Characterization of Mitochondrial Bioenergetics in Preeclampsia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Patient Recruitment and Sample Collection
2.3. Isolation of Intact Mitochondria from Human Placenta
2.4. Measurement of ETC Activity
2.5. Measurement of ETC Expression by Immunoblotting
2.6. Measurement of Respiration in Isolated Mitochondria
2.7. Measurement of Mitochondrial ROS Production in Isolated Mitochondria
2.8. Statistical Analysis
3. Results
3.1. Patient Demographics
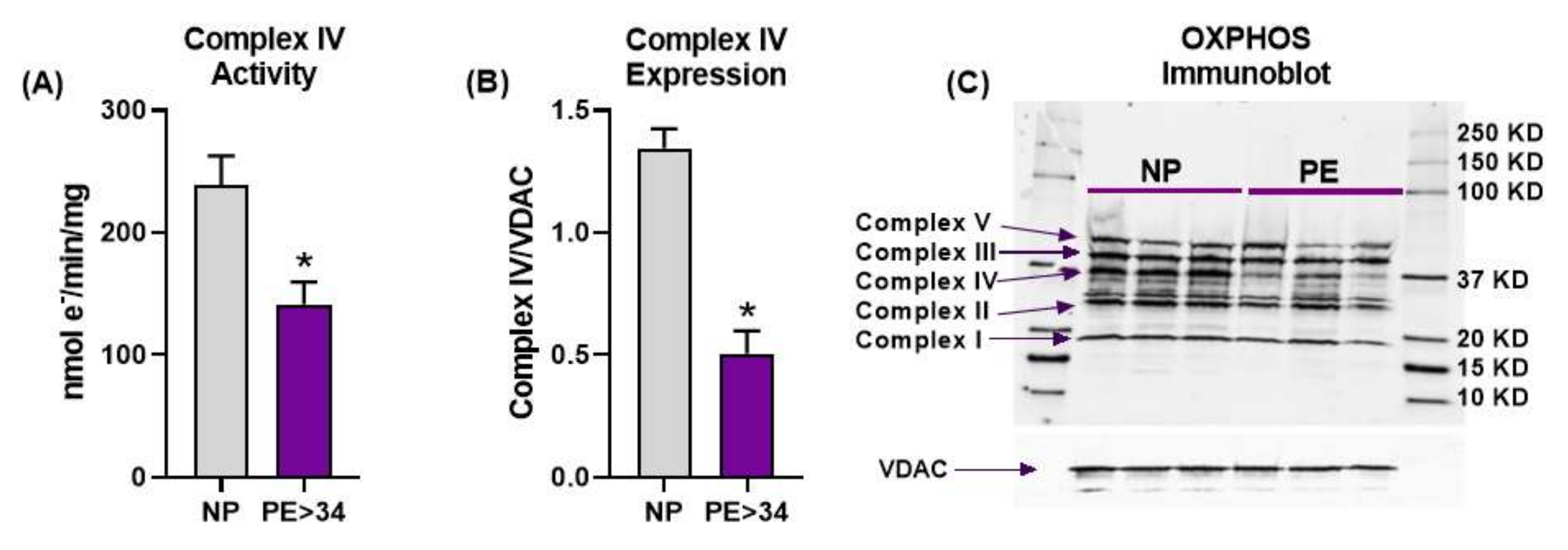
3.2. Placental Mitochondrial Electron Transport Chain Complex IV Activity and Expression Are Reduced in Preeclampsia
3.3. Mitochondrial Respiration Is Reduced in Preeclamptic Placental Mitochondria
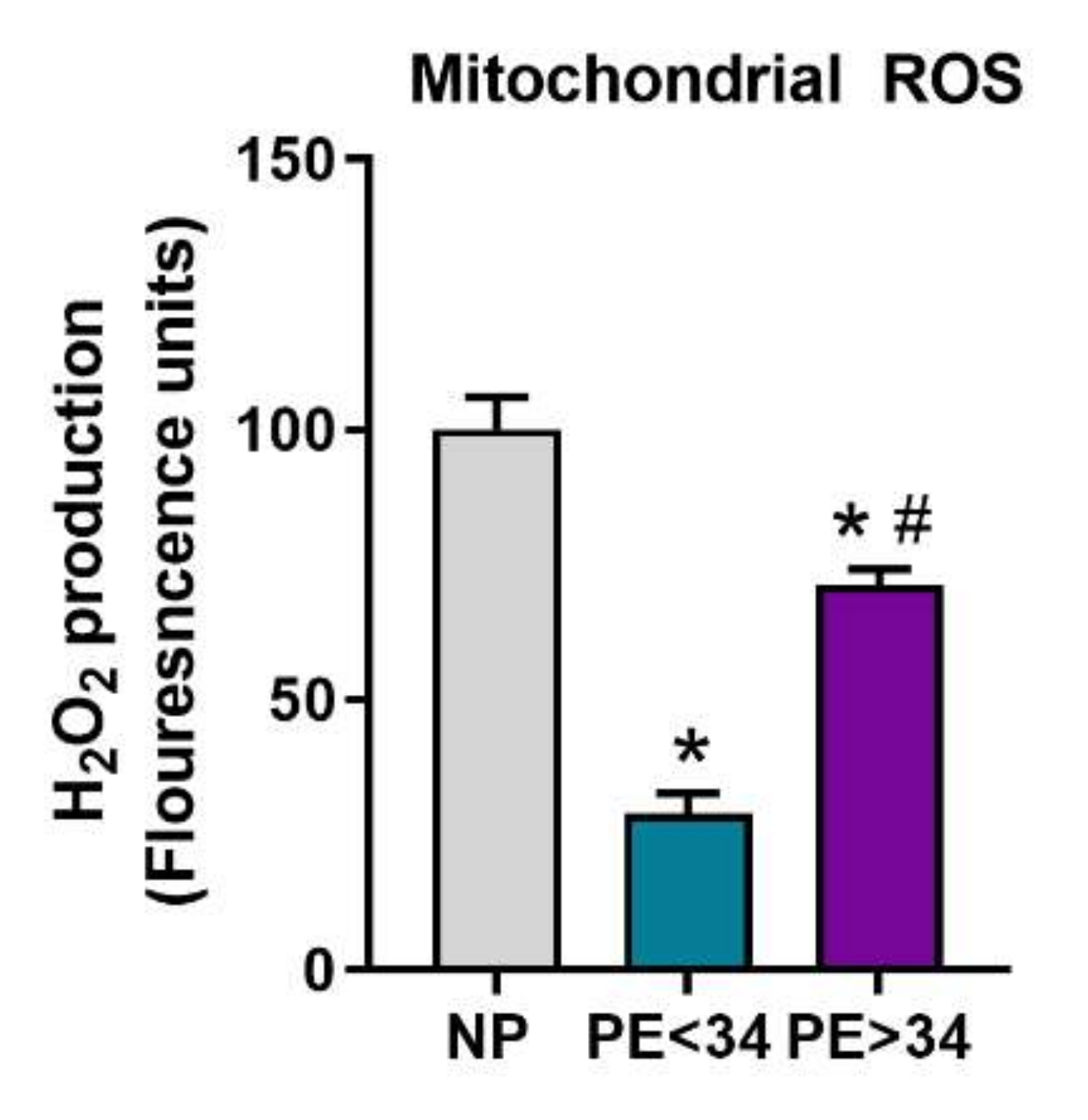
3.4. Mitochondrial ROS Is Reduced in Preeclamptic Placental Mitochondria
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| NP (n = 30) | PE < 34 (n = 10) | PE > 34 (n = 13) | ||||||
|---|---|---|---|---|---|---|---|---|
| Age (years) | 27.84 ± 0.87 | 25.80 ± 2.23 | 24.13 ± 1.18 * | |||||
| Ethnicity | ||||||||
| Caucasian | 2 | 6.7% | 1 | 10% | 2 | 13.3% | ||
| African American | 27 | 90% | 9 | 90.0% | 13 | 86.7% | ||
| other | 1 | 3.3% | ||||||
| BMI | 35.67 ± 1.64 | 34.96 ± 2.64 | 40.39 ± 2.73 | |||||
| Delivery: | ||||||||
| Gestational Age (Wks) | 38.51 ± 0.19 | 30.30 ± 0.81 * | 37.83 ± 0.44 + | |||||
| Platelets (1 × 103/mL) | 247 ± 29.83 | 217.8 ± 45.02 | 242.3 ± 9.15 | |||||
| Fetal Weight (g) | 3169 ± 96.9 | 1516 ± 108.1 * | 3120 ± 153.3 + | |||||
| Route of Delivery | ||||||||
| vaginal | 7 | 23.3% | 2 | 20% | 8 | 53.3% | ||
| cesarean | 23 | 76.7% | 8 | 80% | 7 | 46.7% | ||
| Fetal Gender | male | 14 | 46.7% | 6 | 60% | 6 | 40% | |
| female | 16 | 53.3% | 4 | 40% | 9 | 60% | ||
| Blood Pressures (mmHg) | ||||||||
| MAP at presentation | 86.4 ± 1.93 | 107.6 ± 3.34 * | 96.33 ± 3.74 *,+ | |||||
| MAP at delivery | 86.4 ± 1.93 | 107.6 ± 3.33 * | 96.33 ± 3.74 * | |||||
| Systolic presentation | 124.9 ± 2.7 | 155.8 ± 5.7 * | 140.7 ± 2.64 *,+ | |||||
| Systolic delivery | 118.1 ± 3.19 | 147.5 ± 3.55 * | 143.1 ± 5.17 * | |||||
| Diastolic presentation | 74.43 ± 1.62 | 94.9 ± 3.49 * | 86.15 ± 3.12 * | |||||
| Diastolic delivery | 71.5 ± 1.95 | 87.2 ± 3.43 * | 78.08 ± 2.84 | |||||
References
- American College of Obstetricians and Gynecolgists. Task Force on Hypertension in Pregnancy; American College of Obstetricians and Gynecolgists: Washington, DC, USA, 2013. [Google Scholar]
