Therapeutic and Reconstructive Management Options in Scleroderma (Morphea) en Coup de Sabre in Children and Adults. A Systematic Literature Review
Abstract
1. Introduction
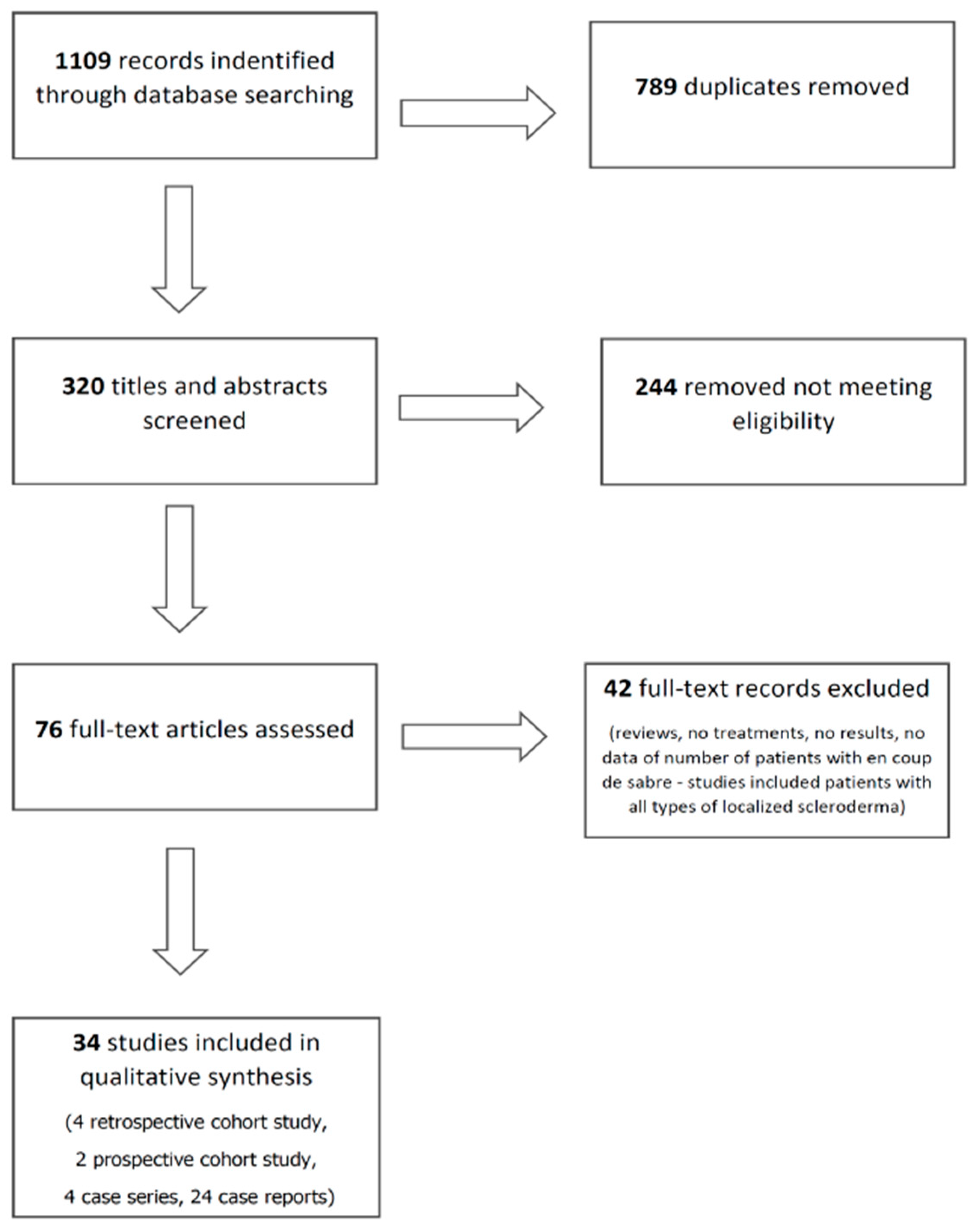
2. Methods
3. Results
3.1. Pharmacological Treatments
3.1.1. Methotrexate
3.1.2. Systemic Glucocorticosteroids
3.1.3. Cyclosporine
3.1.4. Mycophenolate Mofetil
3.1.5. Hydroxychloroquine
3.1.6. Abatacept
3.1.7. Tocilizumab
3.1.8. Interferon Gamma
3.1.9. UVA1-Therapy
3.1.10. PUVA-Therapy
3.1.11. NB-UVB Therapy
3.1.12. Pulsed Dye Laser (595 nm)
3.2. Reconstructive Treatments
3.2.1. Fat Grafting
3.2.2. Hyaluronic Acid Filler
3.2.3. Polymethylmethacrylate
3.2.4. Tissue Cocktail Injection
3.2.5. Toxinum Botulinum
3.2.6. Surgical Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LScs | localized scleroderma/morphea en coup de sabre |
| PFH | progressive facial hemiatrophy |
| MTX | methotrexate |
| MRI | Magnetic Resonance Imaging |
| IFN-ϒ | Interferon gamma |
| TGFb | transforming growth factor beta |
| NB-UVB | narrow-band ultraviolet B |
References
- Tollefson, M.M.; Witman, P.M. En coup de sabre morphea and Parry-Romberg syndrome: A retrospective review of 54 patients. J. Am. Acad. Dermatol. 2007, 56, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Rattanakaemakorn, P.; Jorizzo, J.L. The efficacy of methotrexate in the treatment of en coup de sabre (linear morphea subtype). J. Dermatolog Treat. 2018, 29, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Khamaganova, I. Progressive Hemifacial Atrophy and Linear Scleroderma en coup de sabre: A Spectrum of the Same Disease? Front. Med. 2017, 4, 258. [Google Scholar] [CrossRef]
- Mears, K.A.; Servat, J.J.; Black, E.H. Linear scleroderma en coup de sabre affecting the upper eyelid and lashes. Graefes Arch. Clin. Exp. Ophthalmol. 2012, 250, 1097–1099. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, A.; Mitrakos, G.; Hofmann, S.C.; Lehmann, P.; Sticherling, M.; Krieg, T.; Lahner, N.; Tigges, C.; Hunzelmann, N.; Moinzadeh, P. Localized Scleroderma of the Head and Face Area: A Retrospective Cross-sectional Study of 96 Patients from 5 German Tertiary Referral Centres. Acta Derm. Venereol. 2018, 98, 603–605. [Google Scholar] [CrossRef]
- Fain, E.T.; Mannion, M.; Pope, E.; Young, D.W.; Laxer, R.M.; Cron, R.Q. Brain cavernomas associated with en coup de sabre linear scleroderma: Two case reports. Pediatr. Rheumatol. Online J. 2011, 9, 18. [Google Scholar] [CrossRef]
- Allmendinger, A.M.; Ricci, J.A.; Desai, N.S.; Viswanadhan, N.; Rodriguez, D. Atypical Neuroimaging Manifestations of Linear Scleroderma “en coup de sabre”. Iran. J. Child. Neurol. 2015, 9, 62–68. [Google Scholar]
- Bielsa Marsol, I. Update on the classification and treatment of localized scleroderma. Actas Dermosifiliogr. 2013, 104, 654–666. [Google Scholar] [CrossRef] [PubMed]
