Association of Serum High-Density Lipoprotein Cholesterol with High Blood Pressures at Checkup: Results of Kanagawa Investigation of Total Checkup Data from the National Database-9 (KITCHEN-9)
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Subjects
2.2. Measurements
2.3. Statistical Analysis
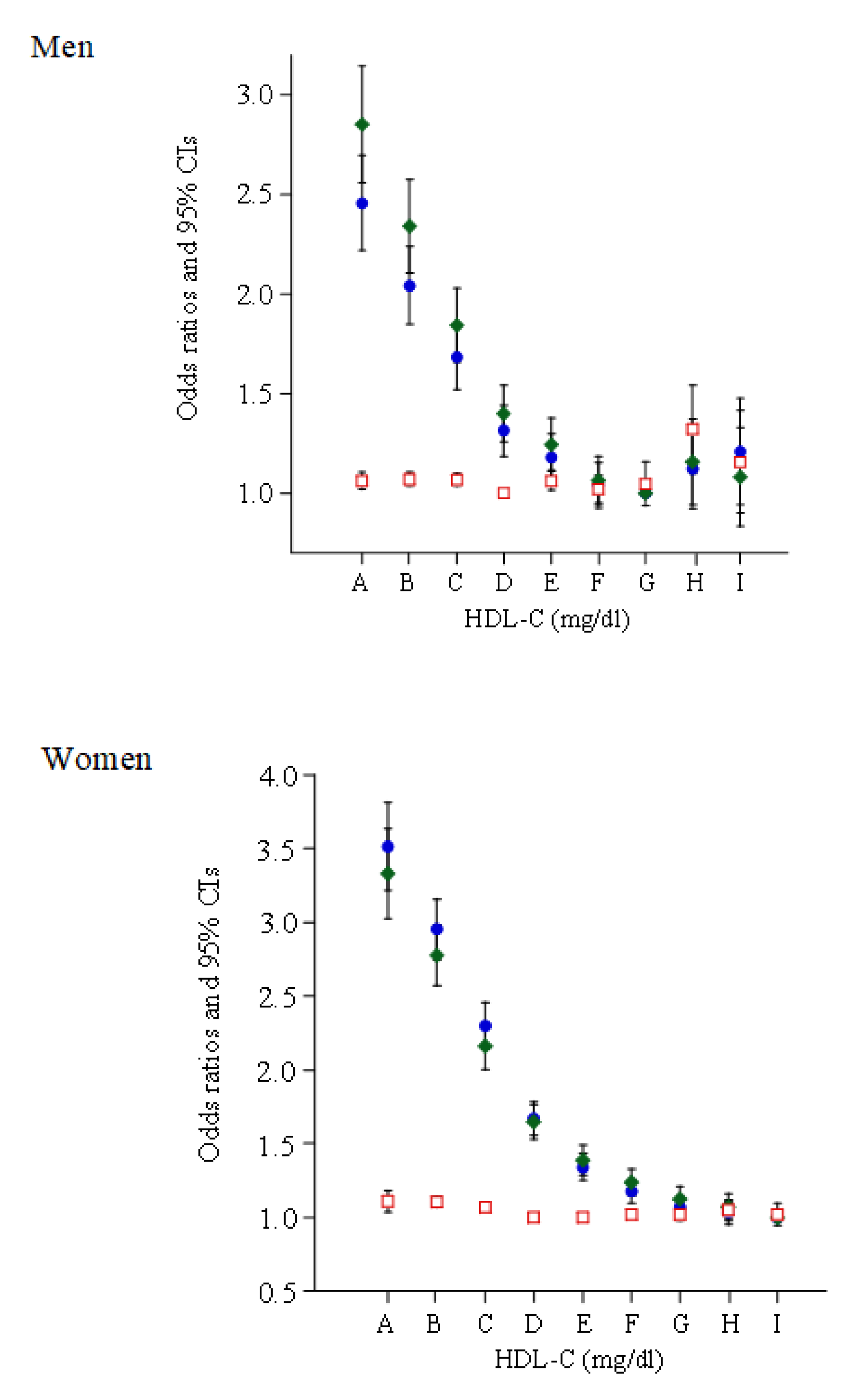
3. Results
3.1. Subgroup Analysis
3.2. Discussion
3.3. Limitations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Martinez, L.; Jacquet, S.; Collet, X.; Perret, B.; Barbaras, R. New insight on the molecular mechanisms of high-density lipoprotein cellular interactions. Cell. Mol. Life Sci. 2004, 61, 2343–2360. [Google Scholar] [CrossRef]
- Bermúdez, V.; Cano, R.; Cano, C.; Bermúdez, F.; Arraiz, N.; Acosta, L.; Finol, F.; Pabón, M.R.; Amell, A.; Reyna, N.; et al. Pharmacologic management of isolated low high-density lipoprotein syndrome. Am. J. Ther. 2008, 15, 377–388. [Google Scholar] [CrossRef]
- Tomlinson, B. Raising high-density lipoprotein: For better or worse? Heart 2010, 96, 1339–1340. [Google Scholar] [CrossRef]
- Feig, J.E.; Hewing, B.; Smith, J.D.; Hazen, S.L.; Fisher, E.A. High-density lipoprotein and atherosclerosis regression: Evidence from preclinical and clinical studies. Circ. Res. 2014, 114, 205–213. [Google Scholar] [CrossRef]
- Toth, P.P.; Barylski, M.; Nikolic, D.; Rizzo, M.; Montalto, G.; Banach, M. Should low high-density lipoprotein cholesterol (HDL-C) be treated? Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Russo, A.; Pahor, M.; Capoluongo, E.; Liperoti, R.; Cesari, M.; Bernabei, R.; Onder, G. Serum high-density lipoprotein cho-lesterol levels and mortality in frail, community-living elderly. Gerontology 2008, 54, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Rahilly-Tierney, C.R.; Spiro, A., 3rd; Vokonas, P.; Gaziano, J.M. Relation between high-density lipoprotein cholesterol and survival to age 85 years in men (from the VA normative aging study). Am. J. Cardiol. 2011, 107, 1173–1177. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, Y.; Pei, W.; Wu, Y.; Zhao, J. Is Elevated High-density Lipoprotein Cholesterol Always Good for Coronary Heart Disease? Clin. Cardiol. 2007, 30, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Ko, D.T.; Alter, D.A.; Guo, H.; Koh, M.; Lau, G.; Austin, P.C.; Booth, G.L.; Hogg, W.; Jackevicius, C.A.; Lee, D.S.; et al. High-Density Lipoprotein Cholesterol and Cause-Specific Mortality in Individuals Without Previous Cardiovascular Conditions: The CANHEART Study. J. Am. Coll Cardiol. 