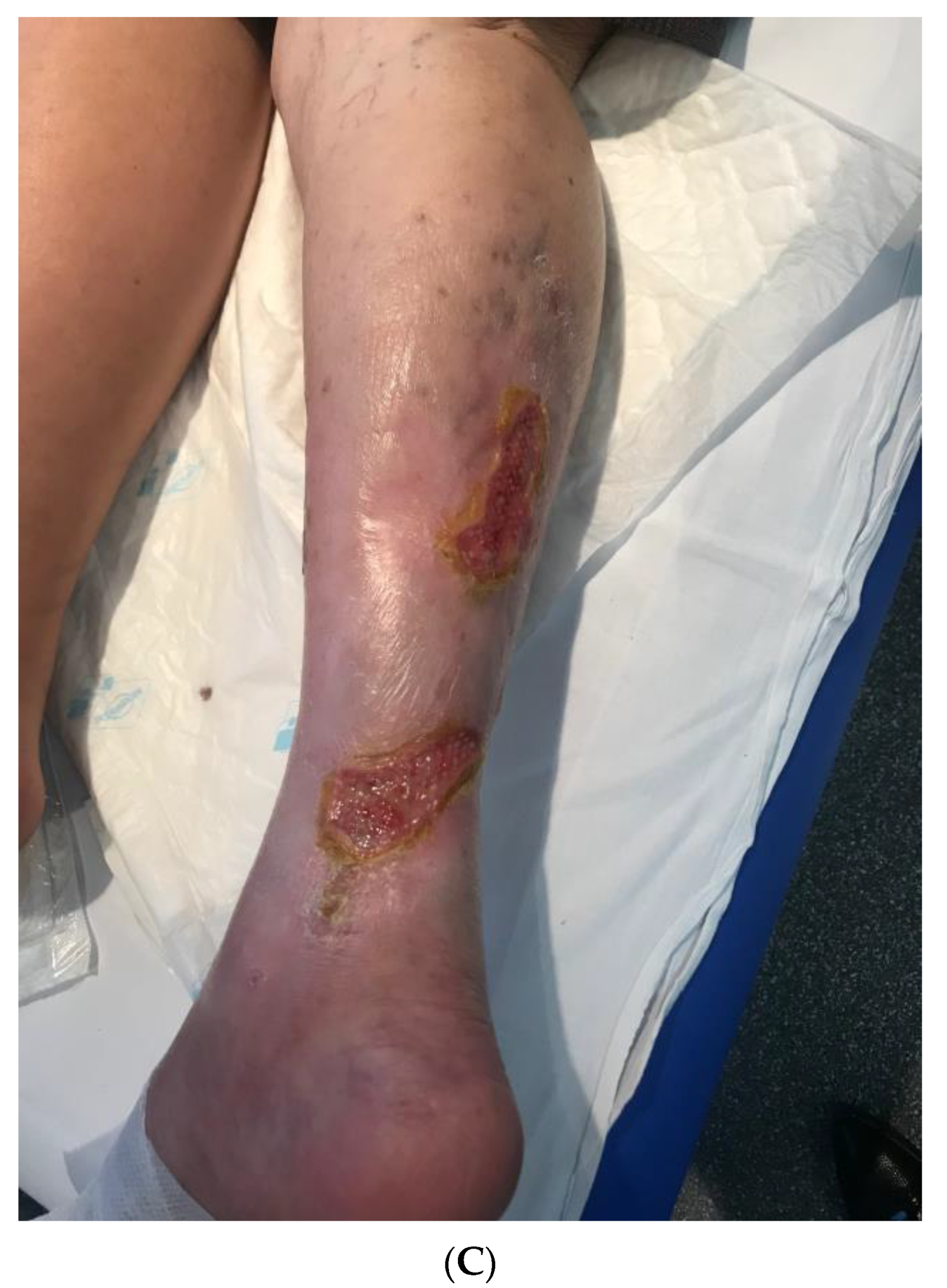
3.4. Wound-Related Factors
4. Discussion
4.1. Difficult-to-Heal Wounds
4.2. Role of PRP in Wound Care
4.3. Use of al-PRP vs. au-PRP in the Treatment of Chronic Wounds
4.4. PRP Preparation
4.5. Implications for Practice
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brod, M. Quality of life issues in patients with diabetes and lower extremity ulcers: Patients and care givers. Qual Life Res. 1998, 7, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Armstrong, D.G.; Lipsky, B.A. Preventing foot ulcers in patients with diabetes. JAMA 2005, 293, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Garber, S.L.; Rintala, D.H. Pressure ulcers in veterans with spinal cord injury: A retrospective study. J. Rehabil. Res. Dev. 2003, 40, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Medina, A.; Scott, P.G.; Ghahary, A.; Tredget, E.E. Pathophysiology of chronic nonhealing wounds. J. Burn Care Rehabil. 2005, 26, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Brtan Romić, R.; Brtan, A.; Romić, I.; Cvitanović, H.; Duvančić, T.; Lugović-Mihić, L. Quality of life and perception of disease in patients with chronic leg ulcer. Acta Clin. Croat 2015, 54, 309–314. [Google Scholar]
- Sierra Martínez, A. Heridas y Cicatrización. Rev. Soc. Española Heridas 2019, 1, 29. [Google Scholar]
- Bolton, L. Platelet-rich plasma: Optimal use in surgical wound care. Wounds 2021, 33, 219–221. [Google Scholar] [CrossRef]
- Harding, K.G.; Morris, H.L.; Patel, G.K. Science, medicine and the future: Healing chronic wounds. BMJ 2002, 324, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Alsousou, J.; Ali, A.; Willett, K.; Harrison, P. The role of platelet-rich plasma in tissue regeneration. Platelets 2013, 24, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.; Loibl, M.; Herrmann, M. Platelet-rich plasma in tissue engineering: Hype and hope. Eur. Surg. Res. 2018, 59, 265–275. [Google Scholar] [CrossRef]
- Etulain, J.; Mena, H.A.; Meiss, R.P.; Frechtel, G.; Gutt, S.; Negrotto, S.; Schattner, M. An optimised protocol for platelet-rich plasma preparation to improve its angiogenic and regenerative properties. Sci. Rep. 2018, 8, 1513. [Google Scholar] [CrossRef]
- Tambella, A.M.; Martin, S.; Cantalamessa, A.; Serri, E.; Attili, A.R. Platelet-rich plasma and other hemocomponents in veterinary regenerative medicine. Wounds 2018, 30, 329–336. [Google Scholar]
- Del Fabbro, M.; Bucchi, C.; Lolato, A.; Corbella, S.; Testori, T.; Taschieri, S. Healing of postextraction sockets preserved with autologous platelet concentrates. a systematic review and meta-analysis. J. Oral. Maxillofac. Surg. 2017, 75, 1601–1615. [Google Scholar] [CrossRef]
- Faillace, V.; Tambella, A.M.; Fratini, M.; Paggi, E.; Dini, F.; Laus, F. Use of autologous platelet-rich plasma for a delayed consolidation of a tibial fracture in a young donkey. J. Vet. Med. Sci. 2017, 79, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Görmeli, G.; Görmeli, C.A.; Ataoglu, B.; Çolak, C.; Aslantürk, O.; Ertem, K. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: A randomized, double-blind, placebo-controlled trial. Knee Surg. Sports Traumatol. Arthrosc 2017, 25, 958–965. [Google Scholar] [CrossRef]
- Alio, J.L.; Rodriguez, A.E.; Arriba, D.; Gisbert, S.; Abdelghany, A.A. Treatment with platelet-rich plasma of surgically related dormant corneal ulcers. Eur. J. Ophthalmol. 2018, 28, 515–520. [Google Scholar] [CrossRef]
