Patterns of Thyroid Hormone Prescription in Patients with Bipolar or Schizoaffective Disorder: Findings from the LiSIE Retrospective Cohort Study
Abstract
1. Introduction
Aims
2. Materials and Methods
2.1. Study Design
2.2. Lithium—Study into Effects and Side Effects Participants
2.3. Patient Selection and Inclusion Criteria
2.4. Exclusion Criteria
2.5. Outcome Definition
2.5.1. Thyroid Status at Which Thyroid Hormone Replacement Therapy Was Started
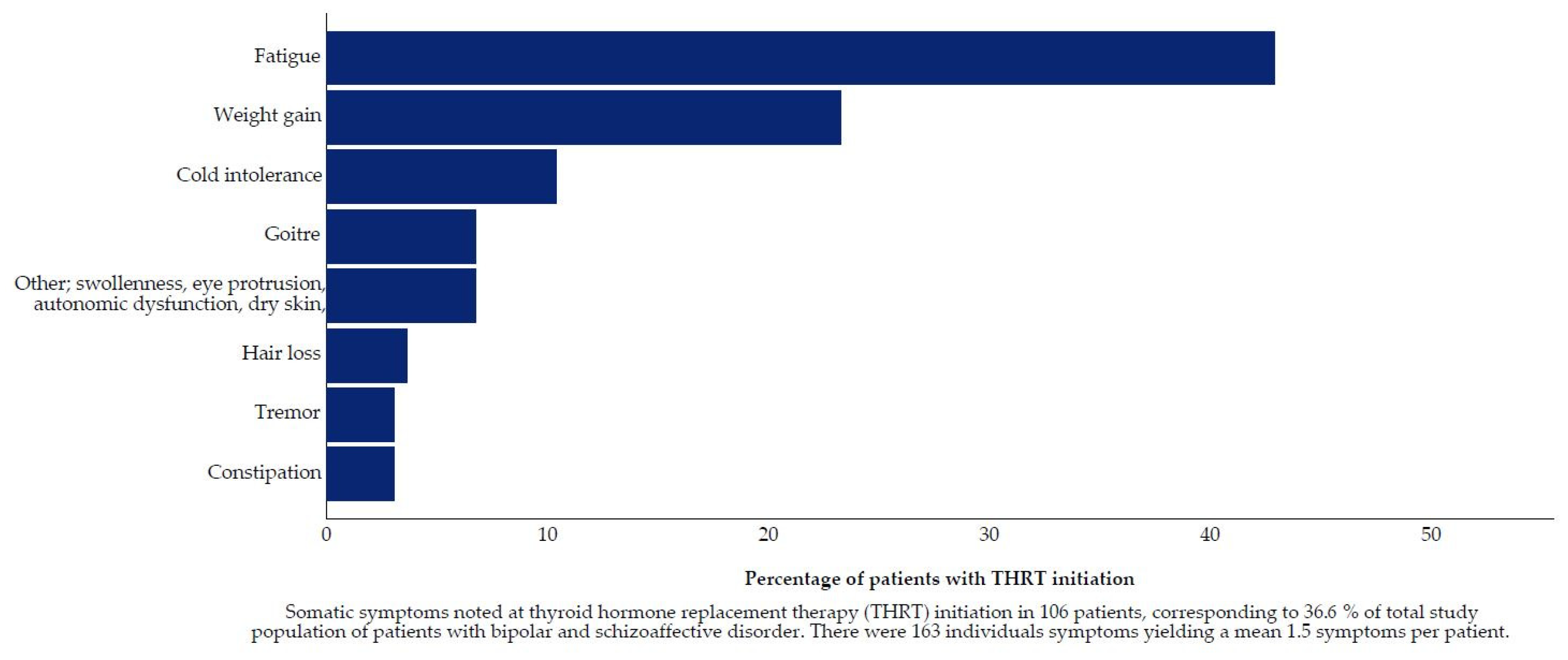
2.5.2. Reasons for Thyroid Hormone Replacement Therapy Initiation
2.5.3. TSH at Thyroid Hormone Replacement Therapy Initiation over Time
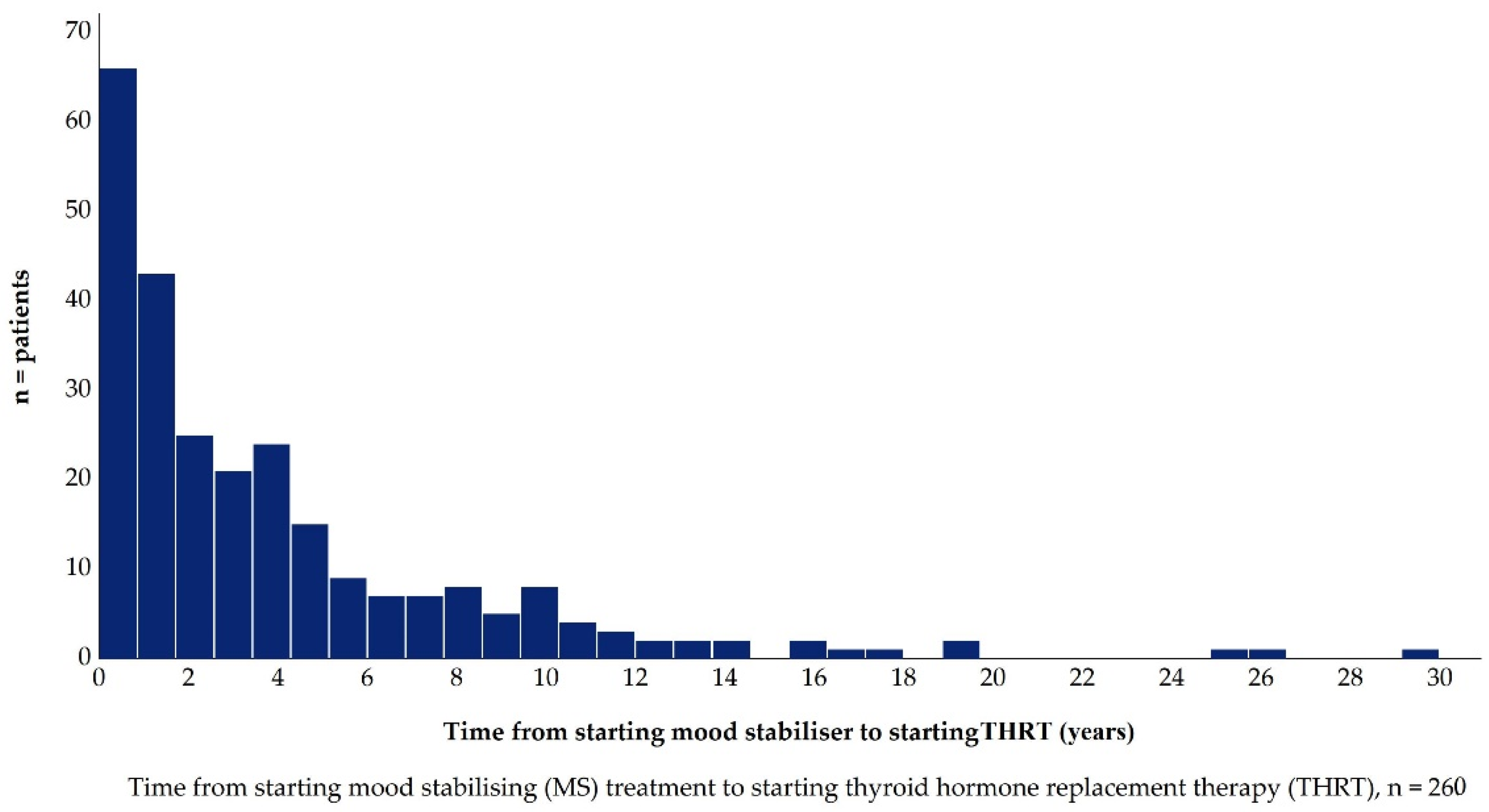
2.5.4. Time from Starting Mood Stabilisers to Starting Thyroid Hormone Replacement Therapy
2.6. Other Variable Definitions
2.6.1. Subtypes of Bipolar Disorder
2.6.2. Age
2.6.3. Thyroid Hormone Replacement Therapy Initiating Clinic
2.6.4. Mood Stabilisers
2.6.5. Mood Stabiliser Combination Therapy at Thyroid Hormone Replacement Therapy Initiation
2.6.6. Mood Stabiliser Treatment Stability
2.6.7. Other Psychotropic Medications
2.7. Validation of Data
2.8. Chart Review and Analysis
2.9. Control for Bias
2.10. Missing Data
2.11. Statistics
3. Results
3.1. Baseline Characteristics at Time of Thyroid Hormone Replacement Therapy Start
3.2. Hypothesis 1 (H1). In the Majority of Patients with Bipolar Disorder/Schizoaffective Disorder, Thyroid Hormone Replacement Therapy Is Prescribed for Only Mild or No Alterations of Thyroid Function Test and/or Unspecific Symptoms
Decision Drivers for Thyroid Hormone Replacement Therapy Initiation
3.3. Hypothesis 2 (H2). The TSH Concentration, at Which Thyroid Hormone Replacement Therapy Is Initiated, Has Decreased over Time
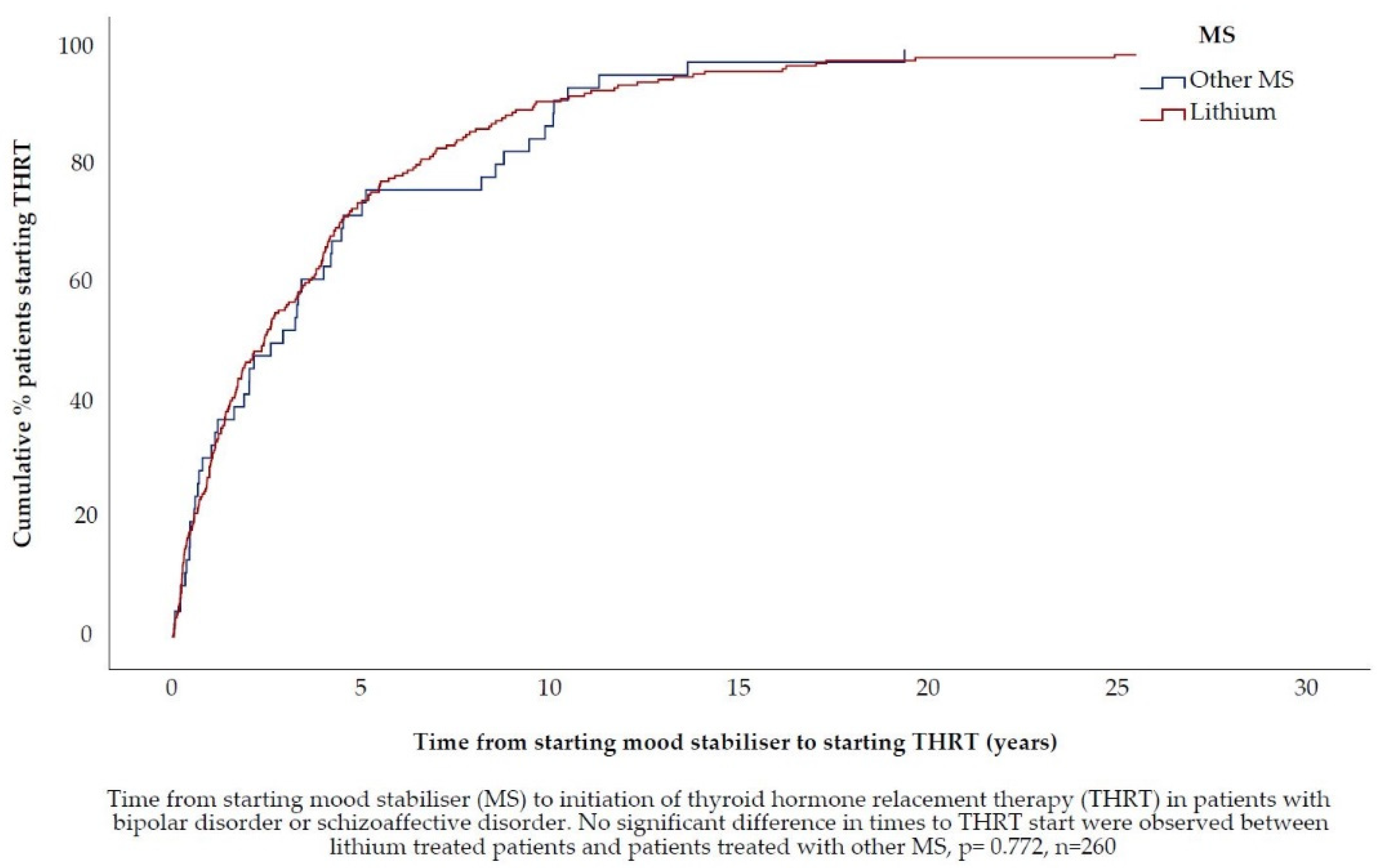
3.4. Hypothesis 3 (H3).In Patients Treated with Lithium, the TSH Concentration, at Which Thyroid Hormone Replacement Therapy Is Initiated, Is Lower
Mood Stabiliser Therapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fairbrother, F.; Petzl, N.; Scott, J.G.; Kisely, S. Lithium can cause hyperthyroidism as well as hypothyroidism: A systematic review of an under-recognised association. Aust. N. Z. J. Psychiatry 2019, 53, 384–402. [Google Scholar] [CrossRef]
- Hayes, J.F.; Marston, L.; Walters, K.; Geddes, J.R.; King, M.; Osborn, D.P.J. Adverse Renal, Endocrine, Hepatic, and Metabolic Events during Maintenance Mood Stabilizer Treatment for Bipolar Disorder: A Population-Based Cohort Study. PLoS Med. 2016, 13, e1002058. [Google Scholar] [CrossRef]
- Grandjean, E.M.; Aubry, J.-M. Lithium: Updated Human Knowledge Using an Evidence-Based Approach: Part III: Clinical safety. CNS Drugs 2009, 23, 397–418. [Google Scholar] [CrossRef]
- Lambert, C.G.; Mazurie, A.J.; Lauve, N.R.; Hurwitz, N.G.; Young, S.S.; Obenchain, R.L.; Hengartner, N.; Perkins, D.J.; Tohen, M.; Kerner, B. Hypothyroidism risk compared among nine common bipolar disorder therapies in a large US cohort. Bipolar Disord. 2016, 18, 247–260. [Google Scholar] [CrossRef]
