Practice Guidelines of the Central European Hepatologic Collaboration (CEHC) on the Use of Thrombopoietin Receptor Agonists in Patients with Chronic Liver Disease Undergoing Invasive Procedures
Abstract
:1. Introduction
2. Summary of Clinical Evidence for Thrombopoietin Receptor Agonists (TPO-RAs)
3. Methods
4. Results
4.1. General Considerations and Comments
4.2. Contraindications for the Use of TPO-RAs
5. Discussion
6. CEHC Recommendations for Using TPO-RA Therapy Prior to Scheduled Invasive Procedures
6.1. Recommendations for Use of TPO-RAs
6.2. Recommendations for Platelet Count Threshold
7. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Marcellin, P.; Kutala, B.K. Liver diseases: A major, neglected global public health problem requiring urgent actions and large-scale screening. Liver Int. 2018, 38 (Suppl. 1), 2–6. [Google Scholar] [CrossRef] [Green Version]
- Brown, C.L.; Hammill, B.G.; Qualsls, L.G.; Curtis, L.H.; Muir, A.J. Significant morbidity and mortality among hospitalized end-stage liver disease patients in Medicare. J. Pain Symptom Manag. 2016, 52, 412–419.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortesi, P.A.; Conti, S.; Scalone, L.; Jaffe, A.; Ciaccio, A.; Okoslicsanyi, S.; Rota, M.; Fabris, L.; Colledan, M.; Fagiuoli, S.; et al. Health related quality of life in chronic liver diseases. Liver Int. 2020, 40, 2630–2642. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, O.; Feldman, D.M.; Diakow, M.; Sigal, S.H. The pathophysiology of thrombocytopenia in chronic liver disease. Hepat. Med. 2016, 8, 39–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scharf, R.E. Thrombocytopenia and hemostatic changes in acute and chronic liver disease: Pathophysiology, clinical and laboratory features, and management. J. Clin. Med. 2021, 10, 1530. [Google Scholar] [CrossRef]
- Abdela, J. Current advance in thrombopoietin Rreceptor agonists in the management of thrombocytopenia associated with chronic liver disease: Focus on avatrombopag. Clin. Med. Insights Blood Disord. 2019, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.H. Thrombocytopenia in cirrhosis: A review of pathophysiology and management options. Clin. Liver Dis. 2019, 14, 183–186. [Google Scholar] [CrossRef] [Green Version]
- Afdhal, N.; McHutchison, J.; Brown, R.; Jacobson, I.; Manns, M.; Poordad, F.; Weksler, B.; Esteban, R. Thrombocytopenia associated with chronic liver disease. J. Hepatol. 2008, 48, 1000–1007. [Google Scholar] [CrossRef] [Green Version]
- Peck-Radosavljevic, M.; Wichlas, M.; Zacherl, J.; Stiegler, G.; Stohlawetz, P.; Fuchsjäger, M.; Kreil, A.; Metz-Schimmerl, S.; Panzer, S.; Steininger, R.; et al. Thrombopoietin induces rapid resolution of thrombocytopenia after orthotopic liver transplantation through increased platelet production. Blood 2000, 95, 795–801. [Google Scholar] [CrossRef]
- Giannini, E.G.; Savarino, V. Thrombocytopenia in liver disease. Curr. Opin. Hematol. 2008, 15, 473–480. [Google Scholar] [CrossRef]
- Bashour, F.N.; Teran, C.J.; Mullen, K.D. Prevalence of peripheral blood cytopenias (hypersplenism) in patients with nonalcoholic chronic liver disease. Am. J. Gastroenterol. 2000, 95, 2936–2939. [Google Scholar] [CrossRef]
- Qureshi, K.; Bonder, A. Thrombopoietin-receptor agonists in perioperative treatment of patients with chronic liver disease. World J. Meta-Anal. 2020, 8, 220–232. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). Technology Appraisal Guidance TA626 2020: Avatrombopag for Treating Thrombocytopenia in People with Chronic Liver Disease Needing a Planned Invasive Procedure. Available online: https://www.nice.org.uk/guidance/ta626/resources/avatrombopag-for-treating-thrombocytopenia-in-people-with-chronic-liver-disease-needing-a-planned-invasive-procedure-pdf-82609020615877 (accessed on 8 June 2020).
