Preliminary Study with the Use of a Titanium Mesh as Space Maker and Implant Primary Stabilization for One-Stage Sinus Lift in Cases with Less Than 1.5 mm Residual Bone
Abstract
:1. Introduction
- −
- Inflammatory-infective processes, including recurrent or chronic maxillary sinusitis;
- −
- Naso-sinusally located specific systemic granulomatosis diseases;
- −
- Benign and malignant naso-sinus neoplasms involving the maxillary sinus;
- −
- Anatomic-structural impairments of the nasal walls and/or naso-sinus mucosa that may interfere with normal homeostatic naso-sinusal physiology (e.g., post-traumatic or post-surgical scars, sequelae of radiotherapy);
- −
- Favorable thickness: 1.5–2.0 mm;
- −
- Normal thickness: 0.8–1.49 mm, 2.01–2.99 mm;
- −
- Unfavorable thickness: <0.79 mm, >3 mm.
2. Materials and Methods
2.1. Inclusion Criteria
2.1.1. Subject Inclusion Criteria
- −
- Age greater than 18;
- −
- Single or multiple edentulous spaces in maxillary lateral area;
- −
- No contraindications for oral surgery related to systemic health;
- −
- Both patients are smokers and non-smokers;
2.1.2. Study Site Inclusion Criteria
- −
- Residual subantral native bone height of 1.5 mm or less at implant site (based on CBCT measurements);
2.2. Study Design
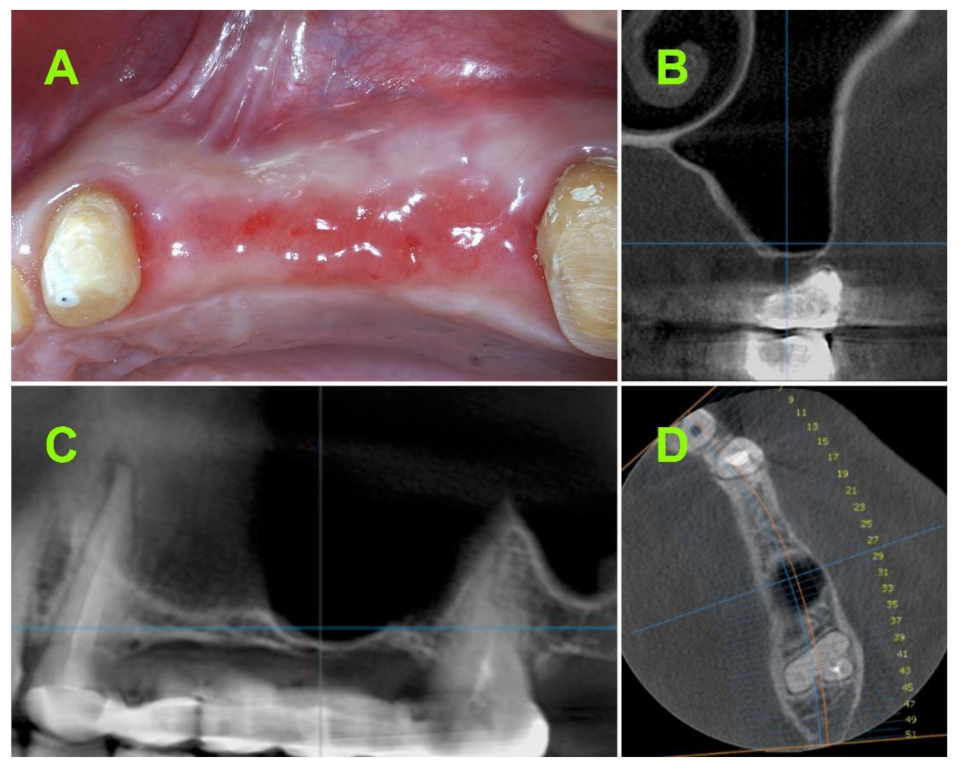
2.2.1. Pre-Operative CBCT Measurements
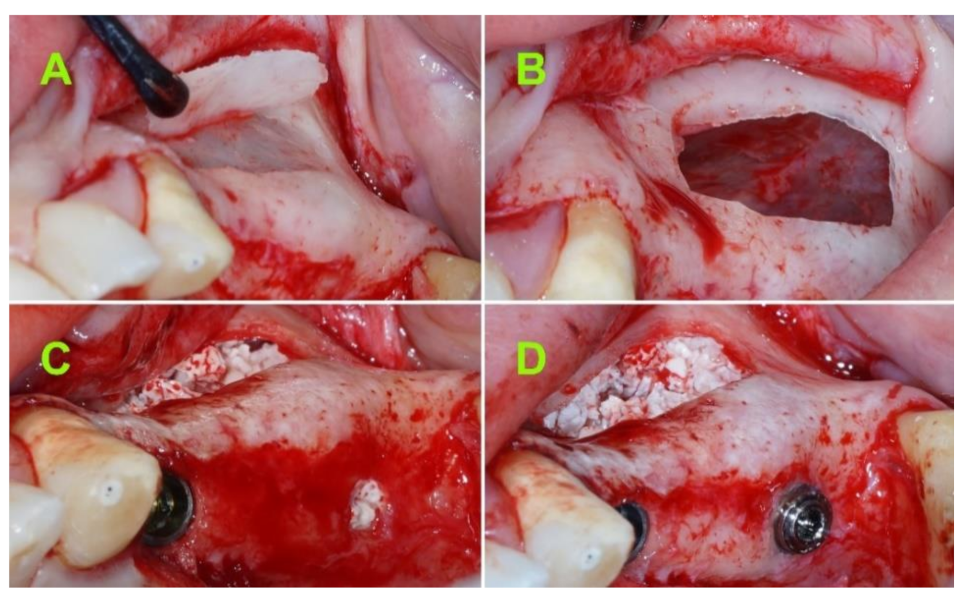
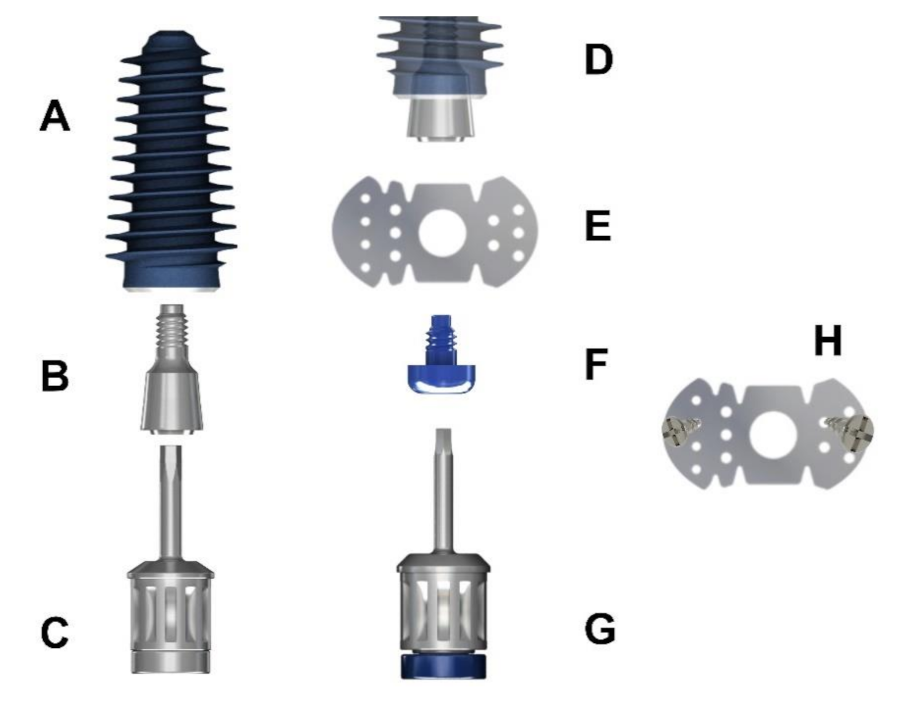
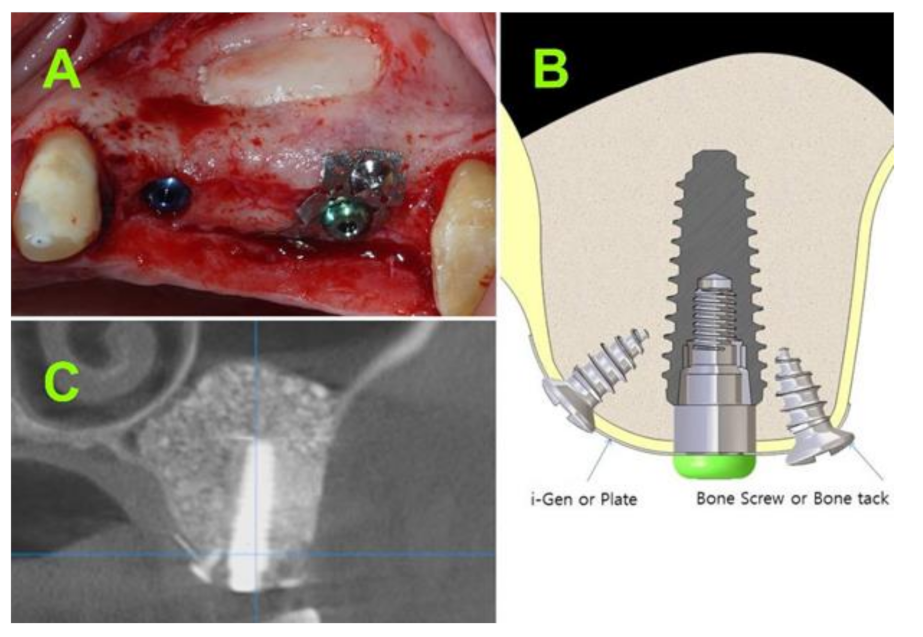
2.2.2. Surgical Techniques
2.2.3. Postsurgical Infection Control
2.2.4. Healing Abutment Phase
2.2.5. Bone Height Evaluation of Newly Regenerated Bone
2.2.6. Evaluation of 1-Year Bone Remodelling
2.2.7. Implant Stability Evaluation
2.2.8. Complications
2.2.9. Data Analysis
3. Results
3.1. Assessment of Bone Height at Implant Site