- Bocchetta, A.; Traccis, F.; Mosca, E.; Serra, A.; Tamburini, G.; Loviselli, A. Bipolar disorder and antithyroid antibodies: Review and case series. Int. J. Bipolar Disord. 2016, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S. Thyroid Functions and Bipolar Affective Disorder. J. Thyroid. Res. 2011, 2011, 306367. [Google Scholar] [CrossRef]
- Fagiolini, A.; Kupfer, D.J.; Scott, J.; Swartz, H.A.; Cook, D.; Novick, D.M.; Frank, E. Hypothyroidism in patients with Bipolar I Disorder treated primarily with lithium. Epidemiol. Psichiatr. Soc. 2006, 15, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Glenn, T.; Pilhatsch, M.; Pfennig, A.; Whybrow, P.C. Gender differences in thyroid system function: Relevance to bipolar disorder and its treatment. Bipolar Disord. 2014, 16, 58–71. [Google Scholar] [CrossRef]
- Ozerdem, A.; Tunca, Z.; Cimrin, D.; Hıdıroğlu, C.; Ergor, G.; Çımrın, D. Female vulnerability for thyroid function abnormality in bipolar disorder: Role of lithium treatment. Bipolar Disord. 2014, 16, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Shim, I.H.; Woo, Y.S.; Bae, D.S.; Bahk, W.-M. Thyroid functioning in patients with bipolar disorder with mixed features. Psychiatry Res. 2015, 225, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.; Ayuso-Gutierrez, J.; Abril, A.; Ayuso-Mateos, J.L. Evaluation of thyroid function in lithium-naive bipolar patients. Eur. Psychiatry 1999, 14, 341–345. [Google Scholar] [CrossRef]
- Whybrow, P.C. Sex differences in thyroid axis function: Relevance to affective disorder and its treatment. Depression 1995, 3, 33–42. [Google Scholar] [CrossRef]
- Bauer, M.; Goetz, T.; Glenn, T.; Whybrow, P.C. The Thyroid-Brain Interaction in Thyroid Disorders and Mood Disorders. J. Neuroendocr. 2008, 20, 1101–1114. [Google Scholar] [CrossRef]
- Ezzaher, A.; Mouhamed, D.H.; Mechri, A.; Neffati, F.; Douki, W.; Gaha, L.; Najjar, M.F. Thyroid function and lipid profile in bipolar I patients. Asian J. Psychiatry 2011, 4, 139–143. [Google Scholar] [CrossRef]
- Frye, M.A.; Denicoff, K.D.; Bryan, A.L.; Smith-Jackson, E.E.; Ali, S.O.; Luckenbaugh, D.; Leverich, G.S.; Post, R.M. Association between Lower Serum Free T4 and Greater Mood Instability and Depression in Lithium-Maintained Bipolar Patients. Am. J. Psychiatry 1999, 156, 1909–1914. [Google Scholar] [PubMed]
- Amann, B.L.; Radua, J.; Wunsch, C.; König, B.; Simhandl, C. Psychiatric and physical comorbidities and their impact on the course of bipolar disorder: A prospective, naturalistic 4-year follow-up study. Bipolar Disord. 2017, 19, 225–234. [Google Scholar] [CrossRef]
- Kelly, T.; Lieberman, D.Z. The use of triiodothyronine as an augmentation agent in treatment-resistant bipolar II and bipolar disorder NOS. J. Affect. Disord. 2009, 116, 222–226. [Google Scholar] [CrossRef]
- Walshaw, P.D.; Gyulai, L.; Bauer, M.; Bauer, M.S.; Calimlim, B.; Sugar, C.; Whybrow, P.C. Adjunctive thyroid hormone treatment in rapid cycling bipolar disorder: A double-blind placebo-controlled trial of levothyroxine (L-T4) and triiodothyronine (T3). Bipolar Disord. 2018, 20, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Loh, H.H.; Lim, L.L.; Yee, A. Association between subclinical hypothyroidism and depression: An updated systematic review and meta-analysis. BMC Psychiatry 2019, 19, 12. [Google Scholar] [CrossRef]