- Bhujbal, R.; Malik, A.N.; Kumar, N.; Kv, S.; Parkar, I.M.; Mb, J. Comparative evaluation of platelet rich plasma in socket healing and bone regeneration after surgical removal of impacted mandibular third molars. J. Dent. Res. Dent. Clin. Dent. Prospect. 2018, 12, 153–158. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, X.; Xu, X.; Liu, J. Intra-articular injections of platelet-rich plasma, hyaluronic acid or corticosteroids for knee osteoarthritis: A prospective randomized controlled study. Orthopade 2019, 48, 239–247. [Google Scholar] [CrossRef]
- Marcazzan, S.; Taschieri, S.; Weinstein, R.L.; Del Fabbro, M. Efficacy of platelet concentrates in bone healing: A systematic review on animal studies—Part b: Large-size animal models. Platelets 2018, 29, 338–346. [Google Scholar] [CrossRef]
- Tambella, A.M.; Attili, A.R.; Dini, F.; Palumbo, P.A.; Vullo, C.; Serri, E.; Scrollavezza, P.; Dupré, G. Autologous platelet gel to treat chronic decubital ulcers: A randomized, blind controlled clinical trial in dogs. Vet. Surg. 2014, 43, 726–733. [Google Scholar] [CrossRef]
- Tambella, A.M.; Attili, A.R.; Dupré, G.; Cantalamessa, A.; Martin, S.; Cuteri, V.; Marcazzan, S.; Del Fabbro, M. Platelet-rich plasma to treat experimentally-induced skin wounds in animals: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0191093. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Alonso, N.; Lobato, I.; Hernández, I.; Sebastian, K.S.; Rodríguez, B.; March, A.G.; Perez Salvador, A.; Arce, V.; Garcia-Alvarez, A.; Gomez-Fernandez, M.C.; et al. Autologous platelet-rich plasma in the treatment of venous leg ulcers in primary care: A randomised controlled, pilot study. J. Wound Care 2018, 27, S20–S24. [Google Scholar] [CrossRef] [PubMed]
- Moneib, H.A.; Youssef, S.S.; Aly, D.G.; Rizk, M.A.; Abdelhakeem, Y.I. Autologous platelet-rich plasma versus conventional therapy for the treatment of chronic venous leg ulcers: A comparative study. J. Cosmet. Derm. 2018, 17, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Picard, F.; Hersant, B.; Bosc, R.; Meningaud, J.P. The growing evidence for the use of platelet-rich plasma on diabetic chronic wounds: A review and a proposal for a new standard care. Wound Repair. Regen 2015, 23, 638–643. [Google Scholar] [CrossRef]
- Martinez, Z.M.J.; Martí, C.A.J.; Solà, I.; Expósito, J.A. Autologous platelet-rich plasma for treating chronic wounds. Cochrane Database Syst. Rev. 2016, 5, CD006899. [Google Scholar] [CrossRef]
- Deng, W.; Boey, J.; Chen, B.; Byun, S.; Lew, E.; Liang, Z.; Armstrong, D.G. Platelet-rich plasma, bilayered acellular matrix grafting and negative pressure wound therapy in diabetic foot infection. J. Wound Care 2016, 25, 393–397. [Google Scholar] [CrossRef]
- Li, T.; Ma, Y.; Wang, M.; Wang, T.; Wei, J.; Ren, R.; He, M.; Wang, G.; Boey, J.; Armstrong, D.G.; et al. Platelet-rich plasma plays an antibacterial, anti-inflammatory and cell proliferation-promoting role in an in vitro model for diabetic infected wounds. Infect. Drug Resist. 2019, 12, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Prado, R. Addressing reproducibility in stem cell and PRP therapies. Trends Biotechnol. 2019, 37, 340–344. [Google Scholar] [CrossRef]
- Scevola, S.; Nicoletti, G.; Brenta, F.; Isernia, P.; Maestri, M.; Faga, A. Allogenic platelet gel in the treatment of pressure sores: A pilot study. Int. Wound J. 2010, 7, 184–190. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Huang, A.W.; Fan, J.J.; Wei, K.; Jin, D.; Chen, B.; Li, D.; Bi, L.; Wang, J.; Pei, G. The potential use of allogeneic platelet-rich plasma for large bone defect treatment: Immunogenicity and defect healing efficacy. Cell Transpl. 2013, 22, 175–187. [Google Scholar] [CrossRef]
