Progressive Comparison of Density Assessment of Alveolar Bone Graft in Patients with Unilateral and Bilateral Cleft
Abstract
1. Introduction
2. Patients and Methods
2.1. Patient Information and Data Collection
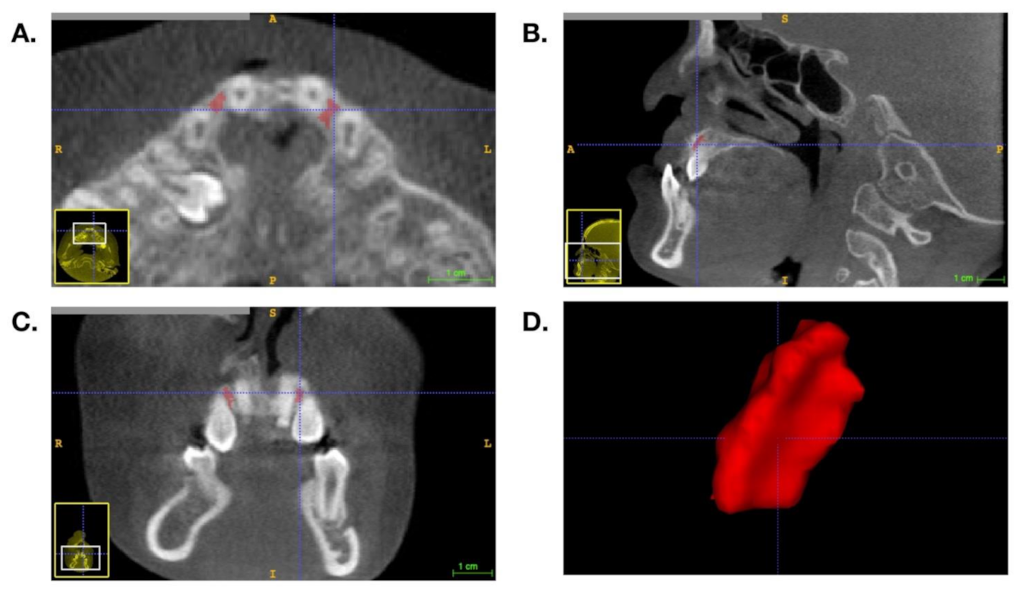
2.2. BMD Measurement and Volumetric Analysis
2.3. Statistical Analysis
3. Results
3.1. Subject Characteristics
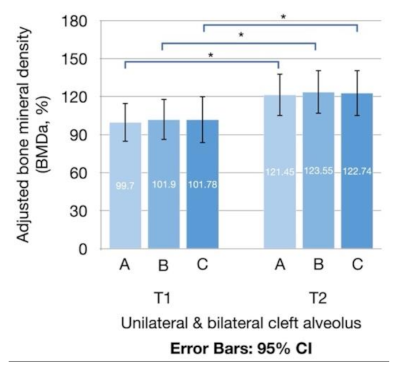
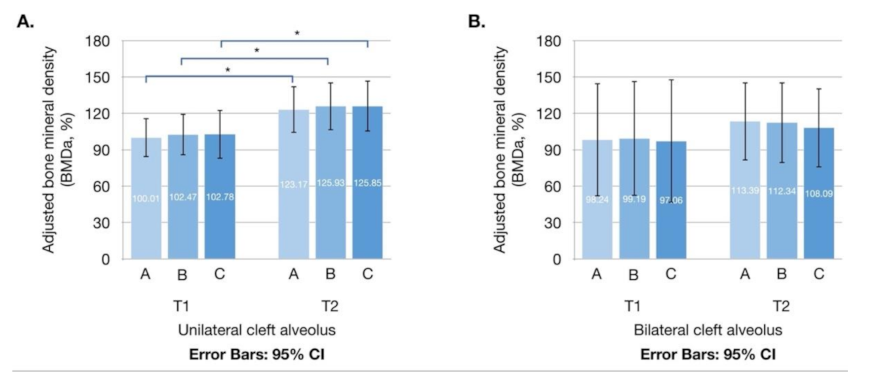
3.2. Adjusted BMD and Density Enhancement Rate
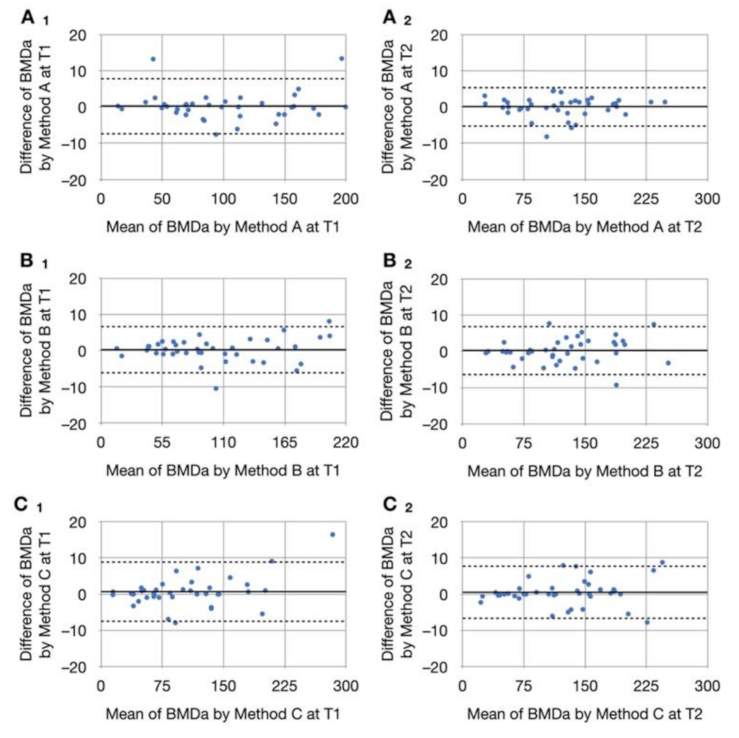
3.3. Intrarater Reliability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bajaj, A.K.; Wongworawat, A.A.; Punjabi, A. Management of alveolar clefts. J. Craniofac. Surg. 2003, 14, 840–846. [Google Scholar] [CrossRef]
- Lonic, D.; Yamaguchi, K.; Chien-Jung Pai, B.; Lo, L.J. Reinforcing the Mucoperiosteal Pocket with the Scarpa Fascia Graft in Secondary Alveolar Bone Grafting: A Retrospective Controlled Outcome Study. Plast. Reconstr. Surg. 2017, 140, 568e–578e. [Google Scholar] [CrossRef] [PubMed]
- Semb, G. Alveolar bone grafting. Front. Oral Biol. 2012, 16, 124–136. [Google Scholar] [CrossRef]
- Chou, P.Y.; Denadai, R.; Hallac, R.R.; Dumrongwongsiri, S.; Hsieh, W.C.; Pai, B.C.; Lo, L.J. Comparative Volume Analysis of Alveolar Defects by 3D Simulation. J. Clin. Med. 2019, 8, 1401. [Google Scholar] [CrossRef] [PubMed]
- Coots, B.K. Alveolar bone grafting: Past, present, and new horizons. Semin. Plast. Surg. 2012, 26, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Jeong, W.; Yeo, H.; Choi, J.; Kim, J.; Son, D.; Oh, S.; Kim, C. Long-term results of secondary alveolar bone grafting using a technique to harvest pure calvarial cancellous bone: Evaluation based on plain radiography and computed tomography. J. Plast. Reconstr. Aesthet. Surg. 2017, 70, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Weissler, E.H.; Paine, K.M.; Ahmed, M.K.; Taub, P.J. Alveolar Bone Grafting and Cleft Lip and Palate: A Review. Plast. Reconstr. Surg. 2016, 138, 1287–1295. [Google Scholar] [CrossRef]
- De Mulder, D.; Cadenas de Llano-Pérula, M.; Jacobs, R.; Verdonck, A.; Willems, G. Three-dimensional radiological evaluation of secondary alveolar bone grafting in cleft lip and palate patients: A systematic review. Dentomaxillofac. Radiol. 2018, 48, 20180047. [Google Scholar] [CrossRef]