- Roberts, J.M.; Pearson, G.; Cutler, J.; Lindheimer, M. NHLBI Working Group on Research on Hypertension During Pregnancy. Summary of the NHLBI Working Group on Research on Hypertension During Pregnancy. Hypertension 2003, 41, 437–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sibai, B.; Dekker, G.; Kupferminc, M. Pre-eclampsia. Lancet 2005, 365, 785–799. [Google Scholar] [CrossRef]
- Sibai, B.M.; Caritis, S.; Hauth, J. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. What we have learned about preeclampsia. Semin. Perinatol. 2003, 27, 239–246. [Google Scholar] [CrossRef]
- Aouache, R.; Biquard, L.; Vaiman, D.; Miralles, F. Oxidative Stress in Preeclampsia and Placental Diseases. Int. J. Mol. Sci. 2018, 19, 1496. [Google Scholar] [CrossRef] [Green Version]
- Matsubara, K.; Higaki, T.; Matsubara, Y.; Nawa, A. Nitric Oxide and Reactive Oxygen Species in the Pathogenesis of Preeclampsia. Int. J. Mol. Sci. 2015, 16, 4600–4614. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.M.; Hubel, C.A. Oxidative stress in preeclampsia. Am. J. Obstet. Gynecol. 2004, 190, 1177–1178. [Google Scholar] [CrossRef]
- Sánchez-Aranguren, L.C.; Prada, C.E.; Riaño-Medina, C.E.; Lopez, M. Endothelial dysfunction and preeclampsia: Role of oxidative stress. Front. Physiol. 2014, 5, 372. [Google Scholar] [CrossRef] [Green Version]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [Green Version]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta (BBA)–Bioenerg. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Beyramzadeh, M.; Dikmen, Z.G.; Erturk, N.K.; Tuncer, Z.S.; Akbiyik, F. Placental respiratory chain complex activities in high risk pregnancies. J. Matern. Neonatal. Med. 2017, 30, 2911–2917. [Google Scholar] [CrossRef] [PubMed]
- Illsinger, S.; Janzen, N.; Sander, S.; Schmidt, K.-H.; Bednarczyk, J.; Mallunat, L.; Bode, J.; Hagebölling, F.; Hoy, L.; Lücke, T.; et al. Preeclampsia and HELLP Syndrome: Impaired Mitochondrial Function in Umbilical Endothelial Cells. Reprod. Sci. 2010, 17, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Mando, C.; Marino, M.A.; Miriam, F.; Palma, C.D.; Borelli, M.; Trabattoni, D.; Stampalija, T.; Ferrazzi, E.; Clementi, E.; Cetin, I. OS048 Mitochondrial content and function in placental cells and tissues of preeclampsia and IUGR. Pregnancy Hypertens. 2012, 2, 203. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, S.; Minakami, H.; Sato, I.; Saito, T. Decrease in cytochrome c oxidase activity detected cytochemically in the placental trophoblast of patients with pre-eclampsia. Placenta 1997, 18, 255–259. [Google Scholar] [CrossRef]