3.2. Evaluation of 1-Year Bone Remodelling
3.3. Implant Stability Evaluation
3.4. Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharan, A.; Madjar, D. Maxillary sinus pneumatization following extractions: A radiographic study. Int. J. Oral Maxillofac. Implant. 2008, 23, 48–56. [Google Scholar]
- Hamdy, R.M.; Abdel-Wahed, N. Three-dimensional linear and volumetric analysis of maxillary sinus pneumatization. J. Adv. Res. 2014, 5, 387–395. [Google Scholar] [CrossRef]
- Boyne, P.J.; James, R.A. Grafting of the maxillary sinus floor with autogenous marrow and bone. J. Oral Surg. 1980, 38, 613–616. [Google Scholar]
- Pignataro, L.; Mantovani, M.; Torretta, S.; Felisati, G.; Sambataro, G. ENT assessment in the integrated management of candidate for (maxillary) sinus lift. Acta Otorhinolaryngol. Ital. 2008, 28, 110–119. [Google Scholar] [PubMed]
- Ta Velli, L.; Borgonovo, A.E.; Re, D.; Maiorana, C. Sinus presurgical evaluation: A literature review and a new classification proposal. Minerva Stomatol. 2017, 66, 115–131. [Google Scholar]
- Savolainen, S.; Eskelin, M.; Jousimies-Somer, H.; Ylikoski, J. Radiological findings in the maxillary sinuses of symptomless young men. Acta Oto-Laryngol. 1997, 117, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.H.; Pan, W.L.; Chan, C.P.; Pan, Y.P.; Lin, C.Y.; Wang, Y.M.; Chang, C.C. Cone-beam computed tomography assessment of Schneiderian membranes: Non-infected and infected membranes, and membrane resolution following tooth extraction: A retrospective clinical trial. Biomed. J. 2019, 42, 328–334. [Google Scholar] [CrossRef]
- Van Den Munckhof, T.; Patel, S.; Koller, G.; Berkhout, E.; Mannocci, F.; Foschi, F. Schneiderian membrane thickness variation following endodontic procedures: A retrospective cone beam computed tomography study. BMC Oral Health 2020, 20, 133. [Google Scholar] [CrossRef]