- Pati, D.; Lorusso, L.N. How to Write a Systematic Review of the Literature. Herd 2018, 11, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Van der Veken, D.; De Haes, P.; Hauben, E.; Teughels, W.; Lambrechts, P. A rare cause of gingival recession: Morphea with intra-oral involvement. Oral Surg Oral Med. Oral Pathol. Oral Radiol. 2015, 119, e257–e264. [Google Scholar] [CrossRef]
- Joly, P.; Bamberger, N.; Crickx, B.; Belaich, S. Treatment of severe forms of localized scleroderma with oral corticosteroids: Follow-up study on 17 patients. Arch. Dermatol. 1994, 130, 663–664. [Google Scholar] [CrossRef] [PubMed]
- Unterberger, I.; Trinka, E.; Engelhardt, K.; Muigg, A.; Eller, P.; Wagner, M.; Sepp, N.; Bauer, G. Linear scleroderma “en coup de sabre” coexisting with plaque-morphea: Neuroradiological manifestation and response to corticosteroids. J. Neurol. Neurosurg. Psychiatry 2003, 74, 661–664. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martini, G.; Ramanan, A.V.; Falcini, F.; Girschick, H.; Goldsmith, D.P.; Zulian, F. Successful treatment of severe or methotrexate-resistant juvenile localized scleroderma with mycophenolate mofetil. Rheumatology 2009, 48, 1410–1413. [Google Scholar] [CrossRef] [PubMed]
- Fage, S.W.; Arvesen, K.B.; Olesen, A.B. Abatacept Improves Skin-score and Reduces Lesions in Patients with Localized Scleroderma: A Case Series. Acta Derm. Venereol. 2018, 98, 465–466. [Google Scholar] [CrossRef]
- Obermoser, G.; Pfausler, B.E.; Linder, D.M.; Sepp, N.T. Scleroderma en coup de sabre with central nervous system and ophthalmologic involvement: Treatment of ocular symptoms with interferon gamma. J. Am. Acad. Dermatol. 2003, 49, 543–546. [Google Scholar] [CrossRef]
- Magro, C.M.; Halteh, P.; Olson, L.C.; Kister, I.; Shapiro, L. Linear scleroderma “en coup de sabre” with extensive brain involvement-Clinicopathologic correlations and response to anti-Interleukin-6 therapy. Orphanet J. Rare Dis. 2019, 14, 110. [Google Scholar] [CrossRef]
- Kumar, A.B.; Blixt, E.K.; Drage, L.A.; El-Azhary, R.A.; Wetter, D.A. Treatment of morphea with hydroxychloroquine: A retrospective review of 84 patients at Mayo Clinic, 1996–2013. J. Am. Acad. Dermatol. 2019, 80, 1658–1663. [Google Scholar] [CrossRef]
- Su, O.; Onsun, N.; Onay, H.K.; Erdemoglu, Y.; Ozkaya, D.B.; Cebeci, F.; Somay, A. Effectiveness of medium-dose ultraviolet A1 phototherapy in localized scleroderma. Int. J. Dermatol. 2011, 50, 1006–1013. [Google Scholar] [CrossRef]
- Kowalzick, L.; Suckow, S.; Mörtel, J.; Mischke, D.; Waldmann, T.; Pönnighaus, J.; Suckow, M. Sklerodermie en coup de sabre: Erfolgreiche Therapie mit topischem Calcipotriol und Medium-Dose UVA1-Phototherapie. Aktuelle Dermatol. 2002, 28, 193–195. [Google Scholar] [CrossRef][Green Version]
- Brownell, I.; Soter, N.A.; Franks, A.G., Jr. Familial linear scleroderma (en coup de sabre) responsive to antimalarials and narrowband ultraviolet B therapy. Dermatol. Online J. 2007, 13, 11. [Google Scholar]
