Abstract
Background: There are few studies on sex differences in the incidence rates (IR) for salmonellosis over several countries by age and time period. The purpose of this study was to explore the extent and consistency of the sex and age-specific differences. Methods: We analyzed national data from eight countries between 1994 and 2016. We computed country-specific male to female incidence rate ratios (IRRs) for each age group and pooled the data using meta-analytic methods. Variations of the IRRs by age, country and time period were evaluated using meta-regression. Results: The pooled male to female incidence RRs for ages 0–1, 1–4, 5–9 and 10–14, were 1.04 (1.02–1.06), 1.02 (1.01–1.03), 1.07 (1.05–1.08) and 1.28 (1.23–1.33), respectively. For the ages 15–44 and 45–64, the incidence rates were significantly higher in females. Meta-regression analyses indicate that age groups contributed most of the variation in the male to female IRRs. Conclusions: We suggest that genetic and hormonal factors and interactions between hormones and gut microbiota could contribute to the sex differences observed in young children. These findings should provide clues about the mechanisms of the infection, and should be useful in targeting treatments and development of vaccines. Highlights: (1) This manuscript provides consistent estimates of the excess salmonellosis incidence rates in male children up to age 15, which suggests an impact of sex hormones or genetic differences. (2) Our findings should promote the further investigations on sex-related determinants of infectious diseases.
1. Introduction
Salmonellosis is a common foodborne disease that affects the intestinal tract and most often presents as acute gastroenteritis. Humans become infected most frequently through contaminated water or food. Pathogens belonging to the Salmonella genus are facultative Gram-negative anaerobes, typically live in animal and human intestines and may shed through feces [1,2]. Within the Salmonella genus, Salmonella enterica is subdivided into six subspecies and includes more than 2500 serovares. The majority of the Salmonella strains that cause infection and represent a significant concern belong to the Salmonella enterica species. Salmonella has been identified as a commonly detected infection and the main cause of high morbidity and mortality in developing countries [1,2].
The observation that males appear to be more affected by COVID-19 than females once again emphasizes the importance of examining sex differences in infectious diseases [3]. Information on sex differences in salmonellosis incidence may provide guidance on the mechanisms of immune response to the infection. Studies on sex differences in salmonellosis incidence rates usually focus on individual countries, restricted to selected age groups and time periods [4,5,6,7,8,9,10]. The main goal of this study was to examine the extent and consistency of sex- and age-specific differences in salmonellosis over countries and years.
2. Materials and Methods
2.1. Search Strategy
We limited our search to published studies or through direct contact with national health organizations to obtain national data on salmonellosis incidence rates disaggregated by age and sex. We identified eight countries with well-established reporting and surveillance systems, with advanced health system and diagnostic facilities. The reporting system and the diagnostic methods of salmonellosis may be different over countries and time, however, there is no reason to believe that they differ by sex.
2.2. Sources of Data
2.2.1. Source of Data
We managed to access detailed data on reported cases of salmonellosis, by age, sex and year, from a number of national institutions and surveillance systems, for eight countries. For Australia, data were obtained from the National Notifiable Diseases Surveillance System (NNDSS) for the years 2001–2016 [11], for Canada, from the Public Health Agency of Canada (PHAC) for 1991–2015 [12], for the Czech Republic, from the Institute of Health Information and Statistics for the years 2008–2013 [13], for Finland, from the National Institute for Health and Welfare (THL) for 1995–2016 [14], for Germany, from the German Federal Health Monitoring System for 2001–2016 [15], for New Zealand, from the Institute of Environmental Science and Research (ESR) for the Ministry of Health for 1997–2015 [16], for Poland, data for the years 2006–2016 were obtained from the National Institute of Public Health [17], and for Spain, from the Spanish Epidemiological Surveillance Network for 2005–2015 [18].
Data about the population denominators (disaggregated by age, sex and year) for the Australian population were obtained from the Australian Bureau of Statistics [19], for Canada from Statistics Canada [20], for the Czech Republic from the Czech Statistical Office [21], for Finland from the Statistics Finland’s PX-Web databases [22], for Germany from the German Federal Health Monitoring System [23], for New Zealand from Statistics New Zealand [24], for Poland from official website Statistics Poland [25] and for Spain from the Demographic Statistics Database [26].