- Misch, C.E. Maxillary sinus augmentation for endosteal implants: Organized alternative treatment plans. Int. J. Oral Implantol. 1987, 4, 49–58. [Google Scholar]
- Fugazzotto, P.A. Maxillary sinus grafting with and without simultaneous implant placement: Technical considerations and case reports. Int. J. Periodontics Restor. Dent. 1994, 14, 544–551. [Google Scholar]
- Lombardo, G.; Marincola, M.; Signoriello, A.; Corrocher, G.; Nocini, P.F. Single-crown, short and ultra-short implants, in association with simultaneous internal sinus lift in the atrophic posterior maxilla: A three-year retrospective study. Materials 2020, 13, 2208. [Google Scholar] [CrossRef]
- Peleg, M.; Garg, A.K.; Mazor, Z. Predictability of simultaneous implant placement in the severely atrophic posterior maxilla: A 9-year longitudinal experience study of 2132 implants placed into 731 human sinus grafts. J. Prosthet. Dent. 2007, 97, 24. [Google Scholar] [CrossRef]
- Felice, P.; Pistilli, R.; Piattelli, M.; Soardi, E.; Barausse, C.; Corvino, V.; Esposito, M. 1-stage versus 2-stage lateral sinus lift procedures: 1-year post-loading results of a multicentre randomised controlled trial. Eur. J. Oral Implantol. 2014, 7, 65–75. [Google Scholar]
- Chao, Y.; Chen, H.; Mei, C.; Tu, Y.; Lu, H. Meta-regression analysis of the initial bone height for predicting implant survival rates of two sinus elevation procedures. J. Clin. Periodontol. 2010, 37, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Filipov, I.; Bolognesi, F.; Chirila, L. ‘Butterfly’: A residual bone independent technique for one-stage sinus lift. BMJ Case Reports CP 2020, 13, e236245. [Google Scholar] [CrossRef]