- Barin, E.Z.; Cinal, H.; Cakmak, M.A.; Tan, O. Treatment of Linear Scleroderma (en coup de sabre) With Dermal Fat Grafting. J. Cutan. Med. Surg. 2016, 20, 269–271. [Google Scholar] [CrossRef]
- Karaaltin, M.V.; Akpinar, A.C.; Baghaki, S.; Akpinar, F. Treatment of “en coup de sabre” deformity with adipose-derived regenerative cell-enriched fat graft. J. Craniofac. Surg. 2012, 23, e103–e105. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.T.; Sun, H.; Chung, E.H. A surgical approach to linear scleroderma using Medpor and dermal fat graft. Arch. Craniofac. Surg. 2019, 20, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Consorti, G.; Tieghi, R.; Clauser, L.C. Frontal linear scleroderma: Long-term result in volumetric restoration of the fronto-orbital area by structural fat grafting. J. Craniofac. Surg. 2012, 23, e263–e265. [Google Scholar] [CrossRef] [PubMed]
- Robitschek, J.; Wang, D.; Hall, D. Treatment of linear scleroderma “en coup de sabre” with AlloDerm tissue matrix. Otolaryngol. Head Neck Surg. 2008, 138, 540–541. [Google Scholar] [CrossRef]
- Thareja, S.K.; Sadhwani, D.; Alan Fenske, N. En coup de sabre morphea treated with hyaluronic acid filler. Report of a case and review of the literature. Int. J. Dermatol. 2015, 54, 823–826. [Google Scholar] [CrossRef]
- Sivek, R.; Emer, J. Use of a blunt-tipped microcannula for soft tissue filler injection in the treatment of linear scleroderma (en coup de sabre). Dermatol. Surg. 2014, 40, 1439–1441. [Google Scholar] [CrossRef]
- Oh, H.M.; Chung, M.E. Botulinum Toxin for Neuropathic Pain: A Review of the Literature. Toxins 2015, 7, 3127–3154. [Google Scholar] [CrossRef]
- Rimoin, L.; Arbiser, J. Improvement of “en coup de sabre” Morphea and Associated Headaches with Botulinum Toxin Injections. Dermatol. Surg. 2016, 42, 1216–1219. [Google Scholar] [CrossRef]
- Hardy, J.; Boralevi, F.; Mallet, S.; Cabrera, N.; Belot, A.; Phan, A.; Barbarot, S.; Duriez-Lasek, A.; Chiaverini, C.; Hubiche, T.; et al. Clinical Profile of Methotrexate-resistant Juvenile Localised Scleroderma. Acta Derm. Venereol. 2019, 99, 539–543. [Google Scholar] [CrossRef]
- Polcari, I.; Moon, A.; Mathes, E.F.; Gilmore, E.S.; Paller, A.S. Headaches as a presenting symptom of linear morphea en coup de sabre. Pediatrics 2014, 134, e1715–e1719. [Google Scholar] [CrossRef]
- Anderson, L.E.; Treat, J.R.; Licht, D.J.; Kreiger, P.A.; Knight, A.M. Remission of seizures with immunosuppressive therapy in Parry-Romberg syndrome and en coup de sabre linear scleroderma: Case report and brief review of the literature. Pediatr. Dermatol. 2018, 35, e363–e365. [Google Scholar] [CrossRef]
- Niklander, S.; Marín, C.; Martínez, R.; Esguep, A. Morphea “en coup de sabre”: An unusual oral presentation. J. Clin. Dent. 2017, 9, e315–e318. [Google Scholar] [CrossRef] [PubMed]
