Is Saffron Able to Prevent the Dysregulation of Retinal Cytokines Induced by Ocular Hypertension in Mice?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Groups
2.3. Treatment with Saffron
2.4. Anaesthetics
2.5. Laser Treatment and Measurement of IOP
2.6. Multiplexed Immunoassay Study
2.6.1. Protein Assay
2.6.2. Multiplexed Magnetic Bead Immunoassay
2.6.3. Immunostaining
2.7. Statistical Analysis
3. Results
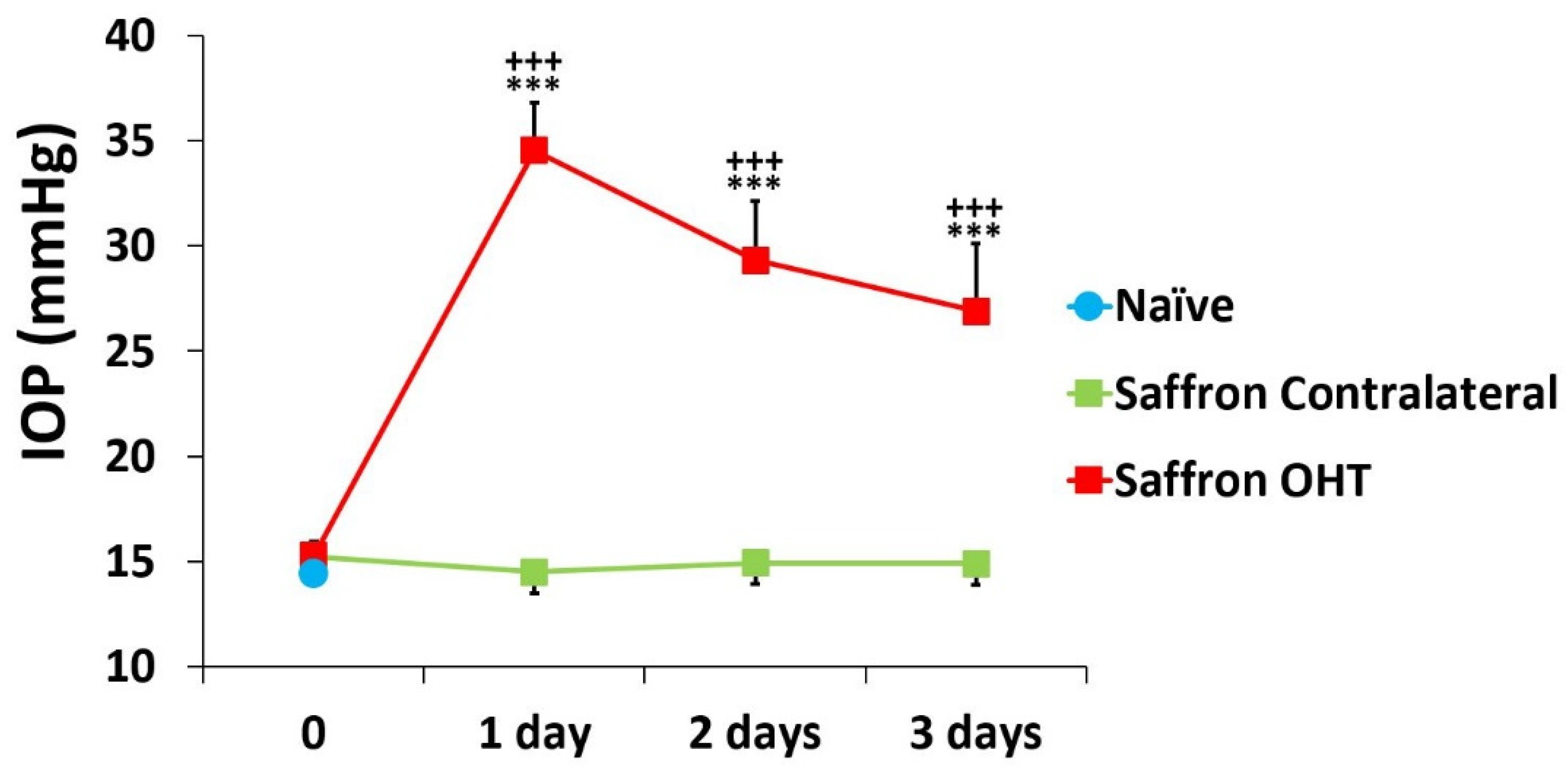
3.1. Intraocular Pressure
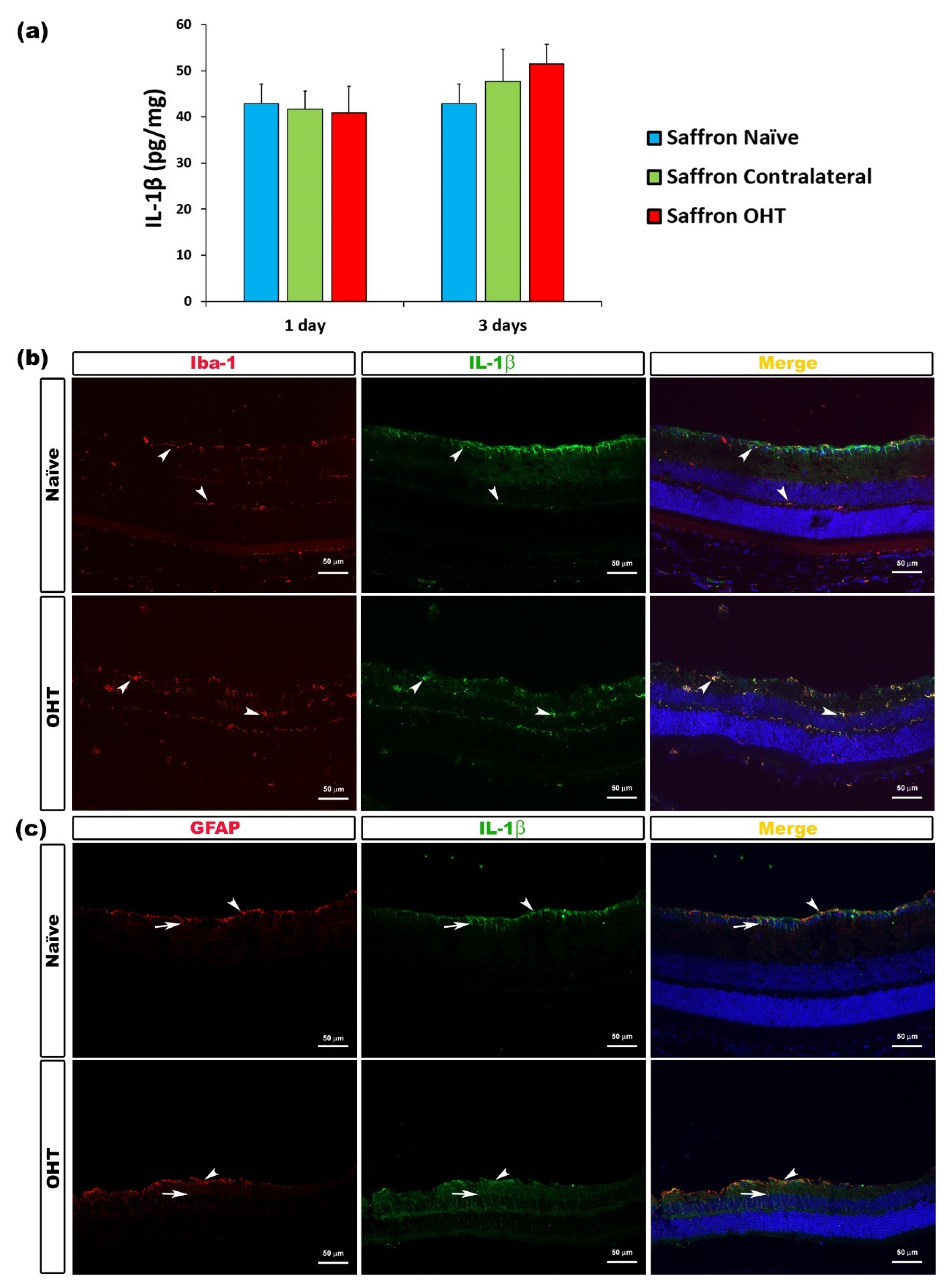
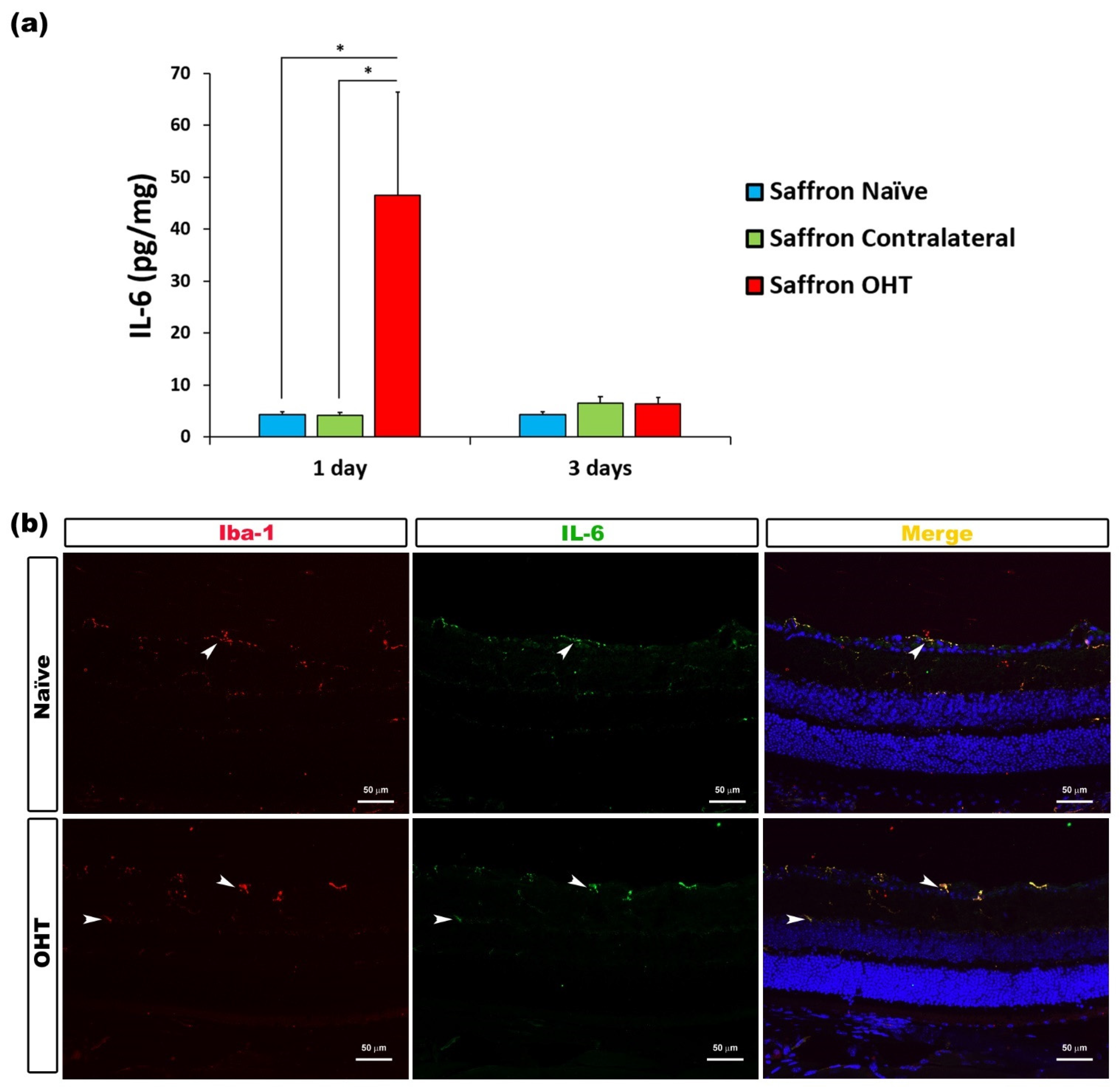