2016, 68, 2073–2083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madsen, C.M.; Varbo, A.; Nordestgaard, B.G. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: Two prospective cohort studies. Eur. Heart J. 2017, 38, 2478–2486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirata, A.; Sugiyama, D.; Watanabe, M.; Tamakoshi, A.; Iso, H.; Kotani, K.; Kiyama, M.; Yamada, M.; Ishikawa, S.; Murakami, Y.; et al. Association of extremely high levels of high-density lipoprotein cholesterol with cardiovascular mortality in a pooled analysis of 9 cohort studies including 43,407 individuals: The EPOCH–JAPAN study. J. Clin. Lipidol. 2018, 12, 674–684.e5. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guan, B.; Wang, Y.; Tse, G.; Zou, F.; Khalid, B.W.; Xia, Y.; Wu, S.; Sun, J. Association between high-density lipoprotein cholesterol and all-cause mortality in the general population of northern China. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Huang, Y.-Q.; Liu, X.-C.; Lo, K.; Liu, L.; Yu, Y.-L.; Chen, C.-L.; Huang, J.-Y.; Feng, Y.-Q.; Zhang, B. The U Shaped Relationship Between High-Density Lipoprotein Cholesterol and All-Cause or Cause-Specific Mortality in Adult Population. Clin. Interv. Aging 2020, 15, 1883–1896. [Google Scholar] [CrossRef]
- Chen, C.-L.; Liu, X.-C.; Liu, L.; Lo, K.; Yu, Y.-L.; Huang, J.-Y.; Huang, Y.-Q.; Chen, J.-Y. U-Shaped Association of High-Density Lipoprotein Cholesterol with All-Cause and Cardiovascular Mortality in Hypertensive Population. Risk Manag. Health Policy 2020, 13, 2013–2025. [Google Scholar] [CrossRef] [PubMed]
- Barter, P.J.; Caulfield, M.; Eriksson, M.; Grundy, S.M.; Kastelein, J.J.P.; Komajda, M.; Lopez-Sendon, J.; Mosca, L.; Tardif, J.-C.; Waters, D.D.; et al. Effects of Torcetrapib in Patients at High Risk for Coronary Events. N. Engl. J. Med. 2007, 357, 2109–2122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lincoff, A.M.; Nicholls, S.J.; Riesmeyer, J.S.; Barter, P.J.; Brewer, H.B.; Fox, K.A.A.; Gibson, C.M.; Granger, C.; Menon, V.; Montalescot, G.; et al. Evacetrapib and Cardiovascular Outcomes in High-Risk Vascular Disease. N. Engl. J. Med. 2017, 376, 1933–1942. [Google Scholar] [CrossRef] [PubMed]
- Riaz, H.; Khan, S.U.; Rahman, H.; Shah, N.P.; Kaluski, E.; Lincoff, A.M.; Nissen, S. Effects of high-density lipoprotein targeting treatments on cardiovascular outcomes: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2019, 26, 533–543. [Google Scholar] [CrossRef]