- Brown, R.S. Current Management of Thrombocytopenia in Chronic Liver Disease. Gastroenterol. Hepatol. 2019, 15, 155–157. [Google Scholar]
- European Medicines Agency (EMA). Doptelet—Product Information 2021. Available online: https://www.ema.europa.eu/en/documents/product-information/doptelet-epar-product-information_en.pdf (accessed on 8 June 2021).
- European Medicines Agency (EMA). Mulpleo—Product Information 2021. Available online: https://www.ema.europa.eu/en/documents/product-information/mulpleo-epar-product-information_en.pdf (accessed on 8 June 2021).
- Dieterich, D.T.; Bernstein, D.; Flamm, S.; Pockros, P.J.; Reau, N. Review article: A treatment algorithm for patients with chronic liver disease and severe thrombocytopenia undergoing elective medical procedures in the United States. Aliment. Pharmacol. Ther. 2020, 52, 1311–1322. [Google Scholar] [CrossRef]
- Terrault, N.; Chen, Y.C.; Izumi, N.; Kayali, Z.; Mitrut, P.; Tak, W.Y.; Allen, L.F.; Hassanein, T. Avatrombopag before procedures reduces need for platelet transfusion in patients with chronic liver disease and thrombocytopenia. Gastroenterology 2018, 155, 705–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poordad, F.; Terrault, N.A.; Alkhouri, N.; Tian, W.; Allen, L.F.; Rabinovitz, M. Avatrombopag, an alternate treatment option to reduce platelet transfusions in patients with thrombocytopenia and chronic liver disease-Integrated analyses of 2 phase 3 studies. Int. J. Hepatol. 2020, 2020, 5421632–5421643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hidaka, H.; Kurosaki, M.; Tanaka, H.; Kudo, M.; Abiru, S.; Igura, T.; Ishikawa, T.; Seike, M.; Katsube, T.; Ochiai, T.; et al. Lusutrombopag Reduces Need for Platelet Transfusion in Patients with Thrombocytopenia Undergoing Invasive Procedures. Clin. Gastroenterol. Hepatol. 2019, 17, 1192–1200. [Google Scholar] [CrossRef] [Green Version]
- Peck-Radosavljevic, M.; Simon, K.; Iacobellis, A.; Hassanein, T.; Kayali, Z.; Tran, A.; Makara, M.; Ben Ari, Z.; Braun, M.; Mitrut, P.; et al. Lusutrombopag for the treatment of thrombocytopenia in patients with chronic liver disease undergoing invasive procedures (L-PLUS 2). Hepatology 2019, 70, 1336–1348. [Google Scholar] [CrossRef] [Green Version]
- Alvaro, D.; Caporaso, N.; Giannini, E.G.; Iacobellis, A.; Morelli, M.; Toniutto, P.; Violi, F. Procedure-related bleeding risk in patients with cirrhosis and severe thrombocytopenia. Eur. J. Clin. Investig. 2021, 51, e13508–e13522. [Google Scholar] [CrossRef]
- Committee for Medicinal Products for Human Use (CHMP). Assessment Report—Lusutrombopag Shionogi (EMA/CHMP/817852/2018) 2018. Available online: https://www.ema.europa.eu/en/documents/assessment-report/lusutrombopag-shionogi-epar-public-assessment-report_en.pdf (accessed on 8 June 2018).