2.2.2. Ethics
National, anonymous data were published by official representatives of each country and there was no need for informed consent and research ethics board approval.
2.3. Statistical Analyses
2.3.1. Calculation of Incidence Rates
Salmonellosis incidence rates (IR) for males and females, for each age group and country, for several years were calculated. Incidence rates per 100,000 of the population of the same sex were obtained as the number of published cases divided by the population size. The grouping by age was performed as follows: infants (<1), early childhood (1–4), late childhood (5–9), puberty (10–14), young adulthood (15–44 or 15–39), middle adulthood (45–64 or 40–59) and senior adulthood (65+ or 60+). The reporting systems of Canada, Finland and New Zealand used different age groups for the young adulthood (15–39), middle adulthood (40–59) and senior adulthood (60+). The male:female salmonellosis incidence rate ratio (RR) was obtained by dividing the male IR by the female IR.
2.3.2. Meta-Analyses
Statistical analyses were performed using meta-analytic methods.
The outcome variable in the study was the male:female salmonellosis IRR. Meta-analyses were performed on the subgroups of age, country and years. Pooled IRRs were calculated for each age subgroup, for all eight countries and time periods. The results are displayed in forest plots. Cochran’s Q test was used to determine the heterogeneity. Between-study variance was evaluated by Tau2 and I2. If I2 ≥ 50% and/or the Q test produced a p-value < 0.1, the random effects model was performed to measure pooled RRs and CI (95% Confidence Interval). To assess the impact of each county or years on the pooled male:female IRRs, we conducted sensitivity analysis and re-evaluated the pooled RRs for each age subgroup. We used meta-regression to identify the source of variation in the IRRs, including age group, country and group of years. The meta-analytic methods and meta-regressions were performed using STATA software version 12.1 (Stata Corp., College Station, TX, USA). The Egger test (illustrated as funnel plots) for asymmetry was performed for exploring possible imbalance in the impact of countries or group of years.
3. Results
Descriptive Statistics
The summary of salmonellosis incidence rates (by sex, per 100,000 population) in different countries for each age subgroup is presented in Table 1. In the Czech Republic at ages <1–14, the incidence rates of salmonellosis were highest compared to other countries. In Spain, at ages 15–65, salmonellosis incidence rates for both sexes were lower than in all other countries.

Table 1.
Descriptive data: details of the countries included in the analysis, disaggregated by sex and age.
The results of the meta-analyses by age groups are displayed as forest plots (Supplementary Materials, Figures S1–S7). The total male:female RR at age < 1 was 1.04 (95% CI 1.02–1.06, Supplementary Figure S1) with low heterogeneity (I2 = 10.8% and Tau2 = 0.0004). The IRRs varied from 1.02 in the Czech Republic to 1.06 in New Zealand, Poland and Spain.
The forest plot for age 1–4 is presented in Supplementary Figure S2. The total male:female RR = 1.02 (95% CI 1.01–1.03), with I2 = 29.7% and Tau2 = 0.0003, and varied from 1 in Germany to 1.05 in Canada.
The forest plot for ages 5–9 is presented in Supplementary Figure S3. The pooled RR was 1.07 (95% CI 1.05–1.08), with I2 = 26.8% and Tau2 = 0.0006. Subgroup pooled IRRs by country varied from 1.01 in Poland to 1.14 in New Zealand.
The forest plot for age group 10–14 is presented in Supplementary Figure S4. The total male:female incidence RR was 1.28 (95% CI 1.23–1.33), with I2 = 79.1% and Tau2 = 0.0108. The sub-group RRs by country varied from 1.12 in Poland to 1.61 in New Zealand.
The forest plot for age for young adulthood is presented in Supplementary Figure S5. The pooled incidence RR = 0.91 for all countries together (95% CI 0.89–0.93), with I2 = 92.5% and Tau2 = 0.068. The subgroup RRs by country ranged from 0.78 for the Czech Republic to 1.08 for New Zealand.
The forest plot for middle adulthood is presented in Supplementary Figure S6. The total male:female incidence RR = 0.89 (95% CI 0.86–0.92), I2 = 92.8% and Tau2 = 0.0121, with RRs ranging from 0.68 in the Czech Republic to 1.16 in Spain.