- Zhao, Q.; Ma, Y.; Lu, Y.; Chai, Y.; Zhou, Y. Successful Treatment of Chronic Lower Extremity Ulcers with Allogeneic Platelet-Rich Plasma and Artificial Dermis: A Case Report. Adv. Ski. Wound Care 2019, 32, 550–552. [Google Scholar] [CrossRef] [PubMed]
- Semenič, D.; Cirman, T.; Rožman, P.; Smrke, D.M. Regeneration of chronic wounds with allogeneic platelet gel versus hydrogel treatment: A prospective study. Acta Clin. Croat 2018, 57, 434–442. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Guo, X.; Li, T.; Jiang, X.; Chen, Y.; Yuan, Y.; Chen, B.; Yang, G.; Fan, Y.; Liang, Z.; et al. Cell Transplant. Comparison of Allogeneic Platelet-rich Plasma with Autologous Platelet-rich Plasma for the Treatment of Diabetic Lower Extremity Ulcers. Cell Transpl. 2020, 29, 963689720931428. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: New York, NJ, USA, 1988. [Google Scholar]
- Hinkle, D.E.; Wiersma, W.; Jurs, S.G. Applied Statistics for the Behavioral Sciences, 5th ed.; Houghton Mifflin: Boston, MA, USA, 2003. [Google Scholar]
- Vidán Estévez, J.; Escalante, F.; Escribano, P.; Cechini, C.; Moro, M.J.; Ahmadi, A.; Ballina, B.; Quiñones, J.; Moro, M.J. Uso de los factores de crecimiento derivados de las plaquetas en úlceras cutáneas y osteonecrosis mandibular secundarias: Una terapia eficaz. In Proceedings of the LV Congreso Nacional de la Sociedad Española de Hematología y Hemoterapia, Sevilla, Spain, 9–17 October 2013. [Google Scholar]
- Lopes, F.C.; Ferreira, R.; Albuquerque, D.M.; Silveira, A.A.; Costa, R.; Soares, R.; Costa, F.F.; Conran, N. In vitro and in vivo anti-angiogenic effects of hydroxyurea. Microvascular. Res. 2014, 94, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Bader, U.; Banyai, M.; Böni, R.; Burg, G.; Hafner, J. Leg ulcers in patients with myeloproliferative disorders: Disease-or treatment-related? Dermatology 2000, 200, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Othman, B.; Lim, J.; Batres, A.; Ponugoti, B.; Zhang, C. Foxo1 inhibits diabetic mucosal wound healing but enhances healing of normoglycemic wounds. Diabetes 2015, 64, 243–256. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Li, H.; Zhu, B.; Wang, L.; Guo, B. GDF11 improves angiogenic function of EPCs in diabetic limb ischemia. Diabetes 2018, 67, 2084–2095. [Google Scholar] [CrossRef] [PubMed]
- Everts, P.A.; Brown Mahoney, C.; Hoffmann, J.J.; Schönberger, J.P.; Box, H.A.; van Zundert, A.; Knape, J.T. Platelet-rich plasma preparation using three devices: Implications for platelet activation and platelet growth factor release. Growth Factors 2006, 24, 165–171. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, Y.; Li, N. Efficacy and long-term longitudinal follow-up of bone marrow mesenchymal cell transplantation therapy in a diabetic patient with recurrent lower limb bullosis diabeticorum. Stem Cell Res. 2018, 9, 99. [Google Scholar] [CrossRef]
- Hu, Z.; Qu, S.; Zhang, J.; Cao, X.; Wang, P.; Huang, S.; Shi, F.; Dong, Y.; Wu, J.; Tang, B.; et al. Efficacy and Safety of Platelet-rich plasma for patients with diabetic ulcers: A systematic review and meta-analysis. Adv. Wound Care 2019, 8, 298–308. [Google Scholar] [CrossRef]
- Marx, R.E. Platelet-rich plasma (PRP): What is PRP and what is not PRP? Implant. Dent. 2001, 10, 225–228. [Google Scholar] [CrossRef] [PubMed]
