Chemokine C-C Motif Ligand 7 (CCL7), a Biomarker of Atherosclerosis, Is Associated with the Severity of Alopecia Areata: A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Measurement of Atherosclerosis and Cardiovascular Risk Markers
2.3. Statistical Analysis
2.4. Ethics Committee Approval
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roifman, I.; Beck, P.L.; Anderson, T.J.; Eisenberg, M.J.; Genest, J. Chronic inflammatory diseases and cardiovascular risk: A systematic review. Can. J. Cardiol. 2011, 27, 174–182. [Google Scholar] [CrossRef]
- Waśkiel-Burnat, A.; Rakowska, A.; Kurzeja, M.; Czuwara, J.; Sikora, M.; Olszewska, M.; Rudnicka, L. The value of dermoscopy in diagnosing eyebrow loss in patients with alopecia areata and frontal fibrosing alopecia. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 213–219. [Google Scholar] [CrossRef] [Green Version]
- Alkhalifah, A. Alopecia areata update. Dermatol. Clin. 2013, 31, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Simakou, T.; Butcher, J.P.; Reid, S.; Henriquez, F.L. Alopecia areata: A multifactorial autoimmune condition. J. Autoimmun 2019, 98, 74–85. [Google Scholar] [CrossRef] [Green Version]
- Rudnicka, L.; Waśkiel-Burnat, A. Systemic aspects of alopecia areata Comment to the article by Lai and Sinclair. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e214–e215. [Google Scholar] [CrossRef] [PubMed]
- Conic, R.R.Z.; Chu, S.; Tamashunas, N.L.; Damiani, G.; Bergfeld, W. Prevalence of cardiac and metabolic diseases among patients with alopecia areata. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e128–e129. [Google Scholar] [CrossRef]
- Glickman, J.W.; Dubin, C.; Renert-Yuval, Y.; Dahabreh, D.; Kimmel, G.W.; Auyeung, K.; Estrada, Y.D.; Singer, G.; Krueger, J.G.; Pavel, A.B.; et al. Cross-sectional study of blood biomarkers of patients with moderate to severe alopecia areata reveals systemic immune and cardiovascular biomarker dysregulation. J. Am. Acad. Dermatol. 2021, 84, 370–380. [Google Scholar] [CrossRef]
- Wei, Y.H.; Tai, Y.H.; Dai, Y.X.; Chang, Y.T.; Chen, T.J.; Chen, M.H. Bidirectional association between alopecia areata and atopic dermatitis: A population-based cohort study in Taiwan. Clin. Exp. Allergy 2020, 50, 1406–1414. [Google Scholar] [CrossRef]