- Arif, T.; Adil, M.; Amin, S.S.; Sami, M.; Raj, D. Concomitant en coup de sabre and plaque type morphea in the same patient: A rare occurrence. Dermatol. Rev./Przegląd Dermatol. 2017, 104, 570–574. [Google Scholar] [CrossRef]
- Crespo, M.P.; Mas, I.B.; Díaz, J.M.; Costa, A.L.; Nortes, I.B. Rapid response to cyclosporine and maintenance with methotrexate in linear scleroderma in a young girl. Pediatr. Dermatol. 2009, 26, 118–120. [Google Scholar] [CrossRef]
- Foeldvari, I.; Anton, J.; Friswell, M.; Bica, B.; de Inocencio, J.; Aquilani, A.; Helmus, N. Tocilizumab is a promising treatment option for therapy resistant juvenile localized scleroderma patients. J. Scleroderma Relat. Disord. 2017, 2, 203–207. [Google Scholar] [CrossRef]
- Osminina, M.; Geppe, N.; Afonina, E. Scleroderma “en coup de sabre” with Epilepsy and Uveitis Successfully Treated with Tocilizumab. Reumatol. Clin. 2020, 16, 356–358. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Terras, S.; Kreuter, A. Treatment regimens, protocols, dosage, and indications for UVA1 phototherapy: Facts and controversies. Clin. Dermatol. 2013, 31, 438–454. [Google Scholar] [CrossRef] [PubMed]
- Kakimoto, C.V.; Victor Ross, E.; Uebelhoer, N.S. En coup de sabre presenting as a port-wine stain previously treated with pulsed dye laser. Dermatol. Surg. 2009, 35, 165–167. [Google Scholar] [PubMed]
- Ayoub, R.; Saba, S.C. Treatment of linear scleroderma “en coup de sabre” with single-stage autologous fat grafting: A case report and review of the literature. J. Cosmet. Dermatol. 2021, 20, 285–289. [Google Scholar] [CrossRef]
- Franco, J.P.; Serra, M.S.; Lima, R.B.; D’Acri, A.M.; Martins, C.J. Scleroderma en coup de sabre treated with polymethylmethacrylate—Case report. An. Bras. Dermatol. 2016, 91, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Dirschka, T.; Jackowski, J.; Bierhoff, E.; Fölster-Holst, R. Surgical correction of scleroderma en coup de sabre. Hautarzt 2007, 58, 611–614. [Google Scholar] [CrossRef]
- Fine, R.M. The use of systemic corticosteroids in dermatology. South. Med. J. 1970, 63, 961–965. [Google Scholar] [CrossRef] [PubMed]
- Strauss, R.M.; Bhushan, M.; Goodfield, M.J. Good response of linear scleroderma in a child to ciclosporin. Br. J. Dermatol. 2004, 150, 790–792. [Google Scholar] [CrossRef] [PubMed]
- Oikarinen, A. Hydroxychloroquine induces autophagic cell death of human dermal fibroblasts: Implications for treating fibrotic skin diseases. J. Investig. Dermatol. 2009, 129, 2333–2335. [Google Scholar] [CrossRef]
- Kalia, S.; Dutz, J.P. New concepts in antimalarial use and mode of action in dermatology. Dermatol. Ther. 2007, 20, 160–174. [Google Scholar] [CrossRef]
- Foeldvari, I. Update on the Systemic Treatment of Pediatric Localized Scleroderma. Paediatr. Drugs 2019, 21, 461–467. [Google Scholar] [CrossRef]