- de Leon, J.M.; Driver, V.R.; Fylling, C.P.; Carter, M.J.; Anderson, C.; Wilson, J.; Dougherty, R.M.; Fuston, D.; Trigilia, D.; Valenski, V.; et al. The clinical relevance of treating chronic wounds with an enhanced near-physiological concentration of platelet-rich plasma gel. Adv. Ski. Wound Care 2011, 24, 357–368. [Google Scholar] [CrossRef]
- Serra, R.; Buffone, G.; Dominijanni, A.; Molinari, V.; Montemurro, R.; de Franciscis, S. Application of platelet-rich gel to enhance healing of transmetatarsal amputations in diabetic dysvascular patients. Int. Wound J. 2013, 10, 612–615. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhao, J.; Xie, J.; Lv, Y.; Cao, D.S. The Efficacy of Platelet-Rich Plasma Dressing for Chronic Nonhealing Ulcers: A Meta-Analysis of 15 Randomized Controlled Trials. Plast. Reconstr. Surg. 2019, 144, 1463–1474. [Google Scholar] [CrossRef]
- Piccin, A.; Di Pierro, A.M.; Canzian, L.; Primerano, M.; Corvetta, D.; Negri, G.; Mazzoleni, G.; Gastl, G.; Steurer, M.; Gentilini, I.; et al. Platelet gel: A new therapeutic tool with great potential. Blood Transfus. 2017, 15, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.J.; Fylling, C.P.; Parnell, L.K. Use of platelet rich plasma gel on wound healing: A systematic review and meta-analysis. Eplasty 2011, 11, e38. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3174862/ (accessed on 15 September 2021).
- Reese, R.J. Autologous platelet rich plasma (PRP): What do we know? Important concepts relevant to hair restoration surgery. Hair Transplant. Forum. Int. 2010, 20, 14–17. [Google Scholar] [CrossRef]
- Özgürtaş, T.; Burkay, U.; Cemil, Y. Platelet-Rich Plasma. In Musculoskeletal Research and Basic Science; Springer International Publishing: New York, NY, USA, 2016; pp. 283–288. [Google Scholar]
- Miller, J.D.; Rankin, T.M.; Hua, N.T.; Ontiveros, T.; Giovinco, N.A.; Mills, J.L.; Armstrong, D.G. Reduction of pain via platelet-rich plasma in split-thickness skin graft donor sites: A series of matched pairs. Diabet. Foot Ankle 2015, 6, 24972. [Google Scholar] [CrossRef]
- Fabbro, M.D.; Bortolin, M.; Taschieri, S.; Ceci, C.; Weinstein, R.L. Antimicrobial properties of platelet-rich preparations. a systematic review of the current pre-clinical evidence. Platelets 2016, 27, 276–285. [Google Scholar] [CrossRef]
- Marques, L.F.; Stessuk, T.; Camargo, I.C.; Sabeh Junior, N.; dos Santos, L.; Ribeiro-Paes, J.T. Platelet-rich plasma (PRP): Methodological aspects and clinical applications. Platelets 2015, 26, 101–113. [Google Scholar] [CrossRef]
- Mariani, E.; Filardo, G.; Canella, V.; Berlingeri, A.; Bielli, A.; Cattini, L.; Landini, M.P.; Kon, E.; Marcacci, M.; Facchini, A. Platelet-rich plasma affects bacterial growth in vitro. Cytotherapy 2014, 16, 1294–1304. [Google Scholar] [CrossRef]
- Farghali, H.A.; AbdElKader, N.A.; AbuBakr, H.O.; Aljuaydi, S.H.; Khattab, M.S.; Elhelw, R.; Elhariri, M. Antimicrobial action of autologous platelet-rich plasma on MRSA-infected skin wounds in dogs. Sci. Rep. 2019, 9, 12722. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Bortolin, M.; Vassena, C.; Romanò, C.L.; Taschieri, S.; Del Fabbro, M. Plasma components and platelet activation are essential for the antimicrobial properties of autologous platelet-rich plasma: An in vitro study. PLoS ONE 2014, 9, e107813. [Google Scholar] [CrossRef]
- Sun, S.; Wang, C.; Chen, D.; Cen, S.; Lv, X.; Wen, X.; Liu, M.; Lu, W.; Zhao, J.; Ran, X. Combating superbug without antibiotic on a postamputation wound in a patient with diabetic foot. Int. J. Low. Extrem. Wounds 2016, 15, 74–77. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, Y.; Kuss, M.; Shi, W.; Aldrich, A.L.; Untrauer, J.; Kielian, T.; Duan, B. Platelet-rich plasma for the treatment of tissue infection: Preparation and clinical evaluation. Tissue Eng. Part B Rev. 2019, 25, 225–236. [Google Scholar] [CrossRef]