- Neuberger, J.; Patel, J.; Caldwell, H.; Davies, S.; Hebditch, V.; Hollywood, C.; Hubscher, S.; Karkhanis, S.; Lester, W.; Roslund, N.; et al. Guidelines on the use of liver biopsy in clinical practice from the British Society of Gastroenterology, the Royal College of Radiologists and the Royal College of Pathology. Gut 2020, 69, 1382–1403. [Google Scholar] [CrossRef]
- Gangireddy, V.G.; Kanneganti, P.C.; Sridhar, S.; Talla, S.; Coleman, T. Management of thrombocytopenia in advanced liver disease. Can. J. Gastroenterol. Hepatol. 2014, 28, 558–564. [Google Scholar] [CrossRef] [Green Version]
- Hasson, F.; Keeney, S.; McKenna, H. Research guidelines for the Delphi survey technique. J. Adv. Nurs. 2000, 32, 1008–1015. [Google Scholar]
- Stanworth, S.J.; New, H.V.; Apelseth, T.O.; Brunskill, S.; Cardigan, R.; Doree, C.; Germain, M.; Goldman, M.; Massey, E.; Prati, D.; et al. Effects of the COVID-19 pandemic on supply and use of blood for transfusion. Lancet Haematol. 2020, 7, e756–e764. [Google Scholar] [CrossRef]
- Lim, M.Y.; Gilreath, J.A. Periprocedural use of avatrombopag for neurosurgical interventions: A strategy to avoid platelet utilization. Blood Adv. 2020, 4, 4438–4441. [Google Scholar] [CrossRef] [PubMed]
- Pavord, S.; Thachil, J.; Hunt, B.J.; Murphy, M.; Lowe, G.; Laffan, M.; Makris, M.; Newland, A.C.; Provan, D.; Grainger, J.D.; et al. Practical guidance for the management of adults with immune thrombocytopenia during the COVID-19 pandemic. Br. J. Haematol. 2020, 189, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Poordad, F.; Theodore, D.; Sullivan, J.; Grotzinger, K. Evaluating medical resource utilization and costs associated with thrombocytopenia in chronic liver disease patients. J. Med. Econ. 2012, 15, 112–124. [Google Scholar] [CrossRef]
- Bleibel, W.; Caldwell, S.H.; Curry, M.P.; Northup, P.G. Peripheral platelet count correlates with liver atrophy and predicts long-term mortality on the liver transplant waiting list. Transpl. Int. 2013, 26, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.S., Jr. Review article: A pharmacoeconomic analysis of thrombocytopenia in chronic liver disease. Aliment. Pharmacol. Ther. 2007, 26, 41–48. [Google Scholar] [CrossRef]