The forest plot for senior adulthood is presented in Supplementary Figure S7. The pooled incidence RR was 1.03, 95% CI 1–1.07, I2 = 89.5% and Tau2 = 0.0109. The subtotal RRs by country varied from 0.83 in the Czech Republic to 1.35 in Spain.
In order to identify factors that may have an unduly influence on the pooled male:female incidence RRs, leave-one-out sensitivity analysis was used.
One country or year period’s omission at a time caused only slight changes in the total male:female incidence RRs (Table 2 and Table 3, respectively). Data from the Czech Republic indicate a high IR of salmonellosis cases. Sensitivity analysis determined that the data from the Czech Republic did not affect overall results by age groups.

Table 2.
Sensitivity analysis, by age group and country.

Table 3.
Sensitivity analysis, by age group and years.
Meta-regression analyses revealed that age groups contributed most of the differences in the male:female incidence RRs. For age < 1, the IRR was higher than in young and middle adulthood (p < 0.0001), but lower than in puberty (p < 0.0001). The incidence RRs for ages 1–4 and 5–9 were also significantly higher than for ages 15–39/44 and 40–59/45–64 (p < 0.0001). The incidence RRs for puberty were higher than in young, middle and senior adulthood (p < 0.0001). There was no association of incidence RRs for reported years.
The p-value for asymmetry (Egger’s test) was significant for early childhood and puberty, p = 0.095 and p = 0.001, respectively. Data regarding the asymmetry test for all age groups are presented in Figure 1.
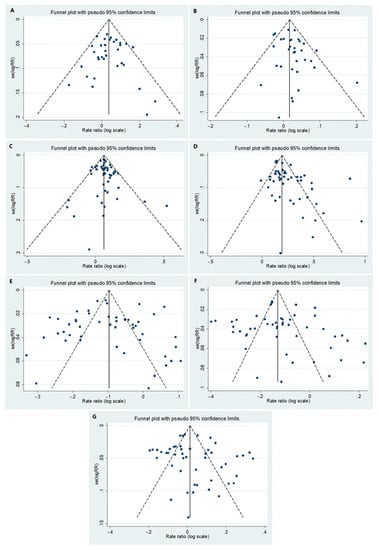
Figure 1.
Funnel plots for the following age groups: (A) <1, (B) 1–4, (C) 5–9, (D) 10–14, (E) 15–39/15–44, (F) 40–59/45–64 and (G) 60+/65+.
4. Discussion
In this study, we studied the sex differences in salmonellosis incidence rates in eight countries by age group over a period of 6 to 22 years. Our results show a consistent excess in disease incidence rates in males under the age of 15. In older ages, in early and middle adulthood, there is an excess in females. In the oldest group, 65 and over, there is again an excess in males.
This study has a number of strengths and limitations. The strengths lie in the use of national incidence rates of reported cases from eight countries over a number of years. This should avoid some of the selection bias in smaller studies. In order to understand the magnitude of the findings in all countries that represent the population of Europe, Canada and Australia/New Zealand, we focused on countries with developed health systems. In these countries, there is no reason to assume that there is selective treatment or attitude according to the sex. For example, the study on the United States population relates to the issue and shows the identical medical care for salmonellosis for both sexes [8]. There may be heterogeneity in national reporting systems as well as the clinical criteria and laboratory-confirmed cases, but these factors should not differ between sexes. Information bias should be taken into consideration, especially because of non-specific clinical performance of the disease or the lack of laboratory confirmation or under-reporting in all reported countries. We assume that, even if this is the case, there is a low likelihood for it to be different between the sexes. Differences between males and females has been reported in the incidence of bacterial diseases, including in children [27]. Previous studies on the incidence of salmonellosis have focused on a particular country or relied on information from medical institutions [4,5,6,7,8,9,10], which could introduce bias. Most studies did not address the size of the population (as a denominator) of males or females and the findings were inconsistent.