- Medrano Rodríguez, J.C.; Yahuaca Mendoza, P.; Presno Bernal, M.; Alvarado Acosta, J.L. Oxidative stress level and placental histological changes during preeclampsia. Ginecol. Obstet. Mex. 2008, 76, 319–326. [Google Scholar]
- Rani, N.; Dhingra, R.; Arya, D.S.; Kalaivani, M.; Bhatla, N.; Kumar, R. Role of oxidative stress markers and antioxidants in the placenta of preeclamptic patients. J. Obstet. Gynaecol. Res. 2010, 36, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Vishnyakova, P.A.; Volodina, M.A.; Tarasova, N.V.; Marey, M.V.; Kan, N.; Khodzhaeva, Z.S.; Vysokikh, M.Y.; Sukhikh, G.T. Alterations in antioxidant system, mitochondrial biogenesis and autophagy in preeclamptic myometrium. BBA Clin. 2017, 8, 35–42. [Google Scholar] [CrossRef]
- Zsengellér, Z.K.; Rajakumar, A.; Hunter, J.T.; Salahuddin, S.; Rana, S.; Stillman, I.E.; Ananth Karumanchi, S. Trophoblast mitochondrial function is impaired in preeclampsia and correlates negatively with the expression of soluble fms-like tyrosine kinase 1. Pregnancy Hypertens. 2016, 6, 313–319. [Google Scholar] [CrossRef]
- Vaka, V.R.; McMaster, K.M.; Cunningham, M.W., Jr.; Ibrahim, T.; Hazlewood, R.; Usry, N.; Cornelius, D.C.; Amaral, L.M.; LaMarca, B. Role of Mitochondrial Dysfunction and Reactive Oxygen Species in Mediating Hypertension in the Reduced Uterine Perfusion Pressure Rat Model of Preeclampsia. Hypertension 2018, 72, 703–711. [Google Scholar] [CrossRef]
- Deer, E.; Vaka, V.R.; McMaster, K.M.; Wallace, K.; Cornelius, D.C.; Amaral, L.M.; Cunningham, M.W.; LaMarca, B. Vascular endothelial mitochondrial oxidative stress in response to preeclampsia: A role for angiotension II type 1 autoantibodies. Am. J. Obstet. Gynecol. MFM 2021, 3, 100275. [Google Scholar] [CrossRef]
- McCarthy, C.M.; Kenny, L.C. Therapeutically targeting mitochondrial redox signalling alleviates endothelial dysfunction in preeclampsia. Sci. Rep. 2016, 6, 32683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Myatt, L.; Cui, X. Oxidative stress in the placenta. Histochem. Cell Biol. 2004, 122, 369–382. [Google Scholar] [CrossRef]