- Incel-Uysal, P.; Akdogan, N.; Alli, N.; Oktem, A.; Candar, T.; Topcuoglu, C.; Turhan, T. Assessment of Metabolic Profile and Ischemia-modified Albumin Level in Patients with Alopecia Areata: A Case-Control Study. Indian J. Dermatol. 2019, 64, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Olsen, E.A.; Roberts, J.; Sperling, L.; Tosti, A.; Shapiro, J.; McMichael, A.; Bergfeld, W.; Callender, V.; Mirmirani, P.; Washenik, K.; et al. Objective outcome measures: Collecting meaningful data on alopecia areata. J. Am. Acad. Dermatol. 2018, 79, 470–478. [Google Scholar] [CrossRef]
- Kibar, M.; Aktan, S.; Bilgin, M. Dermoscopic findings in scalp psoriasis and seborrheic dermatitis; two new signs; signet ring vessel and hidden hair. Indian J. Dermatol. 2015, 60, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-W.; Kang, T.; Lee, J.S.; Kang, M.J.; Huh, C.-H.; Kim, M.-S.; Kim, H.J.; Ahn, H.S. Time-Dependent Risk of Acute Myocardial Infarction in Patients With Alopecia Areata in Korea. JAMA Dermatol. 2020, 156, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Lin, H.C.; Kao, S.; Tsai, M.C.; Chung, S.D. Alopecia Areata Increases the Risk of Stroke: A 3-year Follow-Up Study. Sci. Rep. 2015, 5, 11718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Kim, Y.C.; Choi, J.W. Alopecia areata is not a risk factor for heart diseases: A 10-year retrospective cohort study. PLoS ONE 2021, 16, e0250216. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.P.; Joyce, C.J.; Topaz, M.; Guo, Y.; Mostaghimi, A. Cardiovascular risk in patients with alopecia areata (AA): A propensity-matched retrospective analysis. J. Am. Acad. Dermatol. 2016, 75, 151–154. [Google Scholar] [CrossRef]
- Wang, E.H.; Santos, L.; Li, X.Y.; Tran, A.; Kim, S.S.Y.; Woo, K.; Shapiro, J.; McElwee, K.J. Alopecia Areata is Associated with Increased Expression of Heart Disease Biomarker Cardiac Troponin I. Acta Derm. Venereol. 2018, 98, 776–782. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Cai, Y.; Liu, L.; Wu, Y.; Xiong, X. Crucial biological functions of CCL7 in cancer. PeerJ 2018, 6, e4928. [Google Scholar] [CrossRef]
- Maddaluno, M.; Di Lauro, M.; Di Pascale, A.; Santamaria, R.; Guglielmotti, A.; Grassia, G.; Ialenti, A. Monocyte chemotactic protein-3 induces human coronary smooth muscle cell proliferation. Atherosclerosis 2011, 217, 113–119. [Google Scholar] [CrossRef]