- Ihn, H.; Sato, S.; Fujimoto, M.; Kikuchi, K.; Takehara, K. Demonstration of interleukin-2, interleukin-4 and interleukin-6 in sera from patients with localized scleroderma. Arch. Dermatol. Res. 1995, 287, 193–197. [Google Scholar] [CrossRef]
- Nagaoka, T.; Sato, S.; Hasegawa, M.; Ihn, H.; Takehara, K. Serum levels of soluble interleukin 6 receptor and soluble gp130 are elevated in patients with localized scleroderma. J. Rheumatol. 2000, 27, 1917–1921. [Google Scholar]
- Hunzelmann, N.; Anders, S.; Fierlbeck, G.; Hein, R.; Herrmann, K.; Albrecht, M.; Bell, S.; Muche, R.; Wehner-Caroli, J.; Gaus, W.; et al. Double-blind, placebo-controlled study of intralesional interferon gamma for the treatment of localized scleroderma. J. Am. Acad. Dermatol. 1997, 36, 433–435. [Google Scholar] [CrossRef]
- Kroft, E.B.; van de Kerkhof, P.C.; Gerritsen, M.J.; de Jong, E.M. Period of remission after treatment with UVA-1 in sclerodermic skin diseases. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Lipner, S.R. Topical Adjuncts to Pulsed Dye Laser for Treatment of Port Wine Stains: Review of the Literature. Dermatol. Surg. 2018, 44, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Asano, Y.; Fujimoto, M.; Ishikawa, O.; Sato, S.; Jinnin, M.; Takehara, K.; Hasegawa, M.; Yamamoto, T.; Ihn, H. Diagnostic criteria, severity classification and guidelines of localized scleroderma. J. Dermatol. 2018, 45, 755–780. [Google Scholar] [CrossRef]
- Keen, M.A. Hyaluronic Acid in Dermatology. Skinmed 2017, 15, 441–448. [Google Scholar] [PubMed]
- Carvalho Costa, I.M.; Salaro, C.P.; Costa, M.C. Polymethylmethacrylate facial implant: A successful personal experience in Brazil for more than 9 years. Dermatol. Surg. 2009, 35, 1221–1227. [Google Scholar] [CrossRef]
- Oh, C.K.; Lee, J.; Jang, B.S.; Kang, Y.S.; Bae, Y.C.; Kwon, K.S.; Jang, H.S. Treatment of atrophies secondary to trilinear scleroderma en coup de sabre by autologous tissue cocktail injection. Dermatol. Surg. 2003, 29, 1073–1075. [Google Scholar]
- Knobler, R.; Moinzadeh, P.; Hunzelmann, N.; Kreuter, A.; Cozzio, A.; Mouthon, L.; Cutolo, M.; Rongioletti, F.; Denton, C.P.; Rudnicka, L.; et al. European Dermatology Forum S1-guideline on the diagnosis and treatment of sclerosing diseases of the skin, Part 1: Localized scleroderma, systemic sclerosis and overlap syndromes. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1401–1424. [Google Scholar] [CrossRef]
- Zulian, F.; Martini, G.; Vallongo, C.; Vittadello, F.; Falcini, F.; Patrizi, A.; Alessio, M.; La Torre, F.; Podda, R.A.; Gerloni, V.; et al. Methotrexate treatment in juvenile localized scleroderma: A randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2011, 63, 1998–2006. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, A.; Krieg, T.; Worm, M.; Wenzel, J.; Moinzadeh, P.; Kuhn, A.; Aberer, E.; Scharffetter-Kochanek, K.; Horneff, G.; Reil, E.; et al. German guidelines for the diagnosis and therapy of localized scleroderma. J. Dtsch. Dermatol. Ges. 2016, 14, 199–216. [Google Scholar] [CrossRef] [PubMed]