- Cristache, C.M.; Burlibasa, M.; Tudor, I.; Totu, E.E.; Di Francesco, F.; Moraru, L. Accuracy, labor-time and patient-reported outcomes with partially versus fully digital workflow for flapless guided dental implants insertion—A randomized clinical trial with one-year follow-up. J. Clin. Med. 2021, 10, 1102. [Google Scholar] [CrossRef]
- Correia, F.; Pozza, D.H.; Gouveia, S.; Felino, A.C.; Faria-Almeida, R. Advantages of porcine xenograft over autograft in sinus lift: A randomised clinical trial. Materials 2021, 14, 3439. [Google Scholar] [CrossRef] [PubMed]
- Renouard, F.; Nisand, D. Impact of implant length and diameter on survival rates. Clin. Oral Implants Res. 2006, 17, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Hadzik, J.; Kubasiewicz-Ross, P.; Nawrot-Hadzik, I.; Gedrange, T.; Pitułaj, A.; Dominiak, M. Short (6 mm) and regular dental implants in the posterior maxilla–7-years follow-up study. J. Clin. Med. 2021, 10, 940. [Google Scholar] [CrossRef]
- Clelland, N.; Chaudhry, J.; Rashid, R.; McGlumphy, E. Split-mouth comparison of splinted and nonsplinted prostheses on short implants: 3-year results. Int. J. Oral Maxillofac. Implant. 2016, 31, 1135–1141. [Google Scholar] [CrossRef] [Green Version]
- Jensen, O.T. The Sinus Bone Graft; Quintessence Publishing: Chicago, IL, USA, 2019. [Google Scholar]
- Summers, R.B. The osteotome technique: Part 4—Future site development. Compend. Contin. Educ. Dent. 1995, 16, 1090–1092. [Google Scholar]
- Raghoebar, G.M.; Onclin, P.; Boven, G.C.; Vissink, A.; Meijer, H.J.A. Long-term effectiveness of maxillary sinus floor augmentation: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 307–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smiler, D.G.; Johnson, P.W.; Lozada, J.L.; Misch, C.; Rosenlicht, J.L.; Tatum, O.H.; Wagner, J.R. Sinus lift grafts and endosseous implants. Treatment of the atrophic posterior maxilla. Dent. Clin. North. Am. 1992, 36, 151–186. [Google Scholar] [PubMed]