- Kim, J.S.; Zhang, Y.; Chang, Y.; Ryu, S.; Guallar, E.; Shin, Y.-C.; Shin, H.; Lim, S.-W.; Cho, J. Subclinical Hypothyroidism and Incident Depression in Young and Middle-Age Adults. J. Clin. Endocrinol. Metab. 2018, 103, 1827–1833. [Google Scholar] [CrossRef]
- Hong, J.W.; Noh, J.H.; Kim, D.-J. Association between subclinical thyroid dysfunction and depressive symptoms in the Korean adult population: The 2014 Korea National Health and Nutrition Examination Survey. PLoS ONE 2018, 13, e0202258. [Google Scholar] [CrossRef]
- Rodriguez-Gutierrez, R.; Maraka, S.; Ospina, N.S.; Montori, V.; Brito, J.P. Levothyroxine overuse: Time for an about face? Lancet Diabetes Endocrinol. 2017, 5, 246–248. [Google Scholar] [CrossRef]
- Calissendorff, J. Hypotyreos—Folksjukdom som ofta överbehandlas. Lakartidningen 2019, 116, FL4C. [Google Scholar]
- Taylor, P.N.; Iqbal, A.; Minassian, C.; Sayers, A.; Draman, M.S.; Greenwood, R.; Hamilton, W.; Okosieme, O.; Panicker, V.; Thomas, S.L.; et al. Falling Threshold for Treatment of Borderline Elevated Thyrotropin Levels—Balancing Benefits and Risks: Evidence from a large community-based study. JAMA Intern. Med. 2014, 174, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Bekkering, G.; Agoritsas, T.; Lytvyn, L.; Heen, A.F.; Feller, M.; Moutzouri, E.; Abdulazeem, H.; Aertgeerts, B.; Beecher, D.; Brito, J.P.; et al. Thyroid hormones treatment for subclinical hypothyroidism: A clinical practice guideline. BMJ 2019, 365, l2006. [Google Scholar] [CrossRef] [PubMed]
- Brito, J.P.; Ross, J.S.; El Kawkgi, O.M.; Maraka, S.; Deng, Y.; Shah, N.D.; Lipska, K.J. Levothyroxine Use in the United States, 2008–2018. JAMA Intern. Med. 2021, 181, 1402. [Google Scholar] [CrossRef]
- Jonklaas, J.; Desale, S. Levothyroxine prescriptions trends may indicate a downtrend in prescribing. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820920551. [Google Scholar] [CrossRef]
- Feller, M.; Snel, M.; Moutzouri, E.; Bauer, D.C.; De Montmollin, M.; Aujesky, D.; Ford, I.; Gussekloo, J.; Kearney, P.; Mooijaart, S.; et al. Association of Thyroid Hormone Therapy With Quality of Life and Thyroid-Related Symptoms in Patients With Subclinical Hypothyroidism: A Systematic Review and Meta-analysis. JAMA 2018, 320, 1349–1359. [Google Scholar] [CrossRef]
- Lieber, I.; Ott, M.; Öhlund, L.; Lundqvist, R.; Eliasson, M.; Sandlund, M.; Werneke, U. Lithium-associated hypothyroidism and potential for reversibility after lithium discontinuation: Findings from the LiSIE retrospective cohort study. J. Psychopharmacol. 2020, 34, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Öhlund, L.; Ott, M.; Bergqvist, M.; Oja, S.; Lundqvist, R.; Sandlund, M.; Renberg, E.S.; Werneke, U. Clinical course and need for hospital admission after lithium discontinuation in patients with bipolar disorder type I or II: Mirror-image study based on the LiSIE retrospective cohort. BJPsych Open 2019, 5, e101. [Google Scholar] [CrossRef]