- Lisman, T.; Porte, R.J. Pathogenesis, prevention, and management of bleeding and thrombosis in patients with liver diseases. Res. Pract. Thromb. Haemost. 2017, 1, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Stravitz, R.T.; Ellerbe, C.; Durkalski, V.; Schilsky, M.; Fontana, R.J.; Peterseim, C.; Lee, W.M.; The Acute Liver Failure Study Group. Bleeding complications in acute liver failure. Hepatology 2018, 67, 1931–1942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inabnet, W.B.; Deziel, D.J. Laparoscopic liver biopsy in patients with coagulopathy, portal hypertension, and ascites. Am. Surg. 1995, 61, 603–606. [Google Scholar] [PubMed]
- Poordad, F. Review article: Thrombocytopenia in chronic liver disease. Aliment. Pharmacol. Ther. 2007, 26, 5–11. [Google Scholar] [CrossRef] [PubMed]
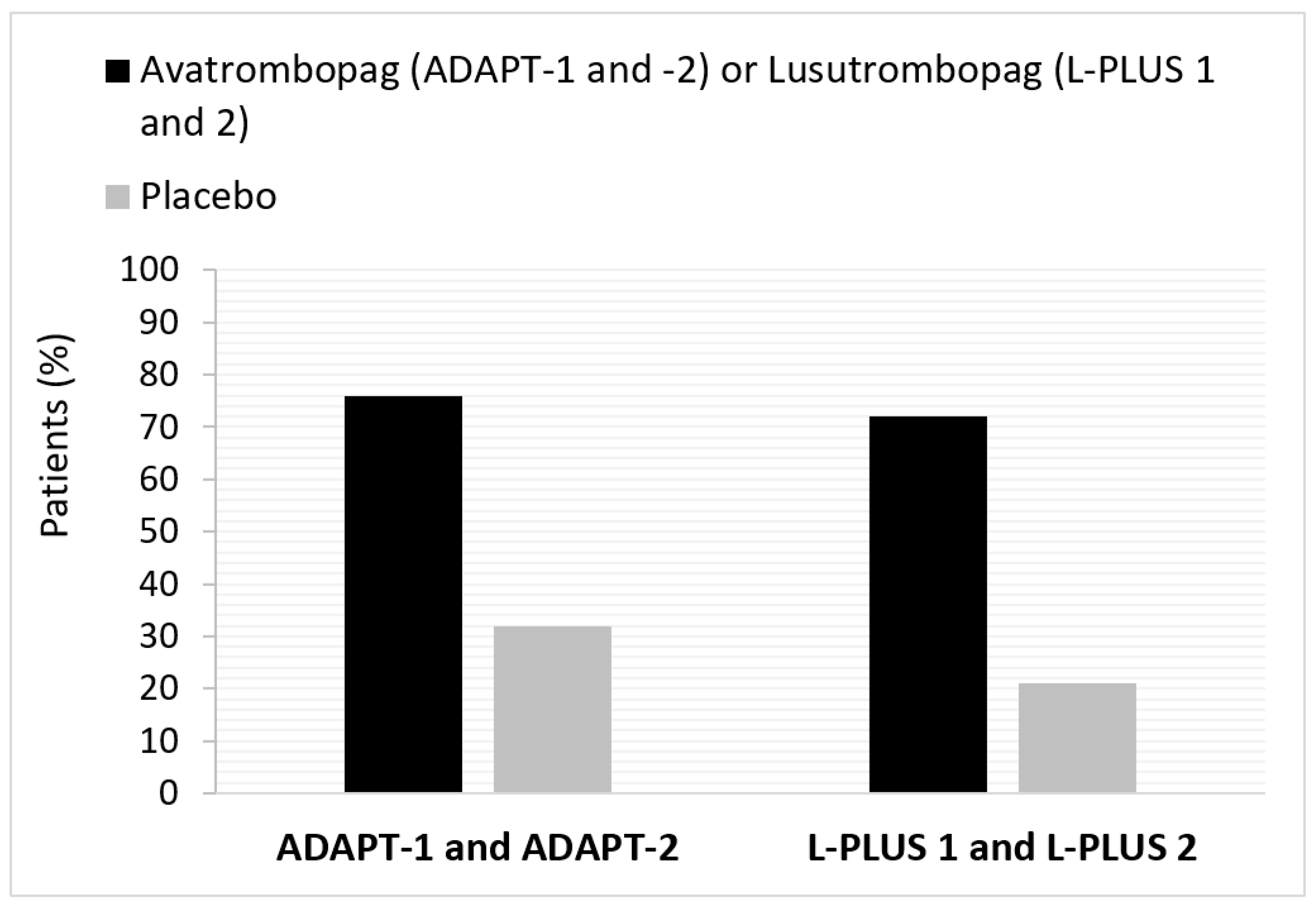
| Study (Publication Year) | Participants | Gender | Age (Years) | Interventions | Mean Baseline Platelet Count ×109/L (Mean ± S.D.) | Primary Efficacy Outcome Measure | Summary of Key Efficacy Results | Ref. |
|---|---|---|---|---|---|---|---|---|
| Avatrombopag—Phase 3 trials: | ||||||||
| ADAPT-1 (2018) | N = 231 | M: 68.4% F: 31.6% | 56.35 ± 9.52 56.22 ± 1.05 | avatrombopag vs. placebo treatment for 5 days | 36.15 ± 8.58 36.80 ± 8.96 | % patients who did not require a platelet transfusion or rescue procedure for bleeding following randomization and up to 7 days after a scheduled procedure | Overall (N = 435): Responders 75.8% avatrombobag vs. 31.7% placebo (treatment difference * Δ44.2; 95% CI: 35.3, 53.0; p < 0.0001) | Terrault et al., 2018; Poordad et al., 2020 [18,19] |
| Low baseline platelet count subgroup (<40 × 109/L; n = 251): Responders 66.9% avatrombobag vs. 28.6% placebo (treatment difference * Δ38.3; 95% CI: 26.5, 50.1; p < 0.0001) | ||||||||
| ADAPT-2 (2020) | N = 204 | M: 62.3% F: 37.7% | 58.28 ± 2.84 58.13 ± 1.25 | avatrombopag vs. placebo treatment for 5 days | 37.98 ± 7.14 38.21 ± 7.74 | High baseline platelet count subgroup (≥40 to <50 × 109/L; n = 184): responders 88.0% avatrombobag vs. 35.8% placebo (treatment difference * Δ52.2; 95% CI: 39.3, 65.1) | ||
| Lusutrombopag—Phase 3 trials: | ||||||||