- Stasiak, M.; Wojtaszek-Słomińska, A.; Racka-Pilszak, B. Current methods for secondary alveolar bone grafting assessment in cleft lip and palate patients—A systematic review. J. Craniomaxillofac. Surg. 2019, 47, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, R.; Jacobs, R.; Singer, S.R.; Mupparapu, M. CBCT-based bone quality assessment: Are Hounsfield units applicable? Dentomaxillofac. Radiol. 2015, 44, 20140238. [Google Scholar] [CrossRef]
- Zhang, D.Z.; Xiao, W.L.; Zhou, R.; Xue, L.F.; Ma, L. Evaluation of Bone Height and Bone Mineral Density Using Cone Beam Computed Tomography After Secondary Bone Graft in Alveolar Cleft. J. Craniofac. Surg. 2015, 26, 1463–1466. [Google Scholar] [CrossRef] [PubMed]
- Pai, B.C.J.; Hung, Y.T.; Wang, R.S.H.; Lo, L.J. Outcome of Patients with Complete Unilateral Cleft Lip and Palate: 20-Year Follow-Up of a Treatment Protocol. Plast. Reconstr. Surg. 2019, 143, 359e-367e. [Google Scholar] [CrossRef]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006, 31, 1116–1128. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Pape, H.C.; Evans, A.; Kobbe, P. Autologous bone graft: Properties and techniques. J. Orthop. Trauma. 2010, 24 (Suppl. S1), S36–S40. [Google Scholar] [CrossRef] [PubMed]
- Benlidayi, M.E.; Tatli, U.; Kurkcu, M.; Uzel, A.; Oztunc, H. Comparison of bovine-derived hydroxyapatite and autogenous bone for secondary alveolar bone grafting in patients with alveolar clefts. J. Oral Maxillofac. Surg. 2012, 70, e95–e102. [Google Scholar] [CrossRef]
- Canan, L.W., Jr.; da Silva Freitas, R.; Alonso, N.; Tanikawa, D.Y.; Rocha, D.L.; Coelho, J.C. Human bone morphogenetic protein-2 use for maxillary reconstruction in cleft lip and palate patients. J. Craniofac. Surg. 2012, 23, 1627–1633. [Google Scholar] [CrossRef] [PubMed]
- Rychlik, D.; Wójcicki, P. Bone graft healing in alveolar osteoplasty in patients with unilateral lip, alveolar process, and palate clefts. J. Craniofac. Surg. 2012, 23, 118–123. [Google Scholar] [CrossRef]
- Shawky, H.; Seifeldin, S.A. Does Platelet-Rich Fibrin Enhance Bone Quality and Quantity of Alveolar Cleft Reconstruction? Cleft. Palate Craniofac. J. 2016, 53, 597–606. [Google Scholar] [CrossRef]