- Biondi, B.; Cappola, A.R.; Cooper, D.S. Subclinical Hypothyroidism: A Review. JAMA 2019, 322, 153–160. [Google Scholar] [CrossRef]
- Tsui, K.Y.Q. The impact of Lithium on thyroid function in Chinese psychiatric population. Thyroid. Res. 2015, 8, 14. [Google Scholar] [CrossRef][Green Version]
- Radhakrishnan, R.; Calvin, S.; Singh, J.K.; Thomas, B.; Srinivasan, K. Thyroid dysfunction in major psychiatric disorders in a hospital based sample. Indian J. Med. Res. 2013, 138, 888–893. [Google Scholar] [PubMed]
- Duval, F.; Morkani, M.-C.; Erb, A.; Danila, V.; Lopera, F.G.; Jeanjean, L. Dopaminergic, Noradrenergic, Adrenal, and Thyroid Abnormalities in Psychotic and Affective Disorders. Front. Psychiatry 2020, 11, 533872. [Google Scholar] [CrossRef] [PubMed]
- Öhlund, L. Factors Affecting the Pharmacological Treatment of Bipolar Disorder. Ph.D. Thesis, Umeå University, Umeå, Sweden, 2020; p. 113. [Google Scholar]
- Angst, J. Historical aspects of the dichotomy between manic—Depressive disorders and schizophrenia. Schizophr. Res. 2002, 57, 5–13. [Google Scholar] [CrossRef]
- Narayana, S.K.; Woods, D.R.; Boos, C.J. Management of amiodarone-related thyroid problems. Ther. Adv. Endocrinol. Metab. 2011, 2, 115–126. [Google Scholar] [CrossRef]
- Khalil, R.B.; Richa, S. Thyroid Adverse Effects of Psychotropic Drugs: A review. Clin. Neuropharmacol. 2011, 34, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Sauvage, M.-F.; Marquet, P.; Rousseau, A.; Raby, C.; Buxeraud, J.; Lachâtre, G. Relationship between Psychotropic Drugs and Thyroid Function: A Review. Toxicol. Appl. Pharmacol. 1998, 149, 127–135. [Google Scholar] [CrossRef]
- Van Melick, E.J.M.; Wilting, I.; Meinders, A.E.; Egberts, T.C.G. Prevalence and determinants of thyroid disorders in elderly patients with affective disorders: Lithium and nonlithium patients. Am. J. Geriatr. Psychiatry 2010, 18, 395–403. [Google Scholar] [CrossRef]
- Kraszewska, A.; Abramowicz, M.; Sowiński, J.; Rybakowski, J.K.; Chlopocka-Wozniak, M. A cross-sectional study of thyroid function in 66 patients with bipolar disorder receiving lithium for 10-44 years. Bipolar Disord. 2015, 17, 375–380. [Google Scholar] [CrossRef]
- Wysokiński, A.; Kłoszewska, I. Level of thyroid-stimulating hormone (TSH) in patients with acute schizophrenia, unipolar depression or bipolar disorder. Neurochem. Res. 2014, 39, 1245–1253. [Google Scholar] [CrossRef]
- Cremaschi, L.; Kardell, M.; Johansson, V.; Isgren, A.; Sellgren, C.M.; Altamura, A.C.; Hultman, C.M.; Landén, M. Prevalences of autoimmune diseases in schizophrenia, bipolar I and II disorder, and controls. Psychiatry Res. 2017, 258, 9–14. [Google Scholar] [CrossRef]
- Åsvold, B.O.; Vatten, L.J.; Bjøro, T. Changes in the prevalence of hypothyroidism: The HUNT Study in Norway. Eur. J. Endocrinol. 2013, 169, 613–620. [Google Scholar] [CrossRef]