| L-PLUS 1 (JapicCTI-132323; 2019) | N = 97 (49 lusutrombopag; 48 placebo) | M: 53.1% F: 46.9% | 67.8 ± 8.60 | lusutrombopag vs. placebo treatment for up to 7 days | 40.4 ± 6.60 (17.7% <35 × 109/L; 53.1% ≥35 to <45 × 109/L; 29.2% >45 × 109/L) | % patients who did not require a platelet transfusion before the primary invasive procedure | Overall (N = 97): Responders 79.2% lusutrombopag vs. 12.5% placebo (treatment difference Δ66.7; p < 0.0001) | Hidaka et al., 2019 [20] |
| L-PLUS 2 (2019) | N = 215 (108 lusutrombopag; 107 placebo) | M: 62.3% F: 37.7% | 51.8 ± 11.3 | lusutrombopag vs. placebo treatment for up to 7 days | 37.55 (34.4% <35 × 109/L; 64.7% ≥35 × 109/L) | % patients who did not require a platelet transfusion or rescue procedure for bleeding following randomization and up to 7 days after a scheduled procedure | Overall (N = 215): Responders 64.8% lusutrombopag vs. 29.0% placebo (treatment difference Δ36.7%; 95% CI: 24.9, 48.5; p < 0.0001) | Peck-Radosavljevic et al., 2019 [21] |
| Low baseline platelet count subgroup (<35 × 109/L; n = 74): responders 41.7% lusutrombopag vs. 18.4% placebo (treatment difference Δ23.3) | ||||||||
| High baseline platelet count subgroup (≥35 × 109/L; n = 139): responders 77.5% lusutrombopag vs. 33.8% placebo (treatment difference Δ43.7) | ||||||||
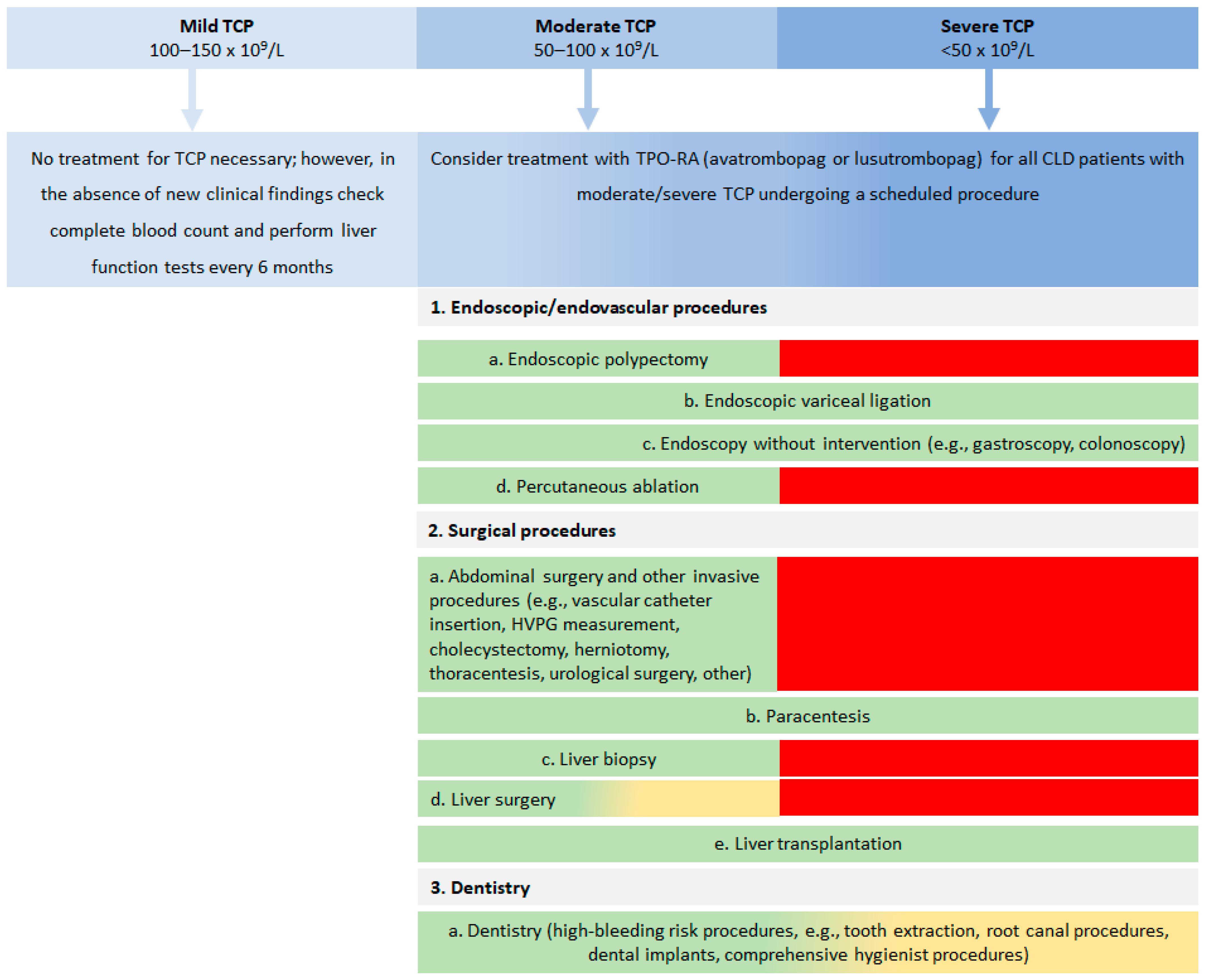
| Procedure | Benchmark * | Minimum Platelet Count for Procedure n (%) | Is TPO-RA Suitable for Platelet Count Elevation? n (%) | Additional Comments and Considerations | ||
|---|---|---|---|---|---|---|
| >30 × 109/L | >50 × 109/L | >80 × 109/L | ||||
| 1. Endoscopic/endovascular procedures: | ||||||
| a. Endoscopic polypectomy | Bleeding risk ~7.5% for patients with platelet count < 50 × 109/L (retrospective data); Immediate post-procedural bleeding rate was 27.5% with RR = 6 | NR | 9 (100.0%) | Yes: 8 (88.9%) No: 1 (11.1%) | ||
| b. Endoscopic variceal ligation | Bleeding risk ~2.75−7.33%; No association between bleeding risk and platelet count | 7 (77.8%) | 2 (22.2%) | Yes: 8 (88.9%) No/NA: 1 (11.1%) | TPO-RA can be used for urgent procedures regardless of platelet count; For elective ligation, TPO-RA is recommended when platelet count is <50 × 109/L; In acute variceal bleeding, ligation may be performed at any platelet count, i.e., as secondary prophylaxis when platelet count is >30 × 109/L | |