Sex differences in the incidence of salmonellosis have been observed in a number of studies. In a study on 116,362 isolates of non-typhoidal Salmonella from England, between years 2004 and 2015, there were more males (unadjusted OR, 1.46; 95% CI, 1.35 to 1.59) [4]. Non-typhoidal Salmonella detected in blood in the United States, in 2003–2013, was associated with male sex and adults ≥ 65 years [5]: 272 adults with non-typhoidal Salmonella bacteremia were included in the study and males predominated (58.5%, with age range of 19–98 years, and a median age of 63 years) [6]. In Nairobi, Kenya, 332 children aged between 1 month and 7 years with salmonellosis recruited in the study showed no difference between sexes (p > 0.05) [7]. In a study that was conducted in the United States over a 33-year period (1968–2000), there was an excess of salmonellosis in adult women [8]. Surveillance data on 11,243 subjects older than 18 years collected over 3 years from two hospitals in Shanghai [9] showed that Salmonella infection was similar between males and females (50.1% and 47.5%). In a study conducted by GBD 2017, non-Typhoidal Salmonella Invasive Disease Collaborators, on 535,000 cases that occurred in the years 1990 to 2017, incidence of Salmonella was not significantly different between sexes in all ages [10].
We hypothesized that the sex differences in salmonellosis incidences include exposure factors, biological (genetic and hormonal differences) factors and interactions between hormones and gut microbiota. As regards exposure, outbreak investigations have shown that the majority of salmonellosis infections result from consuming Salmonella-contaminated foods [28]. We hypothesized that the main cause of female predominance (in young and middle adulthood groups) in Salmonella incidence is excessive exposure. In general, females consume more fruits and vegetables than males [29]. The differences between sexes in eating habits [30,31] and overall exposure influence salmonellosis incidence. Females’ greater exposure to poultry during food-handling practices [32] and childcare place adult women (especially in young and middle adulthood) at increased risk of salmonellosis.
We suggest that the heterogeneity over time is likely a consequence of random variation. The heterogeneity in puberty and in older ages may result from random variation in the Salmonella male:female RR between reported year groups and between countries.
Biological differences between sexes should not be ignored. Biological and hormonal sex differences can lead to more robust immune responses in females and to differences in disease outcomes between sexes due to infections [33]. However, sex-specific differences can provide an additional biological mechanism mediating differences in microbiota composition. Healthy females have greater pro-inflammatory genes’ expression in gastrointestinal tract samples compared with males [34]. It is possible that gut inflammatory status influences the sex-specific pattern in healthy and asymptomatic individuals and may contribute to female immune systems’ overreaction. These differences between male and female microbial composition, in part driven by sex hormones, may affect intestinal inflammatory disease outcomes and severity [35,36].
There do not appear to be any reasons for suspecting sex differences in exposure in infants and young children. Contact with live animals can result in salmonellosis in childhood and among children aged < 1 year, but the exposure is not likely to differ between the sexes and it seems to be a marginal factor to explain the male predominance in disease incidence [37].
We hypothesized that the higher salmonellosis incidence rate in young males under the age of 15 may be explained by genetic and hormonal factors. Both sex hormones and the X chromosome [38] may contribute to the immune response even in young age groups. The differential responses of immune cells to pathogens in the presence of hormones contribute to explaining age and sex differences in immune responses and in diseases [38]. In infants, the short activation of the pituitary-gonadal hormone axis is defined as “mini-puberty” [39]. Higher estrogen levels are found in girls even before physical manifestation of pubertal maturation [40].
Sex hormones, mainly estrogen, regulate immune responses, cytokine production and induction [33]. High levels of IFN-γ, TNF-α and the inflammatory interleukins IL-18, IL-12 and IL-15 participate in Salmonella clearance [41,42], along with CD4+ T cells, the key factor mediating immunity against the Salmonella pathogen via the IFN-γ production. A variety of estrogen-regulated cytokines that are produced as a result of immune response provide the appropriate environmental conditions for CD4+ T cells’ differentiation towards both Th1 and Th17 cells that are essential for the immune response to Salmonella [43].
As hypothesized, sex hormones lead to gut estrogen-regulated microbiota composition differences between girls and boys, even in early life [44]. Since the composition of the human gut microbiota is regulated by estrogen, we can assume that differences in the gut microbiota may be a factor of the sexual dimorphism in salmonellosis [45,46].