- Bennett, M.R.; Sinha, S.; Owens, G.K. Vascular Smooth Muscle Cells in Atherosclerosis. Circ. Res. 2016, 118, 692–702. [Google Scholar] [CrossRef]
- Zouggari, Y.; Ait-Oufella, H.; Bonnin, P.; Simon, T.; Sage, A.P.; Guérin, C.; Vilar, J.; Caligiuri, G.; Tsiantoulas, D.; Laurans, L.; et al. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat. Med. 2013, 19, 1273–1280. [Google Scholar] [CrossRef]
- Wang, J.; Wei, L.; Yang, X.; Zhong, J. Roles of Growth Differentiation Factor 15 in Atherosclerosis and Coronary Artery Disease. J. Am. Heart Assoc. 2019, 8, e012826. [Google Scholar] [CrossRef] [PubMed]
- Tzikas, S.; Palapies, L.; Bakogiannis, C.; Zeller, T.; Sinning, C.; Baldus, S.; Bickel, C.; Vassilikos, V.; Lackner, K.J.; Zeiher, A.; et al. GDF-15 predicts cardiovascular events in acute chest pain patients. PLoS ONE 2017, 12, e0182314. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Patients with Alopecia Areata (n = 60) | Healthy Controls (n = 20) | Statistical Significance |
|---|---|---|---|
| Cardiovascular and Atherosclerosis Markers | |||
| MPO (ng/mL), median (IQR) | 91.27 (68.82–130.9) | 78.95 (45.55–129.4) | 0.21 |
| IL1RL1 (pg/mL), median (IQR) | 4628 (3886–5925) | 4666 (3886–5193) | 0.54 |
| GDF15 (pg/mL), median (IQR) | 40.84 (24.3–68.9) | 37.35 (25.51–42.92) | 0.40 |
| CCL4 (pg/mL), median (IQR) | 338. 3 (223.8–527.8) | 285.2 (228.5–432.6) | 0.19 |
| CCL7 (pg/mL), median (IQR) | 31.69 (20.12–48.13) | 28.45 (21.52–36.59) | 0.67 |
| SORT1 (pg/mL), median (IQR) | 297.8 (282.3–314.35) | 312 (296.6–321.4) | 0.09 |
| Demographic and Clinical Data | |||
| Age (years), mean ± SD | 39 ± 15 | 37 ± 10 | 0.44 |
| Sex (women), n (%) | 41 (68%) | 14 (70%) | 0.88 |
| BMI (kg/m2), median (IQR) | 23.88 (21.09–26.88) | 23.68 (19.46–25.37) | 0.25 |
| Smoking (yes), n (%) | 10 (17%) | 4 (20%) | 0.73 |
| Family history (yes), n (%) | 12 (20%) | 3 (15%) | 0.61 |
| Age at the first episode of alopecia (years), mean (range) | 31 (3–70) | NA | |
| Number of episodes of hair loss, n (range) | 3 (1–20) | NA | |
| Duration of the present episode of alopecia (months), mean (range) | 30 (1–300) | NA | |
| SALT, mean (range) | 43 (5–100) | NA | |
| Activity of the disease, n (%) | NA | ||
| Active | 25 (42%) | ||
| Stable | 27 (45%) | ||