| c. Endoscopy without intervention (e.g., gastroscopy, colonoscopy) | No data was provided in the article; Advisory Board discussed the low risk of bleeding | 9 (100%) | Yes: 4 (44.4%) No/NA: 5 (55.6%) | Not performed in patients with spontaneous bleeding; May be performed at any platelet count | ||
| d. Percutaneous ablation | Rarely performed in patients with platelet count < 50 × 109/L and is usually preceded by platelet transfusions and close monitoring of platelet count; Bleeding risk following radio-frequency ablation of HCC is <1 | NR | 9 (100.0%) | Yes: 97 (100.0%) No: 0 (0.0%) | ||
| 2. Surgical procedures: | ||||||
| a. Abdominal surgery and other invasive procedures ** | Available evidence insufficient to assess association between platelet count and post-procedural bleeding risk | NR | 8 (88.9%) | 1 (11.1%) | Yes: 9 (100.0%) No: 0 (0.0%) | |
| b. Paracentesis | Typically performed in cirrhotic patients with significant portal hypertension and TCP; No bleeding was recorded in patients with platelet count < 50 × 109/L | 9 (100.0%) | Yes: 5 (55.6%) No/NA: 4 (44.4%) | In patients with severe dyspnoea due to large ascites, evacuatory paracentesis is recommended even at lower platelet counts; Paracentesis may be performed at any platelet count; can be safe even if platelet count is <30 × 109/L but can be associated with bleeding in rare situations | ||
| c. Liver biopsy | Bleeding risk ~0.6%; Usually performed in patients without portal hypertension and platelet count > 50 × 109/L | NR | 8 (88.9%) | 1 (11.1%) | Yes: 9 (100%) No: 0 (0%) | For percutaneous liver biopsy; Except for patients with portal hypertension when platelet count should be >80 × 109/L; In the last few years, liver biopsy has become less popular and Central European physicians are more cautious |
| d. Liver surgery | Portal hypertension is the main determinant of outcome; Even mild TCP (platelet count < 150 × 109/L) predicted major postoperative complications and mortality after resection of HCC | NR | 1 (11.1%) | 8 (88.9%) | Yes: 9 (100.0%) No: 0 (0.0%) | |
| e. Liver transplantation | No association between platelet count and intra- or post-transplantation bleeding | 7 (77.8%) | 1 (11.1%) | 1 (11.1%) | Yes: 7 (77.8%) No: 2 (22.2%) | May be performed at any platelet count; Usually not a planned procedure |
| 3. Dentistry: | ||||||