- Cohn, C.S.; Lockhart, E. Autologous platelet-rich plasma: Evidence for clinical use. Curr. Opin. Hematol. 2015, 22, 527–532. [Google Scholar] [CrossRef]
- Schaller, M.; Hofmann, A.; Connor, J.; Nydegger, U.E. Filtered platelet concentrates from pooled buffy coats show comparable storage lesions when stored for 9 d at 20-24 degrees C or when supplemented with thromboSol at 2-6 degrees C. Eur. J. Haematol. 2000, 64, 401–410. [Google Scholar] [CrossRef]
- Brecher, M.E. Technical Manual, 15th ed.; American Association of Blood Banks: Bethesda, MD, USA, 2005; pp. 196–197. [Google Scholar] [CrossRef]
- Jones, C.I. Platelet function and ageing. Mamm. Genome 2016, 27, 358–366. [Google Scholar] [CrossRef]
- Haughey, L.; Barbul, A. Nutrition and lower extremity ulcers: Causality and/or treatment. Int. J. Low. Extrem. Wounds 2017, 16, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Liang, J.X.; Li, S.H.; Huang, S.; Yan, J.X.; Xiao, L.L.; Song, J.X.; Liu, H.W. Allogeneic Platelet-Rich Plasma Therapy as an Effective and Safe Adjuvant Method for Chronic Wounds. J. Surg. Res. 2020, 246, 284–291. [Google Scholar] [CrossRef]
- Steed, D.L.; Goslen, J.B.; Holloway, G.A.; Malone, J.M.; Bunt, T.J.; Webster, M.W. Randomized prospective double-blind trial in healing chronic diabetic foot ulcers. CT-102 activated platelet supernatant, topical versus placebo. Diabetes Care 1992, 15, 1598–1604. [Google Scholar] [CrossRef] [PubMed]
- Margolis, D.J.; Kantor, J.; Santanna, J.; Strom, B.L.; Berlin, J.A. Effectiveness of platelet release for the treatment of diabetic neuropathic foot ulcers. Diabetes Care 2001, 24, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.H.; Baek, D.S.; Kim, N.; Park, J.H.; Park, C. Topical allogeneic platelet-rich plasma treatment for a massive cutaneous lesion induced by disseminated intravascular coagulation in a toy breed dog. Ir. Vet. J. 2015, 68, 4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rajabi, H.; Sheikhani Shahin, H.; Norouzian, M. The healing effects of aquatic activities and allogenic injection of platelet-rich plasma (PRP) on injuries of Achilles tendon in experimental rat. World J. Plast. Surg. 2015, 4, 66–73. [Google Scholar]
- Abouelnasr, K.; Hamed, M.; Lashen, S. Enhancement of abdominal wall defect repair using allogenic platelet-rich plasma with commercial polyester/cotton fabric (Damour) in a canine model. J. Vet. Med. Sci. 2017, 79, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Smrke, D.; Gubina, B.; Domanoviç, D.; Rozman, P. Allogeneic platelet gel with autologous cancellous bone graft for the treatment of a large bone defect. Eur. Surg. Res. 2007, 39, 170–174. [Google Scholar] [CrossRef]
- Jeong, S.H.; Han, S.K.; Kim, W.K. Treatment of diabetic foot ulcers using a blood bank concentrate. Plast. Reconstr. Surg. 2010, 125, 944–952. [Google Scholar] [CrossRef]
- Quattrone, F.; Dini, V.; Barbanera, S.; Zerbinati, N.; Romanelli, M. Cutaneous ulcers associated with hydroxyurea therapy. J. Tissue Viability 2013, 22, 112–121. [Google Scholar] [CrossRef]
- Rebulla, P.; Pupella, S.; Santodirocco, M.; Greppi, N.; Villanova, I.; Buzzi, M.; De Fazio, N.; Grazzini, G. Italian Cord Blood Platelet Gel Study Group. Multicentre standardisation of a clinical grade procedure for the preparation of allogeneic platelet concentrates from umbilical cord blood. Blood Transfus. 2016, 14, 73–79. [Google Scholar] [CrossRef]