- Weaver, C.M.; Gordon, C.M.; Janz, K.F.; Kalkwarf, H.J.; Lappe, J.M.; Lewis, R.; O’Karma, M.; Wallace, T.C.; Zemel, B.S. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: A systematic review and implementation recommendations. Osteoporos. Int. 2016, 27, 1281–1386. [Google Scholar] [CrossRef]
- Feichtinger, M.; Mossböck, R.; Kärcher, H. Assessment of bone resorption after secondary alveolar bone grafting using three-dimensional computed tomography: A three-year study. Cleft. Palate Craniofac. J. 2007, 44, 142–148. [Google Scholar] [CrossRef]
- Tai, C.C.; Sutherland, I.S.; McFadden, L. Prospective analysis of secondary alveolar bone grafting using computed tomography. J. Oral Maxillofac Surg. 2000, 58, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Van der Meij, A.J.; Baart, J.A.; Prahl-Andersen, B.; Valk, J.; Kostense, P.J.; Tuinzing, D.B. Bone volume after secondary bone grafting in unilateral and bilateral clefts determined by computed tomography scans. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2001, 92, 136–141. [Google Scholar] [CrossRef]
- Lee, C.Y.; Prasad, H.S.; Suzuki, J.B.; Stover, J.D.; Rohrer, M.D. The correlation of bone mineral density and histologic data in the early grafted maxillary sinus: A preliminary report. Implant. Dent. 2011, 20, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.T.; Rosenbaum, A.J. Bone grafts, bone substitutes and orthobiologics: The bridge between basic science and clinical advancements in fracture healing. Organogenesis 2012, 8, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Abyholm, F.E.; Bergland, O.; Semb, G. Secondary bone grafting of alveolar clefts. A surgical/orthodontic treatment enabling a non-prosthodontic rehabilitation in cleft lip and palate patients. Scand. J. Plast. Reconstr. Surg. 1981, 15, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.; James, D.R.; Mars, M. Alveolar bone grafting: A review of 115 patients. Eur. J. Orthod. 1998, 20, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Heidbuchel, K.L.; Kuijpers-Jagtman, A.M.; Freihofer, H.P. An orthodontic and cephalometric study on the results of the combined surgical-orthodontic approach of the protruded premaxilla in bilateral clefts. J. Craniomaxillofac. Surg. 1993, 21, 60–66. [Google Scholar] [CrossRef]
- Khorasani, R. Image compression in your PACS: Should you do it? What are the issues? J. Am. Coll. Radiol. 2004, 1, 780–781. [Google Scholar] [CrossRef]