- Rancitelli, D.; Borgonovo, A.E.; Cicciù, M.; Re, D.; Rizza, F.; Frigo, A.C.; Maiorana, C. Maxillary sinus septa and anatomic correlation with the Schneiderian membrane. J. Craniofac. Surg. 2015, 26, 1394–1398. [Google Scholar] [CrossRef] [PubMed]
- Elangovan, S. Dental implant survival in the bone augmented by direct sinus lift is comparable to implants placed in the native bone. J. Evid. Based. Dent. Pract. 2020, 20, 101410. [Google Scholar] [CrossRef]
- Tatum, H. Maxillary and sinus implant reconstructions. Dent. Clin. North. Am. 1986, 30, 207–229. [Google Scholar]
- Jensen, O.T.; Shulman, L.B.; Block, M.S.; Iacono, V.J. Report of the Sinus Consensus Conference of 1996. Int. J. Oral Maxillofac. Implants 1998, 13, 11–45. [Google Scholar] [PubMed]
- Cosci, F.; Luccioli, M. A new sinus lift technique in conjunction with placement of 265 implants. Implant. Dent. 2000, 9, 363–368. [Google Scholar] [CrossRef]
- Canullo, L. Sinus lift surgery in severely resorbed maxillae: One-year follow-up. J. Oral Sci. Rehabil. 2018, 4, 8–14. [Google Scholar]
- Cha, H.S.; Kim, A.; Nowzari, H.; Chang, H.S.; Ahn, K.M. Simultaneous sinus lift and implant installation: Prospective study of consecutive two hundred seventeen sinus lift and four hundred sixty-two implants. Clin. Implant. Dent. Relat. Res. 2014, 16, 337–347. [Google Scholar] [CrossRef]
- Felice, P.; Pistilli, R.; Piattelli, M.; Soardi, E.; Pellegrino, G.; Corvino, V.; Esposito, M. 1-stage versus 2-stage lateral maxillary sinus lift procedures: 4-month post-loading results of a multicenter randomised controlled trial. Eur. J. Oral Implantol. 2013, 6, 153–165. [Google Scholar] [PubMed]