- Somwaru, L.L.; Rariy, C.M.; Arnold, A.M.; Cappola, A.R. The Natural History of Subclinical Hypothyroidism in the Elderly: The Cardiovascular Health Study. J. Clin. Endocrinol. Metab. 2012, 97, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Hak, A.E.; Pols, H.A.; Visser, T.J.; Drexhage, H.A.; Hofman, A.; Witteman, J.C. Subclinical Hypothyroidism Is an Independent Risk Factor for Atherosclerosis and Myocardial Infarction in Elderly Women: The Rotterdam Study. Ann. Intern. Med. 2000, 132, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Duggal, J.; Molnar, J.; Maldonado, F.; Barsano, C.P.; Arora, R. Impact of subclinical thyroid disorders on coronary heart disease, cardiovascular and all-cause mortality: A meta-analysis. Int. J. Cardiol. 2008, 125, 41–48. [Google Scholar] [CrossRef]
- Rodondi, N.; Newman, A.B.; Vittinghoff, E.; De Rekeneire, N.; Satterfield, S.; Harris, T.B.; Bauer, D.C. Subclinical Hypothyroidism and the Risk of Heart Failure, Other Cardiovascular Events, and Death. Arch. Intern. Med. 2005, 165, 2460–2466. [Google Scholar] [CrossRef] [PubMed]
- Gencer, B.; Collet, T.-H.; Virgini, V.; Bauer, D.C.; Gussekloo, J.; Cappola, A.R.; Nanchen, D.; Elzen, W.D.; Balmer, P.; Luben, R.; et al. Subclinical Thyroid Dysfunction and the Risk of Heart Failure Events: An individual participant data analysis from 6 prospective cohorts. Circulation 2012, 126, 1040–1049. [Google Scholar] [CrossRef]
- Stott, D.J.; Rodondi, N.; Kearney, P.; Ford, I.; Westendorp, R.G.; Mooijaart, S.; Sattar, N.; Aubert, C.E.; Aujesky, D.; Bauer, D.C.; et al. Thyroid Hormone Therapy for Older Adults with Subclinical Hypothyroidism. N. Engl. J. Med. 2017, 376, 2534–2544. [Google Scholar] [CrossRef]
- Tost, M.; Monreal, J.A.; Armario, A.; Barbero, J.D.; Cobo, J.; Garcia-Rizo, C.; Bioque, M.; Usall, J.; Huerta-Ramos, E.; Soria, V.; et al. Targeting Hormones for Improving Cognition in Major Mood Disorders and Schizophrenia: Thyroid Hormones and Prolactin. Clin. Drug Investig. 2020, 40, 1–14. [Google Scholar] [CrossRef]
- Pilhatsch, M.; Stamm, T.J.; Stahl, P.; Lewitzka, U.; Berghöfer, A.; Sauer, C.; Gitlin, M.; Frye, M.A.; Whybrow, P.C.; Bauer, M. Treatment of bipolar depression with supraphysiologic doses of levothyroxine: A randomized, placebo-controlled study of comorbid anxiety symptoms. Int. J. Bipolar Disord. 2019, 7, 21. [Google Scholar] [CrossRef]
- Medici, B.B.; Nygaard, B.; la Cour, J.L.; Grand, M.K.; Siersma, V.; Nicolaisdottir, M.D.R.; Lind, B.; Olivarius, N.D.F.; Andersen, C.L. Changes in Prescription Routines for Treating Hypothyroidism Between 2001 and 2015: An Observational Study of 929,684 Primary Care Patients in Copenhagen. Thyroid 2019, 29, 910–919. [Google Scholar] [CrossRef]
- Shine, B.; McKnight, R.F.; Leaver, L.; Geddes, J.R. Long-term effects of lithium on renal, thyroid, and parathyroid function: A retrospective analysis of laboratory data. Lancet 2015, 386, 461–468. [Google Scholar] [CrossRef]
- McKnight, R.F.; Adida, M.; Budge, K.; Stockton, S.; Goodwin, G.M.; Geddes, J.R. Lithium toxicity profile: A systematic review and meta-analysis. Lancet 2012, 379, 721–728. [Google Scholar] [CrossRef]