5. Conclusions
Future research should be performed in order to understand the patterns and pathways that mediate the immune system response and impact immunity to infectious diseases differently in males compared with females. The consistent sex differences observed between males and females in the incidence of Salmonellosis emphasizes the need to consider “sex” as a biological variable in studies of communicable infectious diseases and public health.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jcm10245767/s1, Figure S1: Male:female salmonellosis incidence rate ratios (RR) for different years and countries at age < 1, Figure S2: Male:female salmonellosis incidence rate ratios (RR) for different years and countries at age 1–4, Figure S3: Male:female salmonellosis incidence rate ratios (RR) for different years and countries at age 5–9, Figure S4: Male:female salmonellosis incidence rate ratios (RR) for different years and countries at age 10–14, Figure S5: Male:female salmonellosis incidence rate ratios (RR) for different years and countries at age 15–39/15–44, Figure S6: Male:female salmonellosis incidence rate ratios (RR) for different years and countries at age 40–59/45–64, Figure S7: Male:female salmonellosis incidence rate ratios (RR) for different years and countries at age 60+/65+.
Author Contributions
V.P. participated in the conception and design of the study, acquisition of data, analysis and interpretation of the data and writing the manuscript; N.S. assisted in the data analysis and reviewed the manuscript; M.S.G. designed and supervised the study, interpreted the data and helped in drafting and revising the manuscript for important intellectual content. All authors have seen and approved the content and have contributed significantly to the work. We hereby declare that this manuscript has not been submitted or accepted elsewhere. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
National, anonymous data published by official representatives of each country were collected, therefore ethical review and approval were waived for this study.
Informed Consent Statement
Anonymous data published by national institutes of each country were obtained, therefore patient consent was waived.
Data Availability Statement
All data are available from the original sources or from the authors. Links to publicly archived datasets analyzed or generated during the study are included in References.
Acknowledgments
We thank all the official institutions of all countries for providing their national data on salmonellosis incidence.
Conflicts of Interest
We declare no conflicts of interest.
References
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2015. EFSA J. 2016, 14, e04634. [Google Scholar]
- Kurtz, J.R.; Goggins, J.A.; McLachlan, J.B. Salmonella infection: Interplay between the bacteria and host immune system. Immunol. Lett. 2017, 190, 42–50. [Google Scholar] [CrossRef]
- Gausman, J.; Langer, A. Sex and Gender Disparities in the COVID-19 Pandemic. J. Womens Health 2020, 29, 465–466. [Google Scholar] [CrossRef]
- Katiyo, S.; Muller-Pebody, B.; Minaji, M.; Powell, D.; Johnson, A.P.; de Pinna, E.; Day, M.; Harris, R.; Godbole, G. Epidemiology and Outcomes of Nontyphoidal Salmonella Bacteremias from England, 2004 to 2015. J. Clin. Microbiol. 2019, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelo, K.M.; Reynolds, J.; Karp, B.E.; Hoekstra, R.M.; Scheel, C.M.; Friedman, C. Antimicrobial Resistance Among Nontyphoidal Salmonella Isolated From Blood in the United States, 2003–2013. J. Infect. Dis. 2016, 214, 1565–1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.L.; Lee, H.C.; Lee, N.Y.; WU, C.J.; Lin, S.H.; Shih, H.I.; Lee, C.C.; Ko, W.C.; Chang, C.M. Non-typhoidal Salmonella bacteraemia in elderly patients: An increased risk for endovascular infections, osteomyelitis and mortality. Epidemiol. Infect. 2012, 140, 2037–2044. [Google Scholar] [CrossRef]
- Kariuki, S.; Revathi, G.; Kariuki, N.; Kiiru, J.; Mwituria, J.; Hart, C.A. Characterisation of community acquired non-typhoidal Salmonella from bacteraemia and diarrhoeal infections in children admitted to hospital in Nairobi, Kenya. BMC Microbiol. 2006, 6, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reller, M.E.; Tauxe, R.V.; Kalish, L.A.; Mølbak, K. Excess salmonellosis in women in the United States: 1968–2000. Epidemiol. Infect 2008, 136, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Gu, Y.; Wang, X.; Zhang, Y.; Zhan, L.; Liu, J.; Yan, H.; Liu, Y.; Zhen, S.; Chen, X.; et al. Epidemiological and clinical differences between sexes and pathogens in a three-year surveillance of acute infectious gastroenteritis in Shanghai. Sci. Rep. 2019, 9, 9993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GBD 2017 Non-Typhoidal Salmonella Invasive Disease Collaborators. The global burden of non-typhoidal salmonella invasive disease: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect. Dis. 2019, 19, 1312–1324. [Google Scholar] [CrossRef] [Green Version]
- National Notifiable Diseases Surveillance System (NNDSS), Department of Health. Available online: http://www9.health.gov.au/cda/source/rpt_5_sel.cfm (accessed on 1 April 2018).