| Medicament | Dose | Number of Patients with Localized Scleroderma en Coup de Sabre | Response Rate | Highest Level of Evidence | Literature |
|---|---|---|---|---|---|
| Methotrexate | 15 mg/week | 1 | 100% (1/1) | IV | Rattanakaemakorn P, et al. [2] |
| Methotrexate | 15 mg/week | 1 | 100% (1/1) | V | Van der Veken D, et al. [10] |
| Prednisone | 0.5–1 mg/kg/day | 7 | 100% (7/7) | III | Joly P, et al. [11] |
| Methylprednisolone | 1 g/day for three days followed by 500 mg/day for further three days intravenosus and tapering with oral methylprednisolone | 1 | 100% (1/1) | V | Unterberger I, et al. [12] |
| Mycophenolate mofetil | 600–1200 mg/m2/day | 2 | 100% (2/2) | IV | Martini G, et al. [13] |
| Abatacept | 500 mg (patients weighing <60 kg) or 750 mg (>60 kg) intravenously on days 1, 15, 30 and thereafter every 4–6 weeks | 2 | 100% (2/2) | IV | Fage S, et al. [14] |
| Interferon gamma | 100 mg three times a week subcutaneous (52 mg/m2 body surface area) | 1 | 100% (1/1) | V | Obermoser G, et al. [15] |
| Tocilizumab | 162 mg/week | 1 | 100% (1/1) | V | Margo CM, et al. [16] |
| Hydroxychloroquine | 400 mg/day | 1 | No data (total response 80%, 4/5, children + adults) | IV | Kumar AB, et al. [17] |
| UVA1 therapy | 30 J/cm2 | 3 | 100% (3/3) | III | Su O, et al. [18] |
| UVA1 therapy | 30 J/cm2 | 1 | 100% (1/1) | V | Kowalzick L, et al. [19] |
| NB-UVB therapy | Three times weekly | 1 | 100% (1/1) | V | Brownell I, et al. [20] |
| Dermal fat grafting | No data | 1 | 100% (1/1) | V | Barin EZ, et al. [21] |
| Regenerative cell-enriched autologous fat grafting | No data | 1 | 100% (1/1) | V | Karaaltin MV, et al. [22] |
| Medpor with dermal fat grafting | No data | 1 | 100% (1/1) | V | Kim KT, et al. [23] |
| Structural fat grafting | No data | 1 | 100% (1/1) | V | Consorti G, et al. [24] |
| Alloplastic implantation with AlloDerm tissue matrix | No data | 1 | 100% (1/1) | V | Robitschek J, et al. [25] |
| Hyaluronic acid | No data | 1 | 100% (1/1) | V | Thareja SK, et al. [26] |
| Hyaluronic acid injected with a blunt-tipped microcannula | No data | 1 | 100% (1/1) | V | Sivek R, et al. [27] |
| Tissue cocktail injection | No data | 1 | 100% (1/1) | V | Oh HM, et al. [28] |
| Toxinum botulinum | 25 units and 35 units at 6 and 9 months | 1 | 100% (1/1) | V | Rimoin L, et al. [29] |
| Medicament | Dose | Number of Patients with Scleroderma en Coup de Sabre | Response Rate | Highest Level of Evidence | Literature |
|---|---|---|---|---|---|
| Methotrexate | 2.5 mg/week | 6 | 100% (6/6) | IV | Rattanakaemakorn P, et al. [2] |
| Methotrexate | 7.1–15 mg/m2/week | 12 | 100% (12/12) 8/12 (67%) stability 4/12 (33%) improvement | IV | Hardy J, et al. [30] |