- Chin, M. Surgical Design for Dental Reconstruction with Implants: A New Paradigm, 1st ed.; Quintessence Publishing: Chicago, IL, USA, 2015. [Google Scholar]
- Yoda, N.; Zheng, K.; Chen, J.; Li, W.; Swain, M.; Sasaki, K.; Li, Q. Bone morphological effects on post-implantation remodeling of maxillary anterior buccal bone: A clinical and biomechanical study. J. Prosthodont. Res. 2017, 61, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Lindorf, H.; Muller-Herzog, R. Der autologe Sinus-Implantat-Stabilisator (ASIS). Z. Zahnheilkd. Manag. Kult. 2004, 181–189. [Google Scholar]
- Vollmer, R.; Vollmer, M.; Valentin, R.; Heineman, F. Sinus elevation and single-stage surgical implant placement with a titanium osteosynthesis bar. Pract. Proced. Aesthet. Dent. 2002, 14, 307–311. [Google Scholar] [PubMed]
- Grandi, C.; Pacifici, L. Sinus implants stabilization in Misch IV Class by means of S.I.S. device: A clinical study. Oral Implantol. 2009, 2, 2. [Google Scholar]
- Albrektsson, T.; Zarb, G.; Worthington, P.; Eriksson, A.R. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int. J. Oral Maxillofac. Implant. 1986, 1, 11–25. [Google Scholar]
- Riben, C.; Thor, A. The maxillary sinus membrane elevation procedure: Augmentation of bone around dental implants without grafts—A review of a surgical technique. Int. J. Dent. 2012, 2012, 105483. [Google Scholar] [CrossRef]
- Song, D.-S.; Kim, C.-H.; Kim, B.-J.; Kim, J.-H. Tenting effect of dental implant on maxillary sinus lift without grafting. J. Dent. Sci. 2020, 15, 278–285. [Google Scholar] [CrossRef]
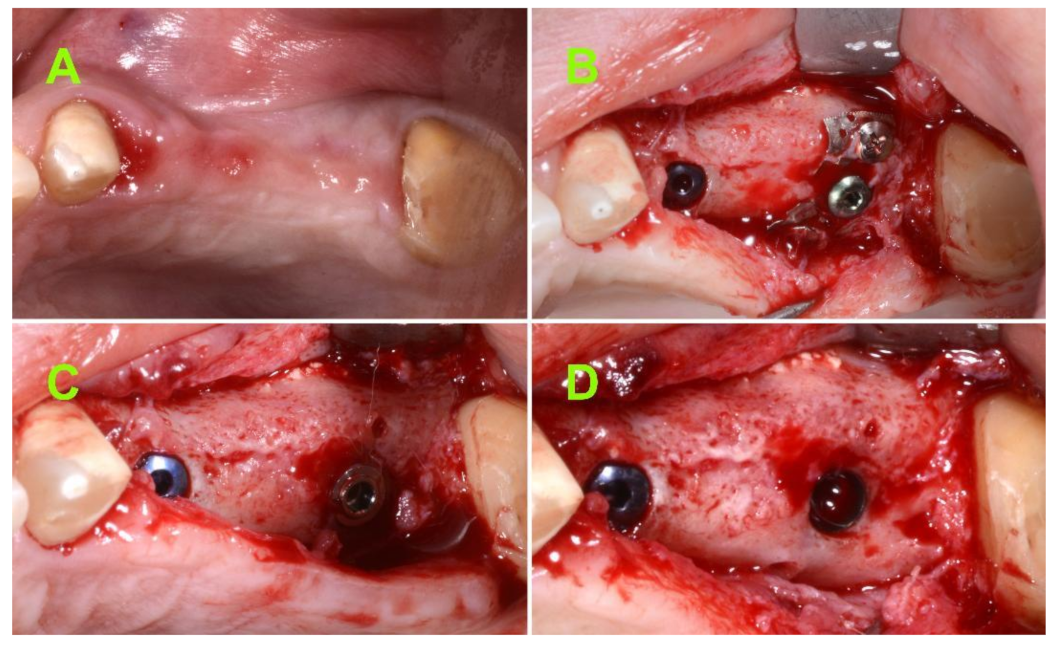
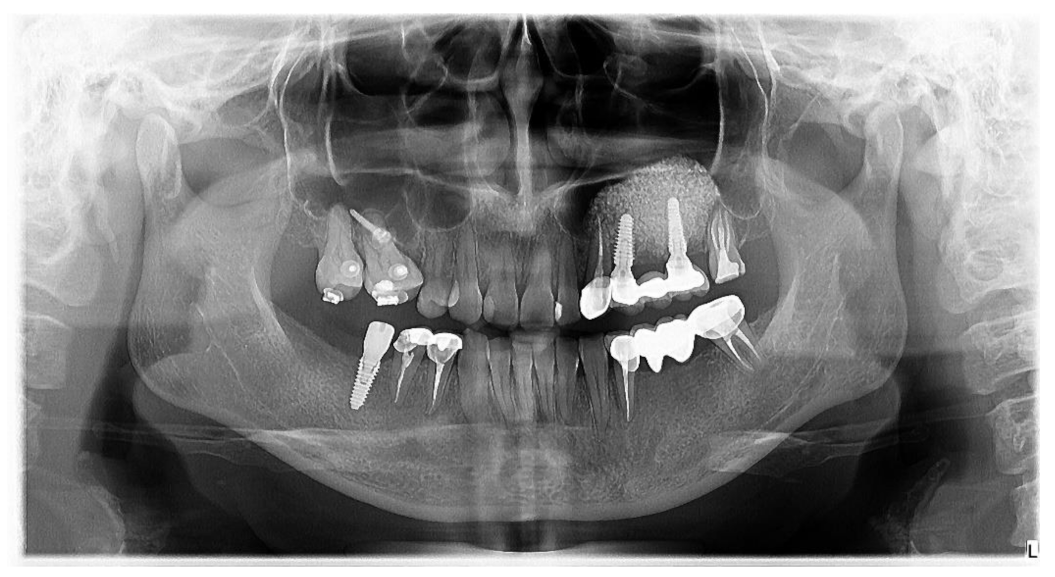