| Remitting | 8 (13%) | ||
| Laboratory Parameters | |||
| Glucose (mg/dL), median (IQR) | 88 (81–95.5) | 84.5 (80–90) | 0.26 |
| Cholesterol (mg/dL), median (IQR) | 186 (162–209) | 190.5 (161–218.5) | 0.55 |
| LDL cholesterol (mg/dL), mean ± SD | 120 ± 40 | 119 ± 39 | 0.96 |
| HDL cholesterol (mg/dL), Median (IQR) | 66.5 (55.5–81.5) | 69.5 (57–83) | 0.77 |
| Triglycerides (mg/dL), median (IQR) | 96 (66–143) | 89 (58–139) | 0.32 |
| Parameter | Disease Duration (Months) | Age at Disease Onset (Years) | Number of Hair Loss Episodes (n) | SALT Score |
|---|---|---|---|---|
| MPO (ng/mL), median (IQR) | 0.170 | −0.132 | 0.955 | 0.214 |
| IL1RL1 (pg/mL), median (IQR) | −0.130 | 0.787 | −0.010 | 0.003 |
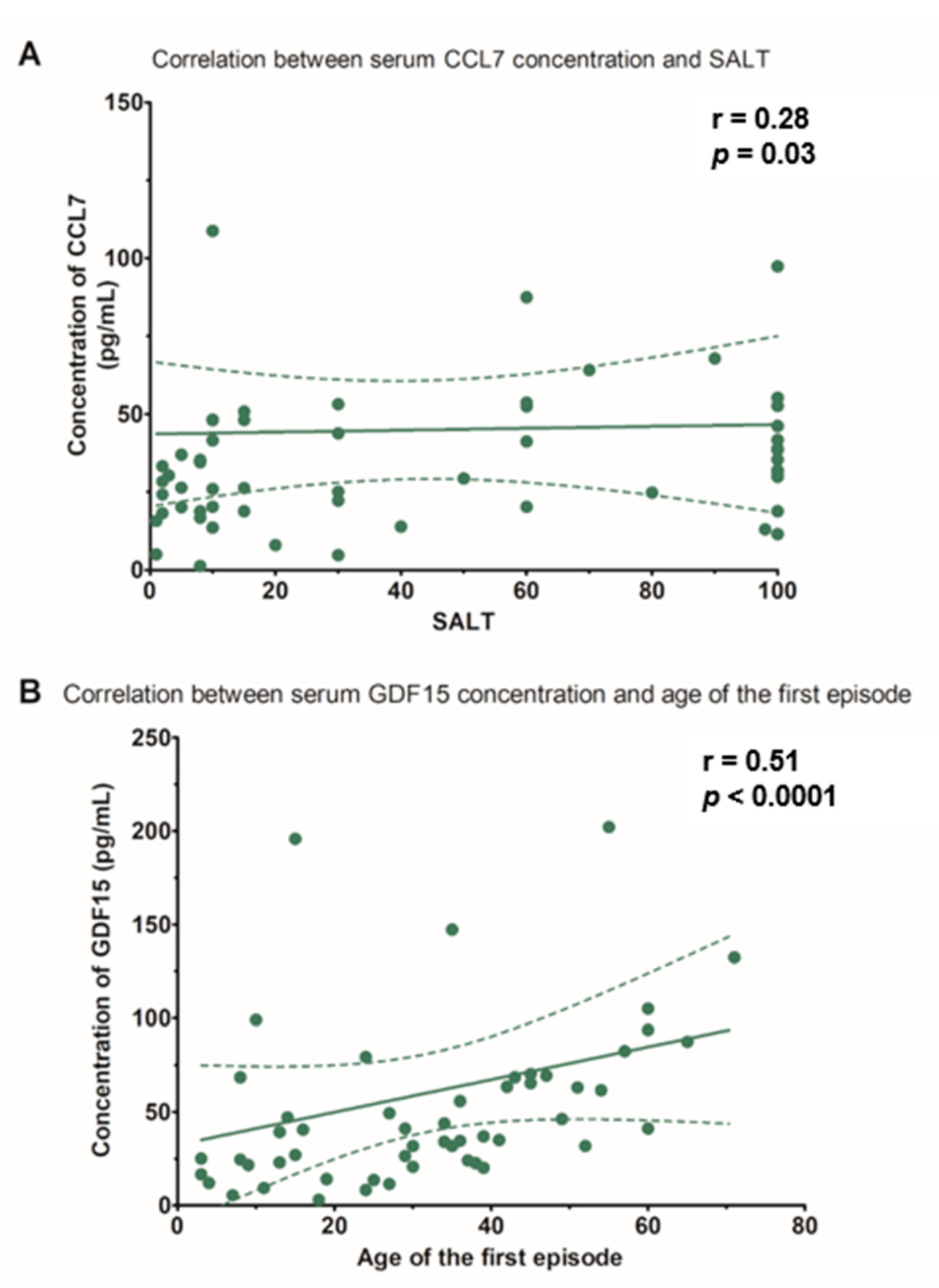
| GDF15 (pg/mL), median (IQR) | −0.147 | 0.509 ** | −0.016 | −0.026 |
| CCL4 (pg/mL), median (IQR) | 0109 | 0.199 | −0.067 | −0.019 |
| CCL7 (pg/mL), median (IQR) | 0.048 | 0.174 | 0.011 | 0.281 * |
| SORT1 (pg/mL), median (IQR) | 0.146 | −0.099 | 0.162 | 0.013 |
| Parameter | Patients with SALT score < 50% (n = 35) | Healthy Controls (n = 20) | Statistical Significance |
|---|---|---|---|
| Cardiovascular and Atherosclerosis Markers | |||
| MPO (ng/mL), median (IQR) | 93.47 (78.77–136.8) | 78.95 (45.55–129.4) | 0.1 |
| IL1RL1 (pg/mL), median (IQR) | 4818 (3726–5341) | 4666 (3886–5193) | 0.75 |
| GDF15 (pg/mL), median (IQR) | 41.52 (21.62–65.28) | 37.35 (25.51–42.92) | 0.52 |
| CCL4 (pg/mL), mean ± SD | 382.49 ± 208.74 | 303.82 ± 176.08 | 0.17 |
| CCL7 (pg/mL), median (IQR) | 26.26 (18.20–43.82) | 28.45 (21.52–36.59) | 0.64 |
| SORT1 (pg/mL), median (IQR) | 297.8 (287.1–313.15) | 312 (296.6–321.4) | 0.15 |
| Demographic and Clinical Data | |||
| Age (years), mean ± SD | 40 ± 15 | 37 ± 10 | 0.41 |
| Sex (women), n (%) | 22 (63%) | 14 (70%) | 0.59 |
| BMI (kg/m2), median (IQR) | 24.81 (21.77–27.77) | 21.29 (19.46–25.37) | 0.06 |
| Smoking (yes), n (%) | 6 (17%) | 4 (20%) | 0.79 |
| Family history (yes), n (%) | 8 (23%) | 3 (15%) | 0.48 |
| Laboratory Parameters | |||
| Glucose (mg/dL), median (IQR) | 92 (83–101) | 84.5 (80–90) | <0.05 |
| Cholesterol (mg/dL), mean ± SD | 191 ± 33 | 192 ± 34 | 0.91 |
| LDL cholesterol (mg/dL), mean ± SD | 124 ± 36 | 119 ± 30 | 0.64 |
| HDL cholesterol (mg/dL), mean ± SD | 64 ± 19 | 69 ± 19 | 0.27 |