- Public Health Agency of Canada. Available online: https://www.canada.ca/en/public-health.html (accessed on 1 June 2018).
- Institute of Health Information and Statistics. Available online: https://www.uzis.cz/en/catalogue/infectious-diseases (accessed on 1 March 2018).
- National Institute for Health and Welfare (THL). Available online: https://www.thl.fi/ttr/gen/rpt/tilastot.html (accessed on 1 May 2018).
- German Federal Health Monitoring System. Available online: http://www.gbe-bund.de/gbe10/pkg_isgbe5.prc_isgbe?p_uid=gast&p_aid=0&p_sprache=D (accessed on 1 February 2018).
- Environmental Science and Research (ESR) for the Ministry of Health. Available online: https://surv.esr.cri.nz/surveillance/annual_surveillance.php (accessed on 30 March 2018).
- National Institute of Public Health. Available online: http://wwwold.pzh.gov.pl/oldpage/epimeld/index_a.html (accessed on 1 December 2018).
- Instituto de Salud Carlos III. Available online: https://eng.isciii.es/eng.isciii.es/Paginas/Inicio.html (accessed on 1 March 2018).
- ABS. Stat (Australian Bureau of Statistics). Available online: http://stat.data.abs.gov.au/Index.aspx?DatasetCode=ABS_ERP_ASGS2016 (accessed on 15 May 2018).
- Statistics, Canada, CANSIM Database. Available online: https://www150.statcan.gc.ca/t1/tbl1/en/cv.action?pid=1710010201 (accessed on 1 June 2018).
- Czech Statistical Office. Available online: https://www.czso.cz/csu/czso/population (accessed on 1 March 2018).
- Statistics Finland’s PX-Web databases. Available online: https://pxnet2.stat.fi/PXWeb/pxweb/en/StatFin/ (accessed on 15 April 2018).
- German Federal Health Monitoring System. Available online: http://www.gbe-bund.de/gbe10/abrechnung.prc_abr_test_logon?p_uid=gast&p_aid=46300054&p_knoten=VR&p_sprache=E&p_suchstring=population (accessed on 1 February 2018).
- Stats NZ, Infoshare. Available online: http://archive.stats.govt.nz/infoshare/SelectVariables.aspx?pxID=b854d8a2-3fdf-402c-af69-604112e80baa (accessed on 15 May 2018).
- Statistics Poland. Available online: https://stat.gov.pl/en/basic-data/ (accessed on 1 December 2018).
- Demographic Statistics Database (United Nations Statistics: Division). Available online: http://data.un.org/Data.aspx?d=POP&f=tableCode%3A22 (accessed on 1 April 2018).
- Peer, V.; Schwartz, N.; Green, M.S. A multi-country, multi-year, meta-analytic evaluation of the sex differences in age-specific pertussis incidence rates. PLoS ONE 2020, 15, e0s231570. [Google Scholar] [CrossRef]
- Bean, N.H.; Goulding, J.S.; Lao, C.; Angulo, F.J. Surveillance for foodborne-disease outbreaks—United States, 1988–1992. MMWR CDC Surveill. Summ. 1996, 45, 1–66. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Serdula, M.; Bland, S.; Mokdad, A.; Bowman, B.; Nelson, D. Trends in fruit and vegetable consumption among adults in 16 US states: Behavioral Risk Factor Surveillance System, 1990–1996. Am. J. Public Health 2000, 90, 777–781. [Google Scholar]
- Sivapalasingam, S.; Barrett, E.; Kimura, A.; van Duyne, S.; de Witt, W.; Ying, M.; Frisch, A.; Phan, Q.; Gould, E.; Shillam, P.; et al. A Multistate Outbreak of Salmonella enterica Serotype Newport Infection Linked to Mango Consumption: Impact of Water-Dip Disinfestation Technology. Clin. Infect. Dis. 2003, 37, 1585–1590. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Outbreaks of Salmonella infections associated with eating Roma tomatoes—United States and Canada, 2004. MMWR Morb. Mortal. Wkly. Rep. 2005, 54, 325–328. [Google Scholar]