| Variables | All (n = 40) | Unilateral (n = 33) | Bilateral (n = 7) | p-Value |
|---|---|---|---|---|
| Age at ABG (year) | 9.45 ± 1.61 | 9.36 ± 1.69 | 9.86 ± 1.12 | 0.467 |
| Men (n (%)) | 24 (60.0%) | 19 (57.6%) | 5 (71.4%) | 0.681 |
| ABG method | ||||
| Scarpa’s fascia (n (%)) | 40 (100%) | 33 (100%) | 7 (100%) | — |
| Bone graft from iliac crest (n (%)) | 40 (100%) | 33 (100%) | 7 (100%) | — |
| Graft volume (mL) [2] | — | 2.0 ± 0.6 | 3.8 ± 1.0 | — |
| CBCT follow-up post-surgery | ||||
| First follow-up (month) | 6.67 ± 0.82 | 6.60 ± 0.79 | 6.97 ± 0.93 | 0.284 |
| Second follow-up (month) | 24.04 ± 5.15 | 23.65 ± 4.65 | 25.86 ± 7.23 | 0.465 |
| Variables | Method | Overall | Unilateral | Bilateral | p-Value |
|---|---|---|---|---|---|
| BMDT1 (HU) | |||||
| A | 293.88 ± 135.27 | 292.63 ± 135.45 | 299.78 ± 145.08 | 0.901 | |
| B | 299.50 ± 137.54 | 298.70 ± 137.77 | 303.25 ± 147.39 | 0.983 | |
| C | 295.84 ± 150.24 | 295.79 ± 150.20 | 296.08 ± 162.45 | 0.996 | |
| BMDT2 (HU) | |||||
| A | 360.90 ± 175.46 | 358.34 ± 184.54 | 373.01 ± 135.14 | 0.844 | |
| B | 366.90 ± 178.55 | 366.16 ± 186.93 | 370.41 ± 144.32 | 0.955 | |
| C | 361.97 ± 185.96 | 362.65 ± 195.78 | 358.78 ± 142.62 | 0.961 | |
| BMDaT1 (%) | |||||
| A | 99.70 ± 49.18 | 100.01 ± 46.98 | 98.24 ± 62.85 | 0.932 | |
| B | 101.90 ± 52.05 | 102.47 ± 50.33 | 99.19 ± 63.97 | 0.882 | |
| C | 101.78 ± 59.74 | 102.78 ± 58.83 | 97.06 ± 68.63 | 0.882 | |
| BMDaT2 (%) | |||||
| A | 121.45 ± 53.98 | 123.17 ± 56.38 | 113.39 ± 43.53 | 0.669 | |
| B | 123.55 ± 55.52 | 125.93 ± 57.82 | 112.34 ± 45.00 | 0.563 | |
| C | 122.74 ± 58.81 | 125.85 ± 61.62 | 108.09 ± 43.88 | 0.475 | |
| Density enhancement rate (%) | |||||
| A | 35.85 ± 51.99 | 36.37 ± 54.51 | 33.41 ± 41.41 | 0.893 | |
| B | 35.61 ± 51.75 | 36.60 ± 53.41 | 30.94 ± 46.47 | 0.796 | |
| C | 37.59 ± 56.41 | 38.25 ± 58.38 | 34.46 ± 49.91 | 0.874 | |
| Pogonion densityT1 (HU) | 312.86 ± 81.68 | 309.06 ± 84.55 | 330.77 ± 69.17 | 0.530 | |
| Pogonion densityT2 (HU) | 309.60 ± 88.60 | 303.99 ± 93.26 | 336.06 ± 60.40 | 0.391 | |
| VolumeT1 (mm3) | – | 107.65 ± 97.67 | 77.68 ± 47.57 | – | |
| VolumeT2 (mm3) | – | 103.52 ± 94.33 | 72.93 ± 31.62 | – |
| Density Enhancement Rate (%) | |||
|---|---|---|---|
| Method A | Method B | Method C | |
| Unilateral cleft alveolus | |||
| Volume loss (%) * | |||
| Correlation coefficient® | –0.038 | 0.019 | 0.008 |