| Triglycerides (mg/dL), median (IQR) | 96 (65–151) | 89 (58–139) | 0.94 |
| Parameter | Patients with SALT score ≥ 50% (n = 25) | Healthy Controls (n = 20) | Statistical Significance |
|---|---|---|---|
| Cardiovascular and Atherosclerosis Markers | |||
| MPO (ng/mL), median (IQR) | 82.78 (57.53–123.3) | 78.95 (45.55–129.4) | 0.48 |
| IL1RL1 (pg/mL), median (IQR) | 4513 (3966–6775) | 4666 (3886–5193) | 0.39 |
| GDF15 (pg/mL), median (IQR) | 37.57 (23.57–74.32) | 37.35 (25.51–42.92) | 0.67 |
| CCL4 (pg/mL), mean ± SD | 371.58 ± 181.81 | 303.82 ± 176.08 | 0.22 |
| CCL7 (pg/mL), median (IQR) | 38.91 (29.36–53.67) | 28.45 (21.52–36.59) | 0.11 |
| SORT1 (pg/mL), median (IQR) | 300.15 (281.1–314.35) | 312 (296.6–321.4) | 0.12 |
| Demographic and Clinical Data | |||
| Age (years), mean ± SD | 39 ± 15 | 37 ± 10 | 0.58 |
| Sex (women), n (%) | 19 (76%) | 14 (65%) | 0.65 |
| BMI (kg/m2), median (IQR) | 22.46 (20.70–25.28) | 21.29 (19.46–25.37) | 0.45 |
| Smoking (yes), n (%) | 4 (16%) | 4 (20%) | 0.72 |
| Family history (yes), n (%) | 4 (16%) | 3 (15%) | 0.92 |
| Laboratory Parameters | |||
| Glucose (mg/dL), mean ± SD | 87 ± 7 | 85 ± 9 | 0.55 |
| Cholesterol (mg/dL), mean ± SD | 189 ± 48 | 192 ± 34 | 0.78 |
| LDL cholesterol (mg/dL), mean ± SD | 113 ± 39 | 119 ± 30 | 0.57 |
| HDL cholesterol (mg/dL), mean ± SD | 70 ± 22 | 69 ± 19 | 0.97 |
| Triglycerides (mg/dL), median (IQR) | 95 (68–140) | 89 (58–139) | 0.34 |
| Parameter | Progressive * (n = 25) | Stable ** (n = 27) | Remitting *** (n = 8) | Statistical Significance |
|---|---|---|---|---|
| Atherosclerosis and Cardiovascular Risk Markers | ||||
| MPO (ng/mL), median (IQR) | 85.21 (65.74–112.7) | 91.69 (73.53–137.10) | 101.62 (65.85–136.20) | 0.77 |
| IL1RL1 (pg/mL), median (IQR) | 4969 (3966–5997) | 4474.5 (3966–5193) | 3645 (3400–7466) | 0.66 |
| GDF15 (pg/mL), median (IQR) | 47.08 (26.48–69.36) | 31.71 (20.14–63.48) | 61.64 (24.54–82.44) | 0.3 |
| CCL4 (pg/mL), mean ± SD | 360.46 ± 210.75 | 388.23 ± 199.43 | 394.48 ± 157.15 | 0.86 |
| CCL7 (pg/mL), median (IQR) | 29.64 (18.84–45.92) | 31.96 (18.84–48.25) | 34.5 (28.39–50.79) | 0.61 |
| SORT1 (pg/mL), median (IQR) | 302.5 (285.9–316.7) | 297.8 (281.1–312) | 293 (276.3–319) | 0.58 |
| Demographic and Clinical Data | ||||
| Age (years), mean ± SD | 37 ± 16 | 43 ± 15 | 36 ± 9 | 0.28 |