- Roy, P.; Dhillon, A.S.; Lauerman, L.H.; Schaberg, D.M.; Bandli, D.; Johnson, S. Results of salmonella isolation from poultry products, poultry, poultry environment, and other characteristics. Avian Dis. 2002, 46, 17–24. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Sankaran-Walters, S.; Macal, M.; Grishina, I.; Nagy, L.; Goulart, L.; Coolidge, K.; Li, J.; Fenton, A.; Williams, T.; Miller, M.K.; et al. Sex differences matter in the gut: Effect on mucosal immune activation and inflammation. Biol. Sex Differ. 2013, 4, 10. [Google Scholar] [CrossRef] [Green Version]
- Mariscal, C.H.; Rangel-Zúñiga, O.A.; Alcalá-Díaz, J.F.; Gómez-Delgado, F.; Pérez-Martinez, P.; Delgado-Lista, J.; Quintana-Navarro, G.M.; Landa, B.B.; Navas-Cortés, J.A.; Tena-Sempere, M.; et al. Intestinal Microbiota Is Influenced by Gender and Body Mass Index. PLoS ONE 2016, 11, e0154090. [Google Scholar]
- Vemuri, R.; Sylvia, K.E.; Klein, S.L.; Forster, S.C.; Plebanski, M.; Eri, R.; Flanagan, K.L. The microgenderome revealed: Sex differences in bidirectional interactions between the microbiota, hormones, immunity and disease susceptibility. Semin. Immunopathol. 2019, 41, 265–275. [Google Scholar] [CrossRef]
- Marus, J.R.; Magee, M.J.; Manikonda, K.; Nichols, M.C. Outbreaks of Salmonella enterica infections linked to animal contact: Demographic and outbreak characteristics and comparison to foodborne outbreaks-United States, 2009–2014. Zoonoses Public Health 2019, 66, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Jaillon, S.; Berthenet, K.; Garlanda, C. Sexual Dimorphism in Innate Immunity. Clin. Rev. Allergy Immunol. 2019, 56, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Copeland, K.C.; Chernausek, S. Mini-Puberty and Growth. Pediatrics 2016, 138, e20161301. [Google Scholar] [CrossRef] [Green Version]
- Frederiksen, H.; Johannsen, T.H.; Andersen, S.E.; Albrethsen, J.; Landersoe, S.K.; Petersen, J.H.; Andersen, A.N.; Vestergaard, E.; Schorring, M.E.; Linneberg, A.; et al. Sex-specific estrogen levels and reference intervals from infancy to late adulthood determined by LC-MS/MS. J. Clin. Endocrinol. Metab. 2020, 105, 754–768. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, Y.; Takada, H.; Nomura, A.; Jin, C.-H.; Hattori, H.; Ihara, K.; Aoki, T.; Eguchi, K.; Hara, T. Th1 and Th1-inducing cytokines in Salmonella infection. Clin. Exp. Immunol. 2003, 131, 111–117. [Google Scholar] [CrossRef]
- Stoycheva, M.; Murdjeva, M. Serum levels of interferon-gamma, interleukin-12, tumour necrosis factor-alpha, and interleukin-10, and bacterial clearance in patients with gastroenteric Salmonella infection. Scand. J. Infect. Dis. 2005, 37, 11–14. [Google Scholar] [CrossRef]
- Thiennimitr, P.; Winter, S.E.; Baumler, A.J. Salmonella, the host and its microbiota. Curr. Opin. Microbiol. 2012, 15, 108–114. [Google Scholar] [CrossRef] [Green Version]
- Martin, R.; Makino, H.; Yavuz, A.C.; Ben-Amor, K.; Roelofs, M.; Ishikawa, E.; Kubota, H.; Swinkels, S.; Sakai, T.; Oishi, K.; et al. Early-Life Events, Including Mode of Delivery and Type of Feeding, Siblings and Gender, Shape the Developing Gut Microbiota. PLoS ONE 2016, 11, e0158498. [Google Scholar] [CrossRef] [Green Version]
- Baker, J.M.; Al-Nakkash, L.; Herbst-Kralovetz, M.M. Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas 2017, 103, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.L.; Madak-Erdogan, Z. Estrogen and Microbiota Crosstalk: Should We Pay Attention? Trends Endocrinol. Metab. 2016, 27, 752–755. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).