- Whyte, M.B. Is high-density lipoprotein a modifiable treatment target or just a biomarker for cardiovascular disease? JRSM Cardiovasc. Dis. 2019, 8, 2048004019869736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Zhang, W.; Zhou, F.; Chen, C.; Zhou, L.; Li, Y.; Liu, L.; Pei, F.; Luo, H.; Hu, Z.; et al. Cholesteryl ester transfer protein in-hibitors in the treatment of dyslipidemia: A systematic review and meta-analysis. PLoS ONE 2013, 8, e77049. [Google Scholar]
- Filippatos, T.D.; Klouras, E.; Barkas, F.; Elisaf, M. Cholesteryl ester transfer protein inhibitors: Challenges and perspectives. Expert Rev. Cardiovasc. Ther. 2016, 14, 953–962. [Google Scholar] [CrossRef]
- Rios, F.; Lopes, R.; Neves, K.B.; Camargo, L.D.L.; Montezano, A.C.; Touyz, R.M. Off-Target Vascular Effects of Cholesteryl Ester Transfer Protein Inhibitors Involve Redox-Sensitive and Signal Transducer and Activator of Transcription 3-Dependent Pathways. J. Pharmacol. Exp. Ther. 2016, 357, 415–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, W.; Gu, D.; Yang, X.; Wu, J.; Kang, L.; Zhang, L. High-density lipoprotein cholesterol levels increase with age, body mass index, blood pressure and fasting blood glucose in a rural Uygur population in China. J. Hypertens. 2005, 23, 1985–1989. [Google Scholar] [CrossRef]
- Oda, E.; Kawai, R. High-density lipoprotein cholesterol is positively associated with hypertension in apparently healthy Japanese men and women. Br. J. Biomed. Sci. 2011, 68, 29–33. [Google Scholar] [CrossRef]
- Kawamoto, R.; Tabara, Y.; Kohara, K.; Miki, T.; Abe, M.; Kusunoki, T. Increased high-density lipoprotein cholesterol is associated with a high prevalence of pre-hypertension and hypertension in community-dwelling persons. Endocrine 2012, 42, 321–328. [Google Scholar] [CrossRef]
- Nakajima, K.; Higuchi, R.; Mizusawa, K.; Nakamura, T. Association between extremely high high-density lipoprotein-cholesterol and hypertensive retinopathy: Results of a cross-sectional study from Kanagawa Investigation of Total Checkup Data from the National Database-6 (KITCHEN-6). BMJ Open 2021, 11, e043677. [Google Scholar] [CrossRef]
- Nakajima, K.; Iwane, T.; Higuchi, R.; Shibata, M.; Takada, K.; Uda, J.; Anan, M.; Sugiyama, M.; Nakamura, T. Kanagawa Investigation of the Total Check-up Data from the National database (KITCHEN): Protocol for data-driven population-based repeated cross-sectional and 6-year cohort studies. BMJ Open 2019, 9, e023323. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, D.-Y.; Li, Y.; Wang, J.-G. The Role of Out-of-Clinic Blood Pressure Measurements in Preventing Hypertension. Curr. Hypertens. Rep. 2018, 20, 85. [Google Scholar] [CrossRef]