- Bauer, M.; Glenn, T.; Alda, M.; Sagduyu, K.; Marsh, W.; Grof, P.; Munoz, R.; Severus, E.; Ritter, P.; Whybrow, P.C. Drug treatment patterns in bipolar disorder: Analysis of long-term self-reported data. Int. J. Bipolar Disord. 2013, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Haeberle, A.; Greil, W.; Russmann, S.; Grohmann, R. Mono- and combination drug therapies in hospitalized patients with bipolar depression. Data from the European drug surveillance program AMSP. BMC Psychiatry 2012, 12, 153. [Google Scholar] [CrossRef]
- Gau, C.-S.; Chang, C.-J.; Tsai, F.-J.; Chao, P.-F.; Gau, S.S.-F. Association between mood stabilizers and hypothyroidism in patients with bipolar disorders: A nested, matched case-control study. Bipolar Disord. 2010, 12, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Kraszewska, E.F.-R.; Rybakowski, J. The effect of including other psychotropic medications into a long-term bipolar disorder lithium treatment on thyroid function. Pharmacother. Psychiatry Neurol. 2019, 2, 111–119. [Google Scholar] [CrossRef]
- Vedal, T.S.J.; Steen, N.E.; Birkeland, K.I.; Dieset, I.; Reponen, E.J.; Laskemoen, J.F.; Rødevand, L.; Melle, I.; Andreassen, O.A.; Molden, E.; et al. Free thyroxine and thyroid-stimulating hormone in severe mental disorders: A naturalistic study with focus on antipsychotic medication. J. Psychiatr. Res. 2018, 106, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.N.; Razvi, S.; Pearce, S.H.; Dayan, C.M. Clinical review: A Review of the Clinical Consequences of Variation in Thyroid Function Within the Reference Range. J. Clin. Endocrinol. Metab. 2013, 98, 3562–3571. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.R.; Camacho, X.; Fischer, H.D.; Austin, P.; Anderson, G.M.; Rochon, P.; Lipscombe, L.L. Levothyroxine dose and risk of fractures in older adults: Nested case-control study. BMJ 2011, 342, d2238. [Google Scholar] [CrossRef]
- Flynn, R.W.; Bonellie, S.R.; Jung, R.T.; MacDonald, T.M.; Morris, A.D.; Leese, G.P. Serum Thyroid-Stimulating Hormone Concentration and Morbidity from Cardiovascular Disease and Fractures in Patients on Long-Term Thyroxine Therapy. J. Clin. Endocrinol. Metab. 2010, 95, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Collet, T.-H.; Gussekloo, J.; Bauer, D.C.; Elzen, W.D.; Cappola, A.R.; Balmer, P.; Iervasi, G.; Åsvold, B.O.; Sgarbi, J.; Völzke, H.; et al. Subclinical Hyperthyroidism and the Risk of Coronary Heart Disease and Mortality. Arch. Intern. Med. 2012, 172, 799–809. [Google Scholar] [CrossRef]
- Jonklaas, J.; Bianco, A.; Bauer, A.J.; Burman, K.D.; Cappola, A.R.; Celi, F.S.; Cooper, D.S.; Kim, B.W.; Peeters, R.P.; Rosenthal, M.S.; et al. Guidelines for the Treatment of Hypothyroidism: Prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid 2014, 24, 1670–1751. [Google Scholar] [CrossRef] [PubMed]
- Ng, F.; Mammen, O.K.; Wilting, I.; Sachs, G.S.; Ferrier, I.N.; Cassidy, F.; Beaulieu, S.; Yatham, L.N.; Berk, M. The International Society for Bipolar Disorders (ISBD) consensus guidelines for the safety monitoring of bipolar disorder treatments. Bipolar Disord. 2009, 11, 559–595. [Google Scholar] [CrossRef]