| Methotrexate | 25 mg/week (2/4) 20 mg/week (1/4) 15 mg/week (1/4) | 4 | 100% (4/4) | IV | Polcari I, et al. [31] |
| Methotrexate | 25 mg/week | 1 | 100% (1/1) | V | Anderson LE, et al. [32] |
| Methotrexate | 20 mg/week | 1 | 100% (1/1) | V | Niklander S, et al. [33] |
| Prednisolone | 30 mg/day | 1 | 100% (1/1) | V | Arif T, et al. [34] |
| Cyclosporine | 3 mg/kg/day for 3 months and 2.5 mg/kg/day for next 4 months | 1 | 100% (1/1) | V | Crespo MP, et al. [35] |
| Tocilizumab | 8–10 mg/kg every 3–4 weeks | 1 | 100% (1/1) | IV | Foeldvari I, et al. [36] |
| Tocilizumab | 10 mg\in 4 weeks | 1 | 100% (1/1) | V | Osminina M, et al. [37] |
| Hydroxychloroquine | 5 mg/kg/day | 4 | general response 80%, (4/5, children + adults) | IV | Kumar AB, et al. [17] |
| PUVA therapy | Topical 8-methoxypsoralen 0.0006%; The initial UVA dose was 0.3 J/cm2 three times weekly (increased by 0.2 j/cm2 after 3 days) | 2 | 100% (2/2) | IV | Gambichler T, et al. [38] |
| Pulsed dye laser (595 nm) | 8-J/cm2 fluence, 1.5 ms pulse duration, 40 ms duration, and 30 ms delay with a dynamic cooling, the fluence was increased to 8.5 J/cm2 and the dynamic cooling increased to 50/30 | 1 | 100% (1/1) | V | Kakimoto CV, et al. [39] |
| Autologous fat grafting | No data | 1 | 100% (1/1) | V | Ayoub R, et al. [40] |
| Polymethylmethacrylate | No data | 1 | 100% (1/1) | V | Franco JP, et al. [41] |
| Resection of the sclerotic area | No data | 1 | 100% (1/1) | V | Dirschka T, et al. [42] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulc, E.; Rudnicka, L.; Waśkiel-Burnat, A.; Warszawik-Hendzel, O.; Niemczyk, A.; Olszewska, M. Therapeutic and Reconstructive Management Options in Scleroderma (Morphea) en Coup de Sabre in Children and Adults. A Systematic Literature Review. J. Clin. Med. 2021, 10, 4517. https://doi.org/10.3390/jcm10194517
Ulc E, Rudnicka L, Waśkiel-Burnat A, Warszawik-Hendzel O, Niemczyk A, Olszewska M. Therapeutic and Reconstructive Management Options in Scleroderma (Morphea) en Coup de Sabre in Children and Adults. A Systematic Literature Review. Journal of Clinical Medicine. 2021; 10(19):4517. https://doi.org/10.3390/jcm10194517
Chicago/Turabian StyleUlc, Ewelina, Lidia Rudnicka, Anna Waśkiel-Burnat, Olga Warszawik-Hendzel, Anna Niemczyk, and Małgorzata Olszewska. 2021. "Therapeutic and Reconstructive Management Options in Scleroderma (Morphea) en Coup de Sabre in Children and Adults. A Systematic Literature Review" Journal of Clinical Medicine 10, no. 19: 4517. https://doi.org/10.3390/jcm10194517
APA StyleUlc, E., Rudnicka, L., Waśkiel-Burnat, A., Warszawik-Hendzel, O., Niemczyk, A., & Olszewska, M. (2021). Therapeutic and Reconstructive Management Options in Scleroderma (Morphea) en Coup de Sabre in Children and Adults. A Systematic Literature Review. Journal of Clinical Medicine, 10(19), 4517. https://doi.org/10.3390/jcm10194517