| Preoperative Measurements (on CBCT) | Postoperative 1st Measurement | Postoperative 2nd Measurement | Postoperative Mean Values (±SD) | |
|---|---|---|---|---|
| 1 | <0.5 | 14 | 15 | 14.5 (±0.71) |
| 2 | 0.5–1 | 16 | 16.5 | 16.2 (±0.35) |
| 3 | 0.5–1 | 16 | 15 | 15.5 (±0.71) |
| 4 | <0.5 | 13 | 11 | 12 (±1.41) |
| 5 | 1–1.5 | 17 | 17 | 17 (±0) |
| 6 | <0.5 | 12 | 13 | 12.5 (±0.71) |
| 7 | 0.5–1 | 17 | 16 | 16.5 (±0.71) |
| 8 | 0.5–1 | 13 | 14 | 13.5 (±0.71) |
| 9 | <0.5 | 18 | 18 | 18 (±0) |
| 10 | <0.5 | 13 | 12 | 12.5 (±0.71) |
| 11 | 1–1.5 | 15 | 14 | 14.5 (±0.71) |
| 12 | <0.5 | 13 | 14 | 13.5 (±0.71) |
| 13 | <0.5 | 11 | 12 | 11.5 (±0.71) |
| 14 | 0.5–1 | 13 | 13 | 13 (±0) |
| 15 | 1–1.5 | 16 | 17 | 16.5 (±0.71) |
| Mean Bone Level at Loading (±SD) | Mean Bone Level at 1-Year Follow-Up (±SD) | Mean 1-Year Marginal Bone Loss | |
|---|---|---|---|
| 1 | 13.48 (±0.36) | 13.16 (±0.18) | 0.32 |
| 2 | 13.71 (±0.45) | 12.75 (±0.32) | 0.97 |
| 3 | 14.41 (±0.05) | 13.34 (±0.14) | 1.07 |
| 4 | 10.95 (±0.62) | 10.84 (±0.06) | 0.11 |
| 5 | 14.60 (±0.38) | 13.39 (±0.40) | 1.21 |
| 6 | 12.18 (±0.30) | 11.25 (±0.36) | 0.93 |
| 7 | 13.73 (±0.55) | 12.45 (±0.33) | 1.28 |
| 8 | 12.90 (±0.08) | 10.90 (±0.09) | 2.00 |
| 9 | 14.56 (±0.47) | 13.33 (±0.17) | 1.23 |
| 10 | 12.14 (±0.71) | 11.36 (±0.02) | 0.78 |
| 11 | 14.38 (±0.06) | 12.88 (±0.13) | 1.50 |
| 12 | 12.64 (±0.12) | 11.71 (±0.20) | 0.93 |
| 13 | 10.80 (±0.60) | 10.23 (±0.06) | 0.57 |
| 14 | 12.44 (±0.06) | 11.64 (±0.33) | 0.80 |
| 15 | 15.35 (±0.31) | 14.97 (±0.14) | 0.38 |
| Mesio-Distal ISQ Measurement | Labio-Palatal ISQ Measurement | Mean ISQ Values (±SD) | |
|---|---|---|---|
| 1 | 73 | 71 | 72 (±1.41) |
| 2 | 76 | 74 | 75 (±1.41) |
| 3 | 68 | 71 | 69.5 (±2.12) |
| 4 | 70 | 73 | 71.5 (±2.12) |
| 5 | 73 | 72 | 72.5 (±0.71) |