- Aggarwal, A.K.; Shashikanth, V.S.; Marwaha, N. Platelet-rich plasma prevents blood loss and pain and enhances early functional outcome after total knee arthroplasty: A prospective randomised controlled study. Int. Orthop. 2014, 38, 387–395. [Google Scholar] [CrossRef]
- Yotsu, R.R.; Hagiwara, S.; Okochi, H.; Tamaki, T. Case series of patients with chronic foot ulcers treated with autologous platelet-rich plasma. J. Derm. 2015, 42, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, N.; Ito, T.; Takemoto, S.; Katakami, M.; Kanda, N.; Tada, H.; Tanaka, S.; Teramukai, S.; Kawai, K.; Nakamura, Y.; et al. An exploratory clinical study on the safety and efficacy of an autologous fibroblast-seeded artificial skin cultured with animal product-free medium in patients with diabetic foot ulcers. Int. Wound J. 2014, 11, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Mazzucco, L.; Balbo, V.; Guaschino, R. “Reasonable compromise” to define the quality standards of platelet concentrate for non-transfusion use (CPunT). Transfus. Apher. Sci. 2012, 47, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, I.A.; Growney Kalaf, E.A.; Bowlin, G.L.; Sell, S.A. Platelet-rich plasma in bone regeneration: Engineering the delivery for improved clinical efficacy. Biomed. Res. Int. 2014, 2014, 392398. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Prado, R.; Orive, G. Allogeneic platelet-rich plasma: At the dawn of an off-the-shelf therapy? Trends Biotechnol. 2017, 35, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, D.; Wang, C.; Yuan, N.; Wang, Y.; He, L.; Yang, Y.; Chen, L.; Liu, G.; Li, X.; et al. Autologous platelet-rich gel for treatment of diabetic chronic refractory cutaneous ulcers: A prospective, randomized clinical trial. Wound Repair. Regen. 2015, 23, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Rožman, P.; Bolta, Z. Use of platelet growth factors in treating wounds and soft-tissue injuries. Acta Dermatovenerol. Alp. Pannonica. Adriat. 2007, 16, 156–165. [Google Scholar]
- Dhurat, R.; Sukesh, M.S. Principles and methods of preparation of platelet-rich plasma: A review and author’s perspective. J. Cutan. Aesthet. Surg. 2014, 7, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E. Platelet-rich plasma: Evidence to support its use. J. Oral. Maxillofac. Surg. 2004, 62, 489–496. [Google Scholar] [CrossRef]
- Wasterlain, A.S.; Braun, H.J.; Dragoo, J.L. Contents and formulations of platelet-rich plasma. Oper. Tech. Orthop. 2012, 22, 33–42. [Google Scholar] [CrossRef]
- European Committee on Blood Transfusion. Guide to the Preparation, Use and Quality Assurance of Blood Transfusion, 204th ed.; EDQM Publications: Strasbourg, France, 2020; p. 149. [Google Scholar]
| VARIABLES | Sample (N = 23 CNHWs) | |
|---|---|---|
| Blood group | ||
| O | 56.5% (13) | |
| A | 34.8% (8) | |
| B | 8.7% (2) | |
| Rh | ||
| Positive | 82.6% (19) | |
| Negative | 17.4% (4) | |
| Irregular antibodies, negative | 100% (23) | |
| Serological results, negative | 100% (23) | |
| Haemoglobin level (g/dL) | ||
| Mean (SD)/Median (Range) | 13.90 (1.19) | 13.8 (10.1–17.2) |
| Normal | 78.3% (18) | |
| Elevated | 4.3% (1) | |
| Reduced | 17.4% (4) | |
| White blood cell count (×103 µL) | ||
| Mean (SD)/Median (Range) | 9.40 (4.14) | 7.6 (4.4–16.2) |
| Normal | 65.2% (15) | |
| Elevated | 34.8% (8) | |
| Platelet count (×103 µL) | ||
| Mean (SD)/Median (Range) | 270 (200) | 213 (120–848) |
| Normal | 56.5% (13) | |
| Elevated | 13.0% (3) | |
| Reduced | 30.4% (7) | |