- Hoshide, S.; Kario, K.; Tomitani, N.; Kabutoya, T.; Chia, Y.; Park, S.; Shin, J.; Turana, Y.; Tay, J.C.; Buranakitjaroen, P.; et al. Highlights of the 2019 Japanese Society of Hypertension Guidelines and perspectives on the management of Asian hypertensive patients. J. Clin. Hypertens. 2020, 22, 369–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsushita, K.; SHosler, A.; Miura, K.; Ito, Y.; Fukuda, T.; Kitamura, A.; Tatara, K. Rationale and Descriptive Analysis of Specific Health Guidance: The Nationwide Lifestyle Intervention Program Targeting Metabolic Syndrome in Japan. J. Atheroscler. Thromb. 2018, 25, 308–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ministry of Health, Labour and Welfare. Health Examination and Guidance Program for Japanese Adults. 2008. Available online: https://www.mhlw.go.jp/bunya/shakaihosho/iryouseido01/info02a.html (accessed on 24 March 2020).
- Available online: http://www.jacd.info/method/ketsuatsusokutei.htm (accessed on 19 September 2021).
- Available online: https://www.mhlw.go.jp/content/10900000/000496780.pdf (accessed on 19 September 2021).
- Flegal, K.M.; Cauley, J. Alcohol Consumption and Cardiovascular Risk Factors. Recent Dev. Alcohol. 1985, 3, 165–180. [Google Scholar] [CrossRef]
- Criqui, M.H. Do Known Cardiovascular Risk Factors Mediate the Effect of Alcohol on Cardiovascular Disease? Novartis Found Symp. 2007, 216, 159–172. [Google Scholar] [CrossRef]
- Kodama, S.; Horikawa, C.; Fujihara, K.; Yoshizawa, S.; Yachi, Y.; Tanaka, S.; Ohara, N.; Matsunaga, S.; Yamada, T.; Hanyu, O.; et al. Meta-Analysis of the Quantitative Relation Between Pulse Pressure and Mean Arterial Pressure and Cardiovascular Risk in Patients With Diabetes Mellitus. Am. J. Cardiol. 2014, 113, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.S.; Medeiros, E.D.; Shah, A.D. Wide pulse pressure: A clinical review. J. Clin. Hypertens. 2020, 22, 1960–1967. [Google Scholar] [CrossRef] [PubMed]
- Nishiwaki, M.; Ishikawa, T.; Ito, T.; Shige, H.; Tomiyasu, K.; Nakajima, K.; Kondo, K.; Hashimoto, H.; Saitoh, K.; Manabe, M.; et al. Effects of alcohol on lipoprotein lipase, hepatic lipase, cholesteryl ester transfer protein, and lecithin:cholesterol acyltransferase in high-density lipoprotein cholesterol elevation. Atherosclerosis 1994, 111, 99–109. [Google Scholar] [CrossRef]