- Cui, X.L.; Brockman, D.; Campos, B.; Myatt, L. Expression of NADPH oxidase isoform 1 (Nox1) in human placenta: Involvement in preeclampsia. Placenta 2006, 27, 422–431. [Google Scholar] [CrossRef] [Green Version]
- Dechend, R.; Viedt, C.; Müller, D.N.; Ugele, B.; Brandes, R.P.; Wallukat, G.; Park, J.-K.; Janke, J.; Barta, P.; Theuer, J.; et al. AT 1 Receptor Agonistic Antibodies From Preeclamptic Patients Stimulate NADPH Oxidase. Circulation 2003, 107, 1632–1639. [Google Scholar] [CrossRef] [Green Version]
- Matsubara, S.; Sato, I. Enzyme histochemically detectable NAD(P)H oxidase in human placental trophoblasts: Normal, preeclamptic, and fetal growth restriction-complicated pregnancy. Histochem. Cell Biol. 2001, 116, 1–7. [Google Scholar] [CrossRef]
- Raijmakers, M.T.; Peters, W.H.; Steegers, E.A.; Poston, L. NAD(P)H oxidase associated superoxide production in human placenta from normotensive and pre-eclamptic women. Placenta 2004, 25, S85–S89. [Google Scholar] [CrossRef] [PubMed]
- Parrish, M.R.; Wallace, K.; Tam, K.B.T.; Herse, F.; Weimer, A.; Wenzel, K.; Wallukat, G.; Ray, L.F.; Arany, M.; Cockrell, K.; et al. Hypertension in response to AT1-AA: Role of reactive oxygen species in pregnancy-induced hypertension. Am. J. Hypertens. 2011, 24, 835–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dikalov, S.I.; Nazarewicz, R.R.; Bikineyeva, A.; Hilenski, L.; Lassègue, B.; Griendling, K.K.; Harrison, D.G.; Dikalova, A.E. Nox2-induced production of mitochondrial superoxide in angiotensin II-mediated endothelial oxidative stress and hypertension. Antioxid. Redox Signal. 2014, 20, 281–294. [Google Scholar] [CrossRef] [Green Version]
- Muralimanoharan, S.; Maloyan, A.; Mele, J.; Guo, C.; Myatt, L. MIR-210 modulates mitochondrial respiration in placenta with preeclampsia. Placenta 2012, 33, 816–823. [Google Scholar] [CrossRef] [Green Version]
- Salgado, S.S.; Salgado, M.K.R. Structural changes in pre-eclamptic and eclamptic placentas—An ultrastructural study. J. Coll. Physicians Surg. Pak. 2011, 21, 482–486. [Google Scholar]
- Shanklin, D.R.; Sibai, B.M. Ultrastructural aspects of preeclampsia. Am. J. Obstet. Gynecol. 1990, 163, 943–953. [Google Scholar] [CrossRef]