| 6 | 69 | 68 | 68.5 (±0.71) |
| 7 | 71 | 71 | 71 (±0) |
| 8 | 72 | 69 | 70.5 (±2.12) |
| 9 | 74 | 76 | 75 (±1.41) |
| 10 | 76 | 75 | 75.5 (±0.71) |
| 11 | 70 | 68 | 69 (±1.41) |
| 12 | 71 | 72 | 71.5 (±0.71) |
| 13 | 69 | 72 | 70.5 (±2.12) |
| 14 | 68 | 65 | 66.5 (±2.12) |
| 15 | 70 | 74 | 72 (±2.83) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filipov, I.; Bolognesi, F.; Chirila, L.; Cristache, C.M.; Corinaldesi, G.; Park, K.B. Preliminary Study with the Use of a Titanium Mesh as Space Maker and Implant Primary Stabilization for One-Stage Sinus Lift in Cases with Less Than 1.5 mm Residual Bone. J. Clin. Med. 2021, 10, 4853. https://doi.org/10.3390/jcm10214853
Filipov I, Bolognesi F, Chirila L, Cristache CM, Corinaldesi G, Park KB. Preliminary Study with the Use of a Titanium Mesh as Space Maker and Implant Primary Stabilization for One-Stage Sinus Lift in Cases with Less Than 1.5 mm Residual Bone. Journal of Clinical Medicine. 2021; 10(21):4853. https://doi.org/10.3390/jcm10214853
Chicago/Turabian StyleFilipov, Iulian, Federico Bolognesi, Lucian Chirila, Corina Marilena Cristache, Giuseppe Corinaldesi, and Kwang Bum Park. 2021. "Preliminary Study with the Use of a Titanium Mesh as Space Maker and Implant Primary Stabilization for One-Stage Sinus Lift in Cases with Less Than 1.5 mm Residual Bone" Journal of Clinical Medicine 10, no. 21: 4853. https://doi.org/10.3390/jcm10214853
APA StyleFilipov, I., Bolognesi, F., Chirila, L., Cristache, C. M., Corinaldesi, G., & Park, K. B. (2021). Preliminary Study with the Use of a Titanium Mesh as Space Maker and Implant Primary Stabilization for One-Stage Sinus Lift in Cases with Less Than 1.5 mm Residual Bone. Journal of Clinical Medicine, 10(21), 4853. https://doi.org/10.3390/jcm10214853