| p-value | 0.844 | 0.921 | 0.966 |
| Bilateral cleft alveolus | |||
| Volume loss (%) * | |||
| Correlation coefficient® | 0.400 | 0.400 | 0.700 |
| p-value | 0.505 | 0.505 | 0.188 |
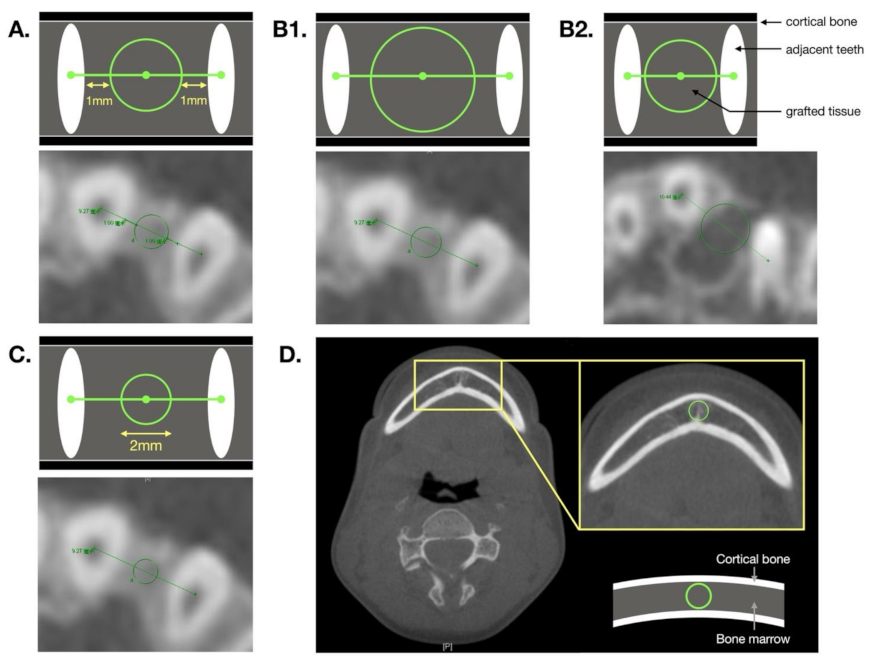
| Method A | Method B | Method C | |
|---|---|---|---|
| Definition | Circular zone is located 1 mm from the adjacent teeth. | The largest circular zone that exactly transects the two adjacent teeth or is tangent to the surrounding cortical bone. | A circle is drawn with a diameter of 2 mm. |
| Positive aspect | BMD measurements are not affected by the density of adjacent teeth. |
|
|
| Negative aspect | The surrounding cortical bone density and air density are often included in examinations of patients with large alveolar gaps. | Easily contains adjacent tooth density or surrounding cortical bone. | Not representative of the overall density in cases with a larger alveolar gap. |
| Operating precautions | — | When measuring, care must be taken of to avoid including sections of the teeth. | Ensure that the distance from the circle to the two adjacent teeth is equal when measuring. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.-R.; Lin, Y.-C.; Pai, B.C.-J.; Tseng, H.-J.; Lo, L.-J.; Chou, P.-Y. Progressive Comparison of Density Assessment of Alveolar Bone Graft in Patients with Unilateral and Bilateral Cleft. J. Clin. Med. 2021, 10, 5143. https://doi.org/10.3390/jcm10215143
Chen P-R, Lin Y-C, Pai BC-J, Tseng H-J, Lo L-J, Chou P-Y. Progressive Comparison of Density Assessment of Alveolar Bone Graft in Patients with Unilateral and Bilateral Cleft. Journal of Clinical Medicine. 2021; 10(21):5143. https://doi.org/10.3390/jcm10215143
Chicago/Turabian StyleChen, Pin-Ru, Yu-Ching Lin, Betty Chien-Jung Pai, Hsiao-Jung Tseng, Lun-Jou Lo, and Pang-Yun Chou. 2021. "Progressive Comparison of Density Assessment of Alveolar Bone Graft in Patients with Unilateral and Bilateral Cleft" Journal of Clinical Medicine 10, no. 21: 5143. https://doi.org/10.3390/jcm10215143
APA StyleChen, P.-R., Lin, Y.-C., Pai, B. C.-J., Tseng, H.-J., Lo, L.-J., & Chou, P.-Y. (2021). Progressive Comparison of Density Assessment of Alveolar Bone Graft in Patients with Unilateral and Bilateral Cleft. Journal of Clinical Medicine, 10(21), 5143. https://doi.org/10.3390/jcm10215143







_周(Chou).jpg)