- Hannuksela, M.L.; Rantala, M.; Kesaniemi, Y.A.; Savolainen, M.J. Ethanol-Induced Redistribution of Cholesteryl Ester Transfer Protein (CETP) Between Lipoproteins. Arter. Thromb. Vasc. Biol. 1996, 16, 213–221. [Google Scholar] [CrossRef]
- Roerecke, M.; Kaczorowski, J.; Myers, M.G. Comparing Automated Office Blood Pressure Readings with Other Methods of Blood Pressure Measurement for Identifying Patients With Possible Hypertension: A Systematic Review and Meta-analysis. JAMA Intern Med. 2019, 179, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Bo, Y.; Kwok, K.O.; Chu, K.K.; Leung, E.Y.; Yu, C.P.; Wong, S.Y.; Lee, E.K. Comparison Between Automated Office Blood Pressure Meas-urements and Manual Office Blood Pressure Measurements-Implications in Individual Patients: A Systematic Review and Meta-analysis. Curr. Hypertens. Rep. 2021, 23, 4. [Google Scholar] [CrossRef]
- Jardim, T.V.; Gaziano, T.A.; Nascente, F.M.; Carneiro, C.D.S.; Morais, P.; Roriz, V.; Mendonça, K.L.; Póvoa, T.I.R.; Barroso, W.K.S.; Sousa, A.L.L.; et al. Office blood pressure measurements with oscillometric devices in adolescents: A comparison with home blood pressure. Blood Press. 2017, 26, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Myers, M.G.; Kaczorowski, J. Are Automated Office Blood Pressure Readings More Variable Than Home Readings? Hypertension 2020, 75, 1179–1183. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, S.; Kadowaki, T.; Hozawa, A.; Fujiyoshi, A.; Hisamatsu, T.; Satoh, A.; Arima, H.; Tanaka, S.; Torii, S.; Kondo, K.; et al. Differences between home blood pressure and strictly measured office blood pressure and their determinants in Japanese men. Hypertens. Res. 2021, 44, 80–87. [Google Scholar] [CrossRef]
- DeRosa, G.; Maffioli, P. Nifedipine and telmisartan for the treatment of hypertension: The TALENT study. Expert Rev. Cardiovasc. Ther. 2011, 9, 1499–1503. [Google Scholar] [CrossRef] [PubMed]
- Grosskopf, I.; Shaish, A.; Charach, G.; Harats, D.; Kamari, Y. Nifedipine Treatment for Hypertension is Associated with Enhanced Lipolytic Activity and Accelerated Clearance of Postprandial Lipemia. Horm. Metab. Res. 2016, 48, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G. Hypertension Management in Chronic Kidney Disease and Diabetes: Lessons from the Systolic Blood Pressure Intervention Trial. Cardiol. Clin. 2019, 37, 307–317. [Google Scholar] [CrossRef]