| Sex (women), n (%) | 13 (52%) | 22 (81%) | 6 (75%) | 0.06 |
| BMI (kg/m2), median (IQR) | 24.37 (21.96–27.68) | 23.73 (20.70–29.76) | 21.77 (20.95–24.63) | 0.30 |
| Smoking (yes), n (%) | 5 (20%) | 4 (15%) | 1 (13%) | 0.83 |
| Family history (yes), n (%) | 6 (24%) | 5 (19%) | 1 (13%) | 0.75 |
| Laboratory Data | ||||
| Glucose (mg/dL), median (IQR) | 92 (85–101) | 84 (79–94) | 86.5 (85–102) | 0.04 |
| Cholesterol, mean ± SD | 186 ± 29 | 192 ± 52 | 196 ± 22 | 0.81 |
| LDL cholesterol (mg/dL), mean ± SD | 122 ± 30 | 119 ± 46 | 116 ± 28 | 0.92 |
| HDL cholesterol (mg/dL), median (IQR) | 60 (49–73) | 63 (51–81) | 80 (76–84.5) | 0.04 |
| Triglycerides (mg/dL), median (IQR) | 97 (68–151) | 97 (65–143) | 71.5 (57–138) | 0.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waśkiel-Burnat, A.; Niemczyk, A.; Blicharz, L.; Chmielińska, P.; Zaremba, M.; Gąsecka, A.; Filipiak, K.J.; Olszewska, M.; Rudnicka, L. Chemokine C-C Motif Ligand 7 (CCL7), a Biomarker of Atherosclerosis, Is Associated with the Severity of Alopecia Areata: A Preliminary Study. J. Clin. Med. 2021, 10, 5418. https://doi.org/10.3390/jcm10225418
Waśkiel-Burnat A, Niemczyk A, Blicharz L, Chmielińska P, Zaremba M, Gąsecka A, Filipiak KJ, Olszewska M, Rudnicka L. Chemokine C-C Motif Ligand 7 (CCL7), a Biomarker of Atherosclerosis, Is Associated with the Severity of Alopecia Areata: A Preliminary Study. Journal of Clinical Medicine. 2021; 10(22):5418. https://doi.org/10.3390/jcm10225418
Chicago/Turabian StyleWaśkiel-Burnat, Anna, Anna Niemczyk, Leszek Blicharz, Paulina Chmielińska, Michał Zaremba, Aleksandra Gąsecka, Krzysztof J. Filipiak, Małgorzata Olszewska, and Lidia Rudnicka. 2021. "Chemokine C-C Motif Ligand 7 (CCL7), a Biomarker of Atherosclerosis, Is Associated with the Severity of Alopecia Areata: A Preliminary Study" Journal of Clinical Medicine 10, no. 22: 5418. https://doi.org/10.3390/jcm10225418
APA StyleWaśkiel-Burnat, A., Niemczyk, A., Blicharz, L., Chmielińska, P., Zaremba, M., Gąsecka, A., Filipiak, K. J., Olszewska, M., & Rudnicka, L. (2021). Chemokine C-C Motif Ligand 7 (CCL7), a Biomarker of Atherosclerosis, Is Associated with the Severity of Alopecia Areata: A Preliminary Study. Journal of Clinical Medicine, 10(22), 5418. https://doi.org/10.3390/jcm10225418