- Shi, Z.; Long, W.; Zhao, C.; Guo, X.; Shen, R.; Ding, H. Comparative Proteomics Analysis Suggests that Placental Mitochondria are Involved in the Development of Pre-Eclampsia. PLoS ONE 2013, 8, e64351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibata, E.; Nanri, H.; Ejima, K.; Araki, M.; Fukuda, J.; Yoshimura, K.; Toki, N.; Ikeda, M.; Kashimura, M. Enhancement of mitochondrial oxidative stress and up-regulation of antioxidant protein peroxiredoxin III/SP-22 in the mitochondria of human pre-eclamptic placentae. Placenta 2003, 24, 698–705. [Google Scholar] [CrossRef]
- Wang, Y.; Walsh, S. Placental mitochondria as a source of oxidative stress in pre-eclampsia. Placenta 1998, 19, 581–586. [Google Scholar] [CrossRef]
- He, L.; Wang, Z.; Sun, Y. Reduced amount of cytochrome c oxidase subunit I messenger RNA in placentas from pregnancies complicated by preeclampsia. Acta Obstet. Gynecol. Scand. 2004, 83, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Holland, O.J.; Cuffe, J.S.; Nitert, M.D.; Callaway, L.; Cheung, K.A.K.; Radenkovic, F.; Perkins, A.V. Placental mitochondrial adaptations in preeclampsia associated with progression to term delivery. Cell Death Dis. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandò, C.; De Palma, C.; Stampalija, T.; Anelli, G.M.; Figus, M.; Novielli, C.; Parisi, F.; Clementi, E.; Ferrazzi, E.; Cetin, I. Placental mitochondrial content and function in intrauterine growth restriction and preeclampsia. Am. J. Physiol. Metab. 2014, 306, E404–E413. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaka, R.; Deer, E.; Cunningham, M.; McMaster, K.M.; Wallace, K.; Cornelius, D.C.; Amaral, L.M.; LaMarca, B. Characterization of Mitochondrial Bioenergetics in Preeclampsia. J. Clin. Med. 2021, 10, 5063. https://doi.org/10.3390/jcm10215063
Vaka R, Deer E, Cunningham M, McMaster KM, Wallace K, Cornelius DC, Amaral LM, LaMarca B. Characterization of Mitochondrial Bioenergetics in Preeclampsia. Journal of Clinical Medicine. 2021; 10(21):5063. https://doi.org/10.3390/jcm10215063
Chicago/Turabian StyleVaka, Ramana, Evangeline Deer, Mark Cunningham, Kristen M. McMaster, Kedra Wallace, Denise C. Cornelius, Lorena M. Amaral, and Babbette LaMarca. 2021. "Characterization of Mitochondrial Bioenergetics in Preeclampsia" Journal of Clinical Medicine 10, no. 21: 5063. https://doi.org/10.3390/jcm10215063
APA StyleVaka, R., Deer, E., Cunningham, M., McMaster, K. M., Wallace, K., Cornelius, D. C., Amaral, L. M., & LaMarca, B. (2021). Characterization of Mitochondrial Bioenergetics in Preeclampsia. Journal of Clinical Medicine, 10(21), 5063. https://doi.org/10.3390/jcm10215063