- Teo, B.W.; Chan, G.C.; Leo, C.C.H.; Tay, J.C.; Chia, Y.; Siddique, S.; Turana, Y.; Chen, C.; Cheng, H.; Hoshide, S.; et al. Hypertension and chronic kidney disease in Asian populations. J. Clin. Hypertens. 2021, 23, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Feig, D. Serum uric acid and the risk of hypertension and chronic kidney disease. Curr. Opin. Rheumatol. 2014, 26, 176–185. [Google Scholar] [CrossRef]
- Bjornstad, P.; Laffel, L.; Lynch, J.; El Ghormli, L.; Weinstock, R.S.; Tollefsen, S.E.; Nadeau, K.J.; TODAY Study Group. Elevated Serum Uric Acid Is Associated with Greater Risk for Hypertension and Diabetic Kidney Diseases in Obese Adolescents with Type 2 Dia-betes: An Observational Analysis from the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) Study. Diabetes Care 2019, 42, 1120–1128. [Google Scholar] [PubMed]
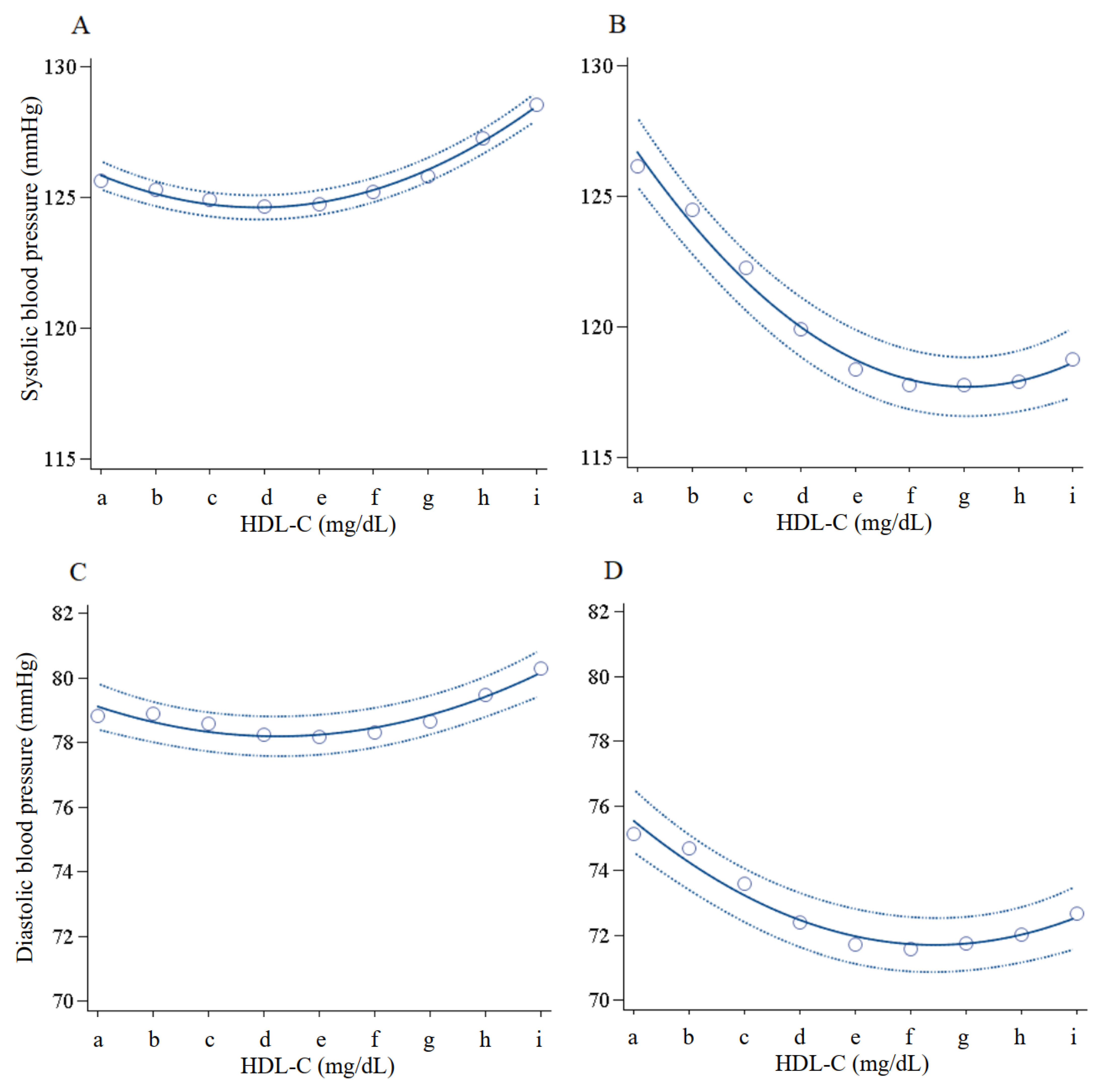

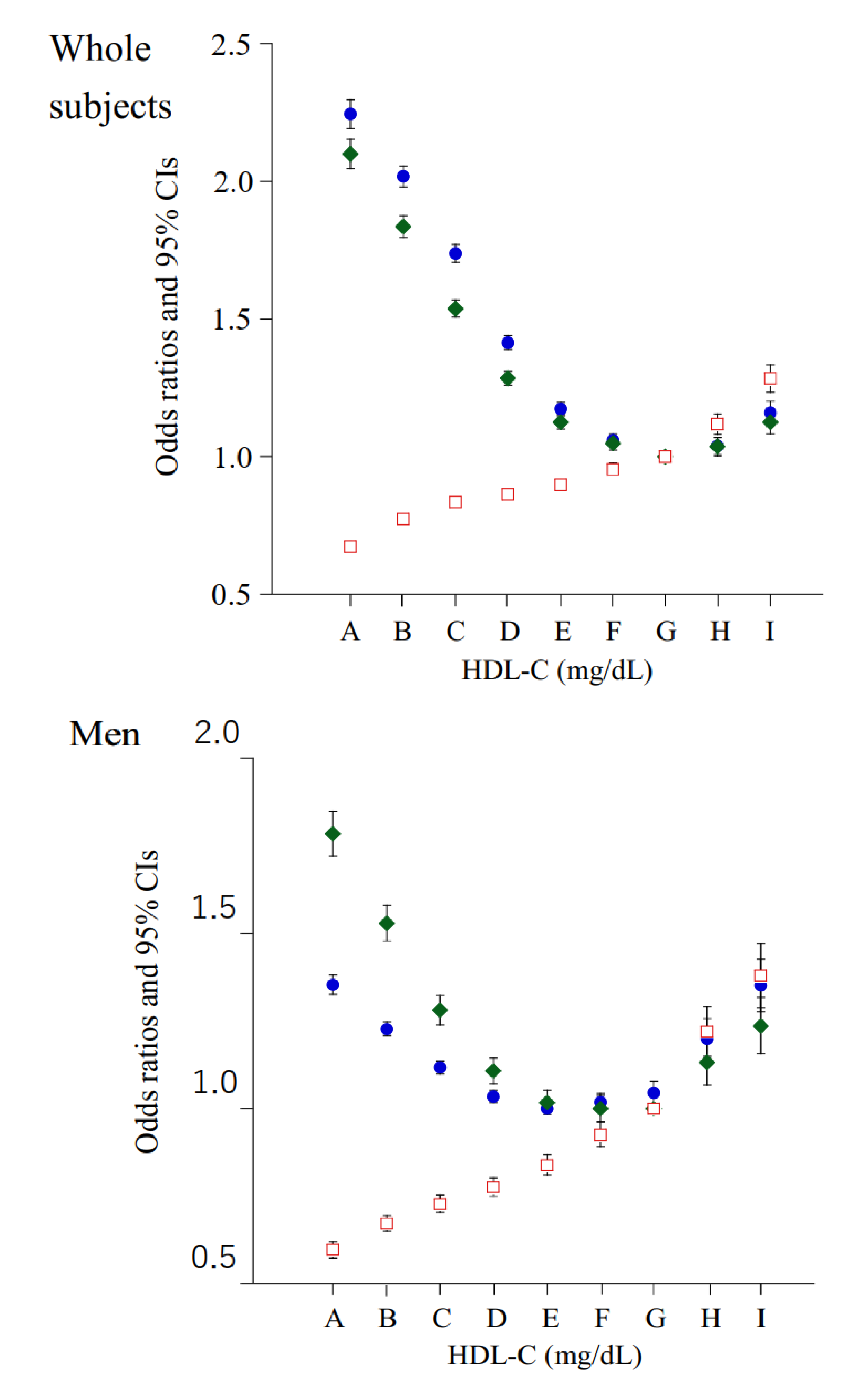
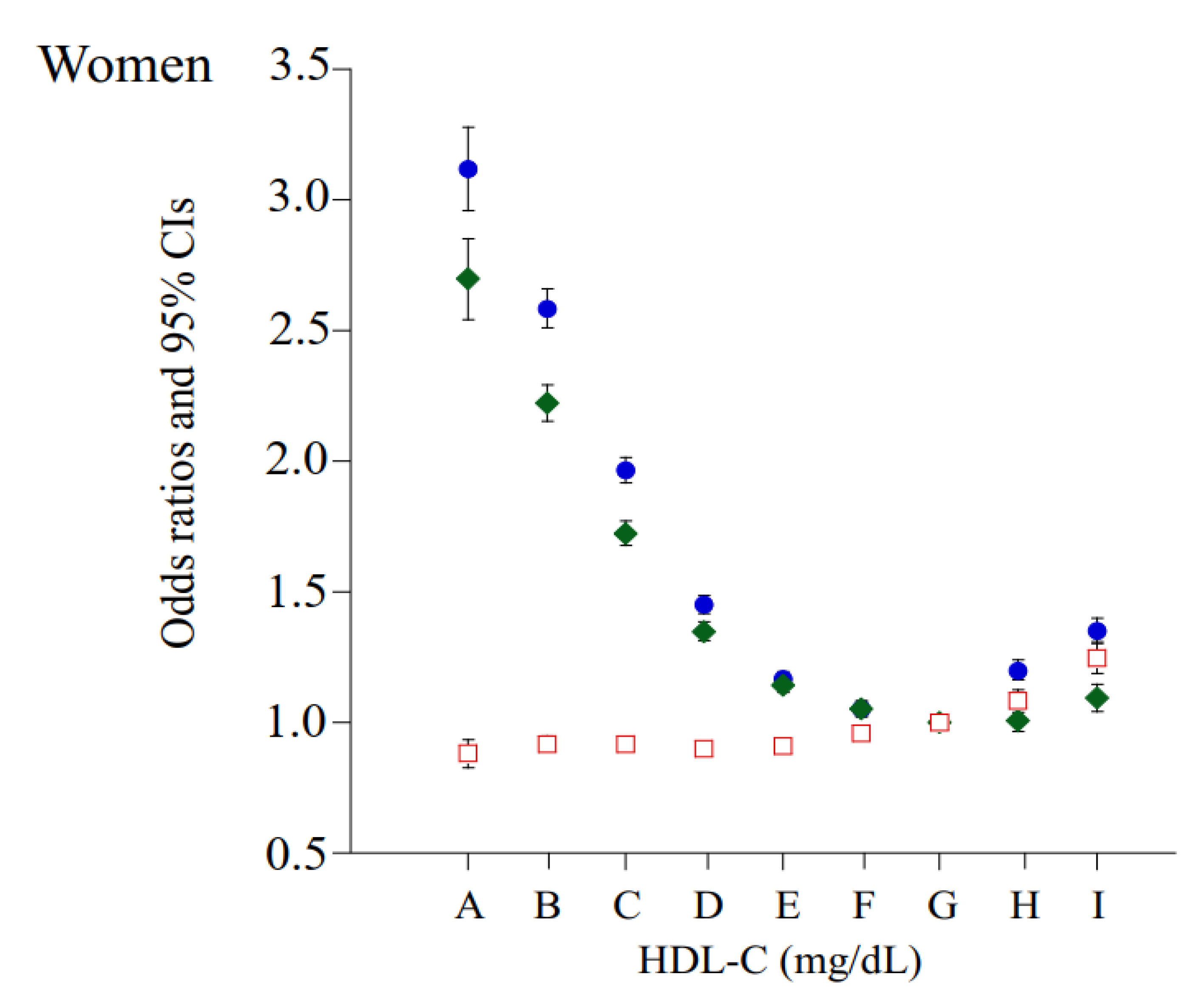
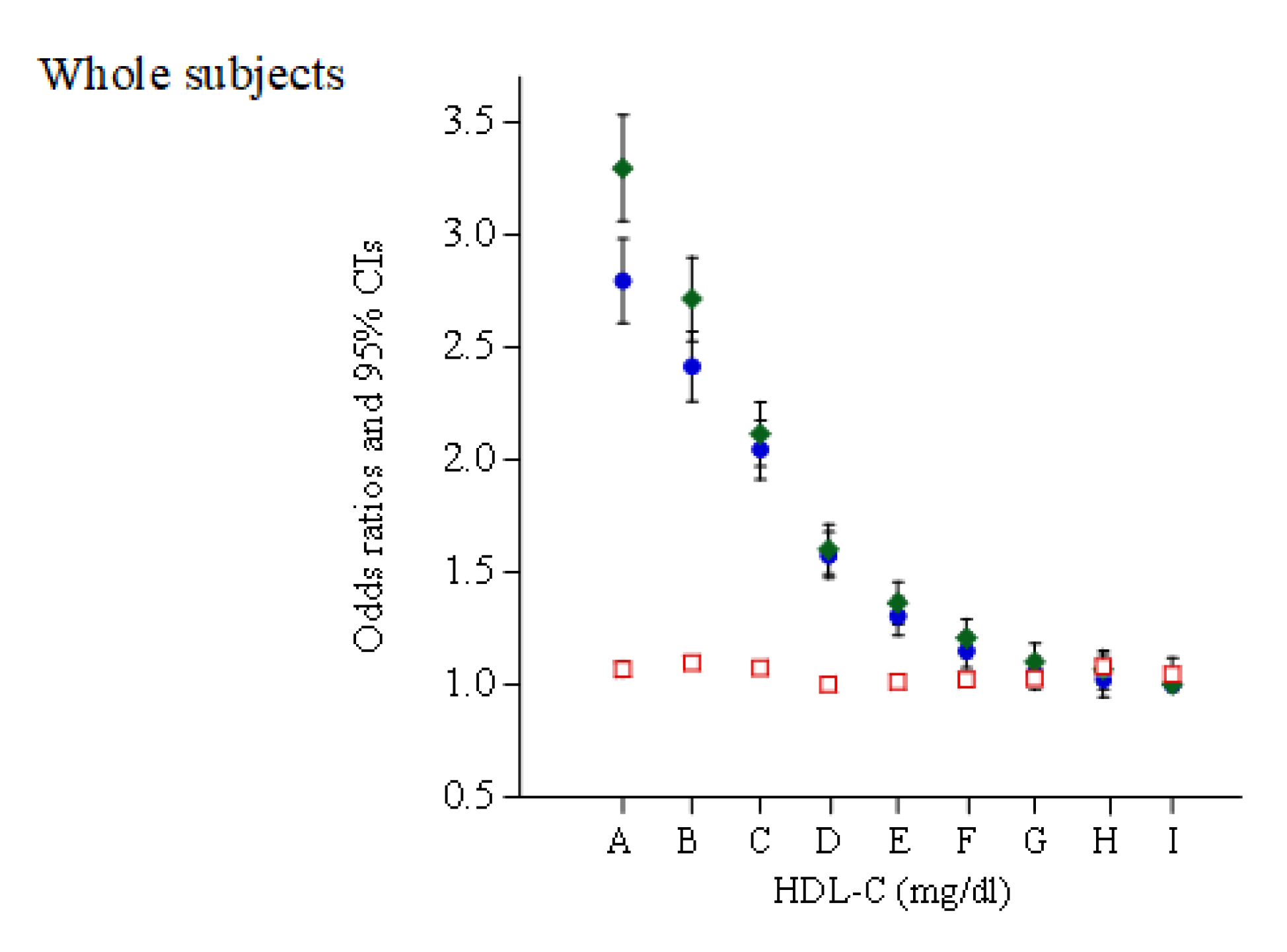
| HDL-C Categories (mg/dL) | 30–39 | 40–49 | 50–59 | 60–69 | 70–79 | 80–89 | 90–99 | 100–109 | ≥110 |
|---|---|---|---|---|---|---|---|---|---|
| N (% of total) | 63,852 (4.3) | 238,101 (16.0) | 348,170 (23.3) | 331,962 (22.2) | 244,322 (16.4) | 144,619 (9.7) | 72,062 (4.8) | 30,510 (2.0) | 19,554 (1.3) |
| s-Age | 54.0 ± 10.0 | 54.2 ± 10.0 | 54.8 ± 10.1 | 55.1 ± 10.2 | 55.1 ± 10.1 | 55.2 ± 10.0 | 55.4 ± 9.7 | 55.6 ± 9.4 | 55.8 ± 9.2 |
| Women, n (%) | 6800 (10.7) | 44,379 (18.6) | 112,421 (32.3) | 160,350 (48.3) | 148,963 (61.0) | 99,424 (68.8) | 52,858 (73.4) | 22,886 (75.0) | 14,402 (73.7) |
| BMI (kg/m2) | 25.9 ± 3.7 | 25.0 ± 3.6 | 23.9 ± 3.4 | 22.8 ± 3.2 | 21.8 ± 3.0 | 21.1 ± 2.8 | 20.7 ± 2.7 | 20.4 ± 2.6 | 20.1 ± 2.6 |
| SBP (mmHg) | 126 ± 16.4 | 125 ± 16.4 | 124 ± 16.8 | 122 ± 17.2 | 121 ± 17.5 | 120 ± 17.6 | 120 ± 17.6 | 120 ± 17.9 | 121 ± 18.2 |
| DBP (mmHg) | 78.4 ± 11.5 | 78.1 ± 11.5 | 77.0 ± 11.6 | 75.4 ± 11.7 | 74.3 ± 11.7 | 73.7 ± 11.7 | 73.6 ± 11.6 | 73.9 ± 11.8 | 74.7 ± 12.0 |
| PP (mmHg) | 47.3 ± 11.1 | 47.0 ± 11.0 | 47.1 ± 11.2 | 47.0 ± 11.3 | 46.6 ± 11.4 | 46.4 ± 11.4 | 46.3 ± 11.4 | 46.4 ± 11.5 | 46.7 ± 11.6 |
| Triglyceride (mg/dL) | 185 (130–270) | 138 (99–193) | 106 (77–146) | 85 (64–117) | 73 (56–98) | 66 (51–87) | 62 (49–81) | 59 (47–76) | 58 (46–75) |
| LDL-C (mg/dL) * | 121 ± 33.4 | 130 ± 32.4 | 130 ± 32.2 | 126 ± 31.6 | 122 ± 30.8 | 120 ± 30.2 | 118 ± 30.3 | 117 ± 30.8 | 112 ± 32.6 |
| HDL-C (mg/dL) | 36.0 ± 2.9 | 45.1 ± 2.8 | 54.6 ± 2.9 | 64.3 ± 2.9 | 74.2 ± 2.8 | 84.0 ± 2.8 | 93.8 ± 2.8 | 103.8 ± 2.8 | 120.4 ± 11.9 |
| HbA1c (NGSP, %) ** | 5.9 ± 0.9 | 5.7 ± 0.8 | 5.6 ± 0.7 | 5.5 ± 0.5 | 5.5 ± 0.5 | 5.5 ± 0.4 | 5.4 ± 0.4 | 5.4 ± 0.4 | 5.4 ± 0.5 |