- Goodwin, G.; Haddad, P.; Ferrier, I.; Aronson, J.; Barnes, T.; Cipriani, A.; Coghill, D.; Fazel, S.; Geddes, J.; Grunze, H.; et al. Evidence-based guidelines for treating bipolar disorder: Revised third edition recommendations from the British Association for Psychopharmacology. J. Psychopharmacol. 2016, 30, 495–553. [Google Scholar] [CrossRef] [PubMed]
- Yatham, L.N.; Kennedy, S.H.; Parikh, S.V.; Schaffer, A.; Bond, D.; Frey, B.N.; Sharma, V.; Goldstein, B.; Rej, S.; Beaulieu, S.; et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018, 20, 97–170. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Bipolar Disorder: The NICE Guideline on the Assessment and Management of Bipolar Disorder in Adults, Children and Young People in Primary and Secondary Care; National Institute for Health and Care Excellence: London, UK, 2014. [Google Scholar]
- Svenska Psykiatriska Föreningen. Bipolär Sjukdom: Kliniska Riktlinjer för Utredning och Behandling. Available online: http://www.svenskpsykiatri.se/wp-content/uploads/2017/02/SPF-kliniska-riktlinjer-om-Bipol%C3%A4r-sjukdom.pdf (accessed on 27 April 2021).
- Pearce, S.; Brabant, G.; Duntas, L.; Monzani, F.; Peeters, R.P.; Razvi, S.; Wemeau, J.-L. 2013 ETA Guideline: Management of Subclinical Hypothyroidism. Eur. Thyroid. J. 2013, 2, 215–228. [Google Scholar] [CrossRef]





| Thyroid Status | Laboratory Values |
|---|---|
| Normal | TSH and fT4 within the normal reference interval |
| Hypothyroidism | |
| Overt | TSH elevated, fT4 lowered |
| Subclinical | |
| Grade 1 | TSH elevated, <10, fT4 normal |
| Grade 2 | TSH elevated, ≥10, fT4 normal |
| Low fT4 | TSH normal, fT4 lowered |
| Unclassified/other | TSH with unknown fT4, fT4 with unknown TSH, lowered TSH with normal fT4, or elevated fT4 with normal TSH but no formal diagnosis of hyperthyroidism/thyrotoxicosis. |
| Hypothesis 1 | Hypothesis 2 | Hypothesis 3 | ||
|---|---|---|---|---|
| n = 291 | n = 281 | n = 260 | ||
| Patients with lithium n = 214 | Patients with MS other than lithium n = 46 | |||
| Sex, n (%) | ||||
| Women | 209 (71.8) * | 200 (71.2) | 148 (69.2) | 34 (73.9) |
| Men | 82 (28.2) * | 81 (28.8) | 66 (30.8) | 12 (26.1) |
| Age (years) at THRT start, | ||||
| median (min, max, IQR) | 46.2 (19.5, 87.2, 22.2) | 46.8 (19.5, 87.2, 23.9) | 45.8 (19.5, 87.2, 19.5) | 45.7 (19.8, 70.1, 18.9) |
| Age at THRT start, n (%) | ||||
| <60 years | 245 (84.2) | 235 (83.6) | 177 (82.7) | 42 (91.3) |
| ≥60 years | 46 (15.8) | 46 (16.4) | 37 (17.3) | 4 (8.7) |
| Type of diagnosis, n (%) | ||||
| BD-I/SZD | 101 (34.7) | 100 (35.6) | 78 (36.4) | 15 (32.6) |
| BD-II/BD other | 190 (65.3) | 181 (64.4) | 136 (63.6) | 31 (67.4) |
| TSHTHRT available, n (%) | 282 (96.9) | 277 (98.6) | 213 (99.5) | 45 (97.8) |
| fT4THRT available, n (%) | 256 (88.0) | 251 (89.3) | 190 (88.8) | 43 (93.5) |
| MS at time of | ||||
| THRT initiation, n (%) | ||||
| Lithium | 214 (100.0) | 0 (0.0) *** | ||
| Carbamazepine | 5 (2.3) | 3 (6.5) | ||
| Lamotrigine | 30 (14.0) | 15 (32.6) * | ||
| Valproate | 36 (16.8) | 17 (37.0) ** | ||