| Lymphocyte count (×103 µL) | ||
| Mean (SD)/Median (Range) | 1.75 (0.59) | 1.7 (0.9–2.9) |
| Normal | 74.2% (23) | 73.9% (17) |
| Lymphopaenia | 25.8% (8) | 26.1% (6) |
| Cholesterol level (mg/dL) | ||
| Mean (SD)/Median (Range) | 156 (37) | 154 (108–217) |
| Normal | 65.2% (15) | |
| Elevated | 21.7% (5) | |
| Reduced | 13.0% (3) | |
| Albumin level (g/dL) | ||
| Mean (SD)/Median (Range) | 4.12 (0.37) | 4.2 (3.3–4.8) |
| Normal | 95.7% (22) | |
| Reduced | 4.3% (1) | |
| FACTOR | Result of PRP Treatment | Hypothesis Test | Effect Size: R2 | ||
|---|---|---|---|---|---|
| Healing (n = 13) | No Healing (n = 10) | Value | p Value | ||
| AGE | |||||
| Mean (SD) | 71.5 (11.6) | 80.3 (4.7) | ZU = 1.85 † | 0.064 | 0.196 |
| Median (Range) | 71 (53–86) | 79 (77–89) | |||
| Intervals | Chi2 = 5.67 † | 0.059 | 0.246 | ||
| 50–69 years | 100% (5) | 0.0% (–) | |||
| 70–79 years | 36.4% (4) | 63.6% (7) | |||
| 89–89 years | 57.1% (4) | 42.9% (3) | |||
| Sex | Chi2 = 3.20 † | 0.074 | 0.139 | ||
| Men | 68.8% (11) | 31.3% (5) | |||
| Women | 28.6% (2) | 71.4% (5) | |||
| FACTOR | Result of PRP Treatment | Hypothesis Test | Effect Size: R2 | ||
|---|---|---|---|---|---|
| Healing (n = 13) | No Healing (n = 10) | Value | p Value | ||
| Comorbidities | Chi2 = 5.79 | 0.122 | 0.252 | ||
| No comorbidities | 80.0% (4) | 20.0% (1) | |||
| DM + cardiovascular diseases | 71.4% (5) | 28.6% (2) | |||
| Only cardiovascular diseases | 50.0% (4) | 50.0% (4) | |||
| Others | 0.0% (–) | 100% (3) | |||
| Comorbidities | Chi2 = 1.43 | 0.231 | 0.062 | ||
| No | 80.0% (4) | 20.0% (1) | |||
| Yes | 50.0% (9) | 50.0% (9) | |||
| High blood pressure | Chi2 = 0.21 | 0.645 | 0.009 | ||
| Yes | 60.0% (9) | 40.0% (6) | |||
| No | 50.0% (4) | 50.0% (4) | |||
| DM | Chi2 = 0.91 | 0.340 | 0.040 | ||
| Yes | 71.4% (5) | 28.6% (2) | |||
| No | 50.0% (8) | 50.0% (8) | |||
| Wound trigger | Chi2 = 2.59 | 0.274 | 0.112 | ||
| No known cause | 70.0% (7) | 30.0% (3) | |||
| Hydroxyurea | 41.7% (5) | 58.3% (7) | |||
| FACTOR | Result of PRP Treatment | Hypothesis Test | Effect Size: R2 | ||
|---|---|---|---|---|---|
| Healing (n = 13) | No Healing (n = 10) | Value | p Value | ||
| Blood group | Chi2 = 2.22 | 0.329 | 0.097 | ||
| O | 46.2% (6) | 53.8% (7) | |||
| A | 62.5% (5) | 37.5% (3) | |||
| B | 100% (2) | 0.0% (–) | |||
| Rh | Chi2 = 1.96 | 0.162 | 0.085 | ||
| Positive | 63.2% (12) | 36.8% (7) | |||
| Negative | 25.0% (1) | 75.0% (3) | |||
| Haemoglobin level (g/dL) | |||||
| Mean (SD) | 13.73 (1.55) | 14.12 (0.40) | ZU = 1.56 | 0.118 | 0.027 |
| Median (Range) | 13.6 (10.1–17.2) | 14.2 (13.4–14.5) | |||
| Categories | Chi2 = 1.64 | 0.441 | 0.071 | ||
| Normal levels | 50.0% (9) | 50.0% (9) | |||
| Elevated levels | 100% (1) | 0.0% (–) | |||
| Reduced levels | 75.0% (3) | 25.0% (1) | |||
| White blood cell count (×103 µL) | |||||
| Mean (SD) | 7.85 (3.01) | 11.41 (4.66) | ZU = 1.97 * | 0.049 | 0.191 |
| Median (Range) | 7.2 (4.4–13.6) | 14.1 (4.4–16.2) | |||
| Categories | Chi2 = 4.96 * | 0.026 | 0.215 | ||
| Normal levels | 73.3% (11) | 26.7% (4) | |||
| Elevated | 25.0% (2) | 75.0% (6) | |||
| Platelet count (×103 µL) | |||||
| Mean (SD) | 280 (193) | 258 (218) | ZU = 0.66 | 0.513 | 0.003 |
| Median (Range) | 218 (147–848) | 213 (120–848) | |||
| Categories | Chi2 = 0.79 | 0.673 | 0.034 | ||
| Normal | 61.5% (8) | 38.5% (5) | |||
| Elevated | 66.7% (2) | 33.3% (1) | |||
| Reduced | 42.9% (3) | 57.1% (4) | |||
| Lymphocyte count (×103 µL) | |||||
| Mean (SD) | 1.52 (0.48) | 2.07 (0.61) | ZU = 1.93 † | 0.053 | 0.214 |