| Pharmacotherapy for | |||||||||
| hypertension, n (%) | 16,700 (26.2) | 56,096 (23.6) | 73,636 (21.2) | 59,303 (17.9) | 36,521 (15.0) | 19,188 (13.3) | 8,810 (12.2) | 3,677 (12.1) | 2,533 (13.0) |
| diabetes, n (%) | 5974 (9.4) | 16,629 (7.0) | 17,094 (4.9) | 11,105 (3.4) | 5720 (2.3) | 2675 (1.9) | 1123 (1.6) | 490 (1.6) | 343 (1.8) |
| dyslipidemia, n (%) | 91,03 (14.3) | 34,431 (14.5) | 49,956 (14.4) | 42,471 (12.8) | 26,229 (10.7) | 13,417 (9.3) | 6005 (8.3) | 2345 (7.7) | 1410 (7.2) |
| Cardiovascular disease, n (%) | 3,293 (5.2) | 9,747 (4.1) | 12,237 (3.5) | 9,867 (3.0) | 6,100 (2.5) | 3,327 (2.3) | 1,532 (2.1) | 597 (2.0) | 349 (1.8) |
| Current smokers, n (%) | 28,658 (44.9) | 81,543 (34.3) | 89,236 (25.6) | 63,958 (19.3) | 37,275 (15.3) | 18,864 (13.0) | 8,550 (11.9) | 3,667 (12.0) | 2,629 (13.4) |
| Alcohol drinkers | |||||||||
| Everyday, n (%) | 11,580 (18.1) | 55,642 (23.4) | 96,004 (27.6) | 96,149 (29.0) | 72,192 (29.6) | 44,524 (30.8) | 23,825 (33.1) | 11,156 (36.6) | 8,577 (43.9) |
| Occasional, n (%) | 21,514 (33.7) | 82,110 (34.5) | 114,385 (32.9) | 103,970 (31.3) | 75,479 (30.9) | 44,631 (30.9) | 21,864 (30.3) | 8,957 (29.4) | 5,325 (27.2) |
| Non, n (%) | 30,758 (48.2) | 100,349 (42.2) | 13,7781 (39.6) | 131,843 (39.7) | 96,651 (39.6) | 55,464 (38.4) | 26,373 (36.6) | 10,397 (34.1) | 5,652 (28.9) |
| Regular exercisers, n (%) *** | 14,582 (22.8) | 60,875 (25.6) | 99,252 (28.5) | 102,162 (30.8) | 78,890 (32.3) | 48,424 (33.5) | 25,094 (34.8) | 10,874 (35.6) | 7,300 (37.3) |
| HDL-C Category (mg/dL) | 20–39 | 40–49 | 50–59 | 60–69 | 70–79 | 80–89 | 90–99 | 100–109 | ≥110 |
|---|---|---|---|---|---|---|---|---|---|
| Whole subjects (n = 1,493,152) | |||||||||
| Case of HBP, n (% in each group) | 26,275 (41.2) | 91,895 (38.6) | 122,287 (35.1) | 101,501 (30.6) | 65,474 (26.8) | 35,924 (24.8) | 17,111 (23.7) | 7461 (24.5) | 5190 (26.5) |
| Men (n = 830,669) | |||||||||
| Case of HBP, n (% in each group) | 23,324 (40.9) | 74,652 (38.5) | 85,647 (36.3) | 59,333 (34.6) | 32,232 (33.8) | 15,473 (34.2) | 6680 (34.8) | 2896 (38.0) | 2104 (40.8) |
| Women (n = 662,483) | |||||||||
| Case of HBP, n (% in each group) | 2951 (43.4) | 17,243 (38.9) | 36,640 (32.6) | 42,168 (26.3) | 33,242 (22.3) | 20,451 (20.6) | 10,431 (19.7) | 4565 (20.0) | 3086 (21.4) |
| HDL-C Category (mg/dL) | 30–39 | 40–49 | 50–59 | 60–69 | 70–79 | 80–89 | 90–99 | 100–109 | ≥110 |
|---|---|---|---|---|---|---|---|---|---|