| Aripiprazole | 3 (1.4) | 3 (6.5) | ||
| Olanzapine | 41 (19.2) | 10 (21.7) | ||
| Quetiapine | 29 (13.6) | 14 (30.4) * | ||
| Risperidone | 14 (6.5) | 6 (13.0) | ||
| MS combination at time of THRT initiation, n (%) | ||||
| Monotherapy | 94 (43.9) | 26 (56.5) | ||
| Combination therapy | 120 (56.1) | 20 (43.5) | ||
| Stability of MS treatment | ||||
| medication, n (%) | ||||
| Stable (≤1 changes) | 151 (70.6) | 34 (73.9) | ||
| Unstable (≥2 changes) | 63 (29.4) | 12 (26.1) | ||
| Time from MS start to THRT start | ||||
| Median (min, max, IQR) | 2.4 (0.04, 29.2, 4.2) | 2.8 (0.06, 19.7, 3.2) | ||
| Clinic initiating THRT, n (%) | ||||
| GP | 107 (36.8) | 104 (37.0) | 60 (28.0) | 29 (63.1) |
| Psychiatric clinic | 170 (58.4) | 164 (58.4) | 144 (67.3) | 15 (32.6) |
| Other | 14 (4.8) | 13 (4.6) | 10 (4.7) | 2 (4.3) |
| Other psychotropic | ||||
| drugs associated with | ||||
| hypothyroidism, n (%) | ||||
| Phenothiazines (derivates) | 16 (5.5) | 14 (5.0) | 9 (4.2) | 2 (4.3) |
| TCA | 9 (3.1) | 9 (3.2) | 6 (2.8) | 3 (6.5) |
| Thyroid Function, n (%) | Whole Sample | BD-I/SZD | BD-II/BD Other | p-Value |
|---|---|---|---|---|
| 291 (100) | 101 (34.7) | 190 (65.3) | ||
| Overt hypothyroidism | 76 (26.1) | 26 (25.7) | 50 (26.3) | 0.916 |
| Subclinical hypothyroidism, total | 118 (40.5) | 39 (38.6) | 79 (41.6) | 0.564 |
| Grade 1 | 99 (34.0) | 29 (28.7) | 70 (36.8) | 0.139 |
| Grade 2 | 19 (6.5) | 10 (9.9) | 9 (4.7) | 0.090 |
| Low fT4 | 18 (6.2) | 3 (3.0) | 15 (7.9) | 0.126 |
| Normal | 37 (12.7) | 13 (12.9) | 24 (12.6) | 0.945 |
| THRT as augmentation | 7 (2.4) | 1 (1.0) | 6 (3.2) | 0.428 |
| Unclassified/other | 35 (12.0) | 19 (18.8) | 16 (8.4) | 0.014 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lieber, I.; Ott, M.; Öhlund, L.; Lundqvist, R.; Eliasson, M.; Sandlund, M.; Werneke, U. Patterns of Thyroid Hormone Prescription in Patients with Bipolar or Schizoaffective Disorder: Findings from the LiSIE Retrospective Cohort Study. J. Clin. Med. 2021, 10, 5062. https://doi.org/10.3390/jcm10215062
Lieber I, Ott M, Öhlund L, Lundqvist R, Eliasson M, Sandlund M, Werneke U. Patterns of Thyroid Hormone Prescription in Patients with Bipolar or Schizoaffective Disorder: Findings from the LiSIE Retrospective Cohort Study. Journal of Clinical Medicine. 2021; 10(21):5062. https://doi.org/10.3390/jcm10215062
Chicago/Turabian StyleLieber, Ingrid, Michael Ott, Louise Öhlund, Robert Lundqvist, Mats Eliasson, Mikael Sandlund, and Ursula Werneke. 2021. "Patterns of Thyroid Hormone Prescription in Patients with Bipolar or Schizoaffective Disorder: Findings from the LiSIE Retrospective Cohort Study" Journal of Clinical Medicine 10, no. 21: 5062. https://doi.org/10.3390/jcm10215062
APA StyleLieber, I., Ott, M., Öhlund, L., Lundqvist, R., Eliasson, M., Sandlund, M., & Werneke, U. (2021). Patterns of Thyroid Hormone Prescription in Patients with Bipolar or Schizoaffective Disorder: Findings from the LiSIE Retrospective Cohort Study. Journal of Clinical Medicine, 10(21), 5062. https://doi.org/10.3390/jcm10215062