| a. Dentistry (high-bleeding risk procedures) ** | Bleeding risk seemed to be inherently related to the procedure or the number of teeth extracted rather than to platelet count; Bleeding risk ~2.9% for a patient with platelet count = 50 × 109/L and INR =2.5 (prospective study data) | 1 (11.1%) | 8 (88.9%) | Yes: 9 (100.0%) No: 0 (0.0%) | Local therapy is generally preferred; Patient and procedure dependent; There is currently no uniformity between dentists; Many Central European dentists request platelet transfusions for platelet count < 80 × 109/L; TPO-RAs should always be considered for patients with Child Pugh score C | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flisiak, R.; Antonov, K.; Drastich, P.; Jarcuska, P.; Maevskaya, M.; Makara, M.; Puljiz, Ž.; Štabuc, B.; Trifan, A. Practice Guidelines of the Central European Hepatologic Collaboration (CEHC) on the Use of Thrombopoietin Receptor Agonists in Patients with Chronic Liver Disease Undergoing Invasive Procedures. J. Clin. Med. 2021, 10, 5419. https://doi.org/10.3390/jcm10225419
Flisiak R, Antonov K, Drastich P, Jarcuska P, Maevskaya M, Makara M, Puljiz Ž, Štabuc B, Trifan A. Practice Guidelines of the Central European Hepatologic Collaboration (CEHC) on the Use of Thrombopoietin Receptor Agonists in Patients with Chronic Liver Disease Undergoing Invasive Procedures. Journal of Clinical Medicine. 2021; 10(22):5419. https://doi.org/10.3390/jcm10225419
Chicago/Turabian StyleFlisiak, Robert, Krasimir Antonov, Pavel Drastich, Peter Jarcuska, Marina Maevskaya, Mihály Makara, Željko Puljiz, Borut Štabuc, and Anca Trifan. 2021. "Practice Guidelines of the Central European Hepatologic Collaboration (CEHC) on the Use of Thrombopoietin Receptor Agonists in Patients with Chronic Liver Disease Undergoing Invasive Procedures" Journal of Clinical Medicine 10, no. 22: 5419. https://doi.org/10.3390/jcm10225419
APA StyleFlisiak, R., Antonov, K., Drastich, P., Jarcuska, P., Maevskaya, M., Makara, M., Puljiz, Ž., Štabuc, B., & Trifan, A. (2021). Practice Guidelines of the Central European Hepatologic Collaboration (CEHC) on the Use of Thrombopoietin Receptor Agonists in Patients with Chronic Liver Disease Undergoing Invasive Procedures. Journal of Clinical Medicine, 10(22), 5419. https://doi.org/10.3390/jcm10225419