| Whole subjects, n (% in total n = 595,268) | 30,758 (5.2) | 100,349 (16.9) | 137,781 (23.1) | 131,843 (22.1) | 96,651 (16.2) | 55,464 (9.3) | 26,373 (4.4) | 10,397 (1.7) | 5,652 (0.9) |
| Case of HBP, n (% in each group) | 12,465 (40.5) | 37,204 (37.1) | 45,813 (33.3) | 36,585 (27.8) | 23,333 (24.1) | 12,139 (21.9) | 5,379 (20.4) | 2,078 (20.0) | 1,108 (19.6) |
| Men, n (% in total n = 227,709) | 25,865 (11.4) | 70,200 (30.8) | 66,599 (29.2) | 38,101 (16.7) | 16,939 (7.4) | 6,499 (2.9) | 2,345 (1.0) | 755 (0.3) | 406 (0.2) |
| Case of HBP, n (% in each group) | 10,235 (39.6) | 24,756 (35.3) | 20,628 (31.0) | 9,887 (26.0) | 4,053 (23.9) | 1,410 (21.7) | 493 (21.0) | 174 (23.1) | 99 (24.4) |
| Women, n (% in total n = 367,559) | 4,893 (1.3) | 30,149 (8.2) | 71,182 (19.4) | 93,742 (25.5) | 79,712 (21.7) | 48,965 (13.3) | 24,028 (6.5) | 9,642 (2.6) | 5,246 (1.4) |
| Case of HBP, n (% in each group) | 2,230 (45.6) | 12,448 (41.3) | 25,185 (35.4) | 26,698 (28.5) | 19,280 (24.2) | 10,729 (21.9) | 4,886 (20.3) | 1,904 (19.8) | 1,009 (19.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakajima, K.; Igata, M.; Higuchi, R.; Tanaka, K.; Mizusawa, K.; Nakamura, T. Association of Serum High-Density Lipoprotein Cholesterol with High Blood Pressures at Checkup: Results of Kanagawa Investigation of Total Checkup Data from the National Database-9 (KITCHEN-9). J. Clin. Med. 2021, 10, 5118. https://doi.org/10.3390/jcm10215118
Nakajima K, Igata M, Higuchi R, Tanaka K, Mizusawa K, Nakamura T. Association of Serum High-Density Lipoprotein Cholesterol with High Blood Pressures at Checkup: Results of Kanagawa Investigation of Total Checkup Data from the National Database-9 (KITCHEN-9). Journal of Clinical Medicine. 2021; 10(21):5118. https://doi.org/10.3390/jcm10215118
Chicago/Turabian StyleNakajima, Kei, Manami Igata, Ryoko Higuchi, Kotone Tanaka, Kaori Mizusawa, and Teiji Nakamura. 2021. "Association of Serum High-Density Lipoprotein Cholesterol with High Blood Pressures at Checkup: Results of Kanagawa Investigation of Total Checkup Data from the National Database-9 (KITCHEN-9)" Journal of Clinical Medicine 10, no. 21: 5118. https://doi.org/10.3390/jcm10215118
APA StyleNakajima, K., Igata, M., Higuchi, R., Tanaka, K., Mizusawa, K., & Nakamura, T. (2021). Association of Serum High-Density Lipoprotein Cholesterol with High Blood Pressures at Checkup: Results of Kanagawa Investigation of Total Checkup Data from the National Database-9 (KITCHEN-9). Journal of Clinical Medicine, 10(21), 5118. https://doi.org/10.3390/jcm10215118