| Median (Range) | 1.6 (0.9–2.7) | 1.7 (1.3–2.9) | |||
| Categories | Chi2 = 2.38 | 0.123 | 0.103 | ||
| Normal | 47.1% (8) | 52.9% (9) | |||
| Lymphopaenia | 83.3% (5) | 16.7% (1) | |||
| Cholesterol level (mg/dL) | |||||
| Mean (SD) | 160 (43) | 151 (27) | ZU = 0.84 | 0.401 | 0.016 |
| Median (Range) | 169 (110–217) | 154 (108–188) | |||
| Categories | Chi2 = 9.44 ** | 0.009 | 0.410 | ||
| Normal | 33.3% (5) | 66.7% (10) | |||
| Elevated | 100% (5) | 0.0% (–) | |||
| Reduced | 100% (3) | 0.0% (–) | |||
| Albumin level (g/dL) | |||||
| Mean (SD) | 4.13 (0.41) | 4.12 (0.34) | ZU = 0.25 | 0.804 | 0.000 |
| Median (Range) | 4.1 (3.6–4.8) | 4.2 (3.3–4.6) | |||
| Categories | Chi2 = 1.36 | 0.244 | 0.059 | ||
| Normal | 59.1% (13) | 40.9% (9) | |||
| Reduced | 0.0% (–) | 100% (1) | |||
| FACTOR | Result of PRP Treatment | Hypothesis Test | Effect Size: R2 | ||
|---|---|---|---|---|---|
| Healing (n = 13) | No Healing (n = 10) | Value | p Value | ||
| Location of the wound | |||||
| Leg + ankle + foot + toes | 54.5% (12) | 45.5% (10) | |||
| Knee | 100% (1) | 0.0% (–) | |||
| Size (cm) | |||||
| Mean (SD) | 3.23 (2.00) | 2.90 (1.68) | ZU = 0.34 | 0.730 | 0.088 |
| Median (Range) | 3.00 (0.5–8.0) | 2.50 (1.0–6.5) | |||
| Categories | Chi2 = 3.24 | 0.198 | 0.141 | ||
| <2 cm | 71.4% (5) | 28.6% (2) | |||
| 2–3 cm | 33.3% (3) | 66.7% (6) | |||
| >3 cm | 71.4% (2) | 28.6% (2) | |||
| Severity | Chi2 = 2.35 | 0.503 | 0.102 | ||
| Mild | 100% (2) | 0.0% (–) | |||
| Moderate | 62.5% (5) | 37.5% (3) | |||
| Moderate-to-Severe | 50.0% (4) | 50.0% (4) | |||
| Severe | 40.0% (2) | 60.0% (3) | |||
| Previous treatments | Chi2 = 1.99 | 0.369 | 0.087 | ||
| Only wound care at home | 71.4% (5) | 28.6% (2) | |||
| Surgery | 33.3% (2) | 66.7% (4) | |||
| Local wound care at hospital | 60.0% (6) | 40.0% (4) | |||
| Duration of previous treatment (months) | |||||
| Mean (SD) | 3.54 (2.50) | 2.90 (0.88) | ZU = 0.33 | 0.974 | 0.027 |
| Median (Range) | 2.0 (1–10) | 3.0 (2–4) | |||
| Categories | Chi2 = 0.78 | 0.676 | 0.034 | ||
| ≤2 months | 63.6% (7) | 36.4% (4) | |||
| 3 months | 40.0% (2) | 60.0% (3) | |||
| >3 months | 57.1% (4) | 42.9% (3) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidán-Estévez, J.; Sánchez-Herráez, S.; Escalante-Barrigón, F.; Seco-Calvo, J. Healing of Chronic Wounds with Platelet-Derived Growth Factors from Single Donor Platelet-Rich Plasma following One Freeze-Thaw Cycle. A Cross-Sectional Study. J. Clin. Med. 2021, 10, 5762. https://doi.org/10.3390/jcm10245762
Vidán-Estévez J, Sánchez-Herráez S, Escalante-Barrigón F, Seco-Calvo J. Healing of Chronic Wounds with Platelet-Derived Growth Factors from Single Donor Platelet-Rich Plasma following One Freeze-Thaw Cycle. A Cross-Sectional Study. Journal of Clinical Medicine. 2021; 10(24):5762. https://doi.org/10.3390/jcm10245762
Chicago/Turabian StyleVidán-Estévez, Julia, Sergio Sánchez-Herráez, Fernando Escalante-Barrigón, and Jesús Seco-Calvo. 2021. "Healing of Chronic Wounds with Platelet-Derived Growth Factors from Single Donor Platelet-Rich Plasma following One Freeze-Thaw Cycle. A Cross-Sectional Study" Journal of Clinical Medicine 10, no. 24: 5762. https://doi.org/10.3390/jcm10245762
APA StyleVidán-Estévez, J., Sánchez-Herráez, S., Escalante-Barrigón, F., & Seco-Calvo, J. (2021). Healing of Chronic Wounds with Platelet-Derived Growth Factors from Single Donor Platelet-Rich Plasma following One Freeze-Thaw Cycle. A Cross-Sectional Study. Journal of Clinical Medicine, 10(24), 5762. https://doi.org/10.3390/jcm10245762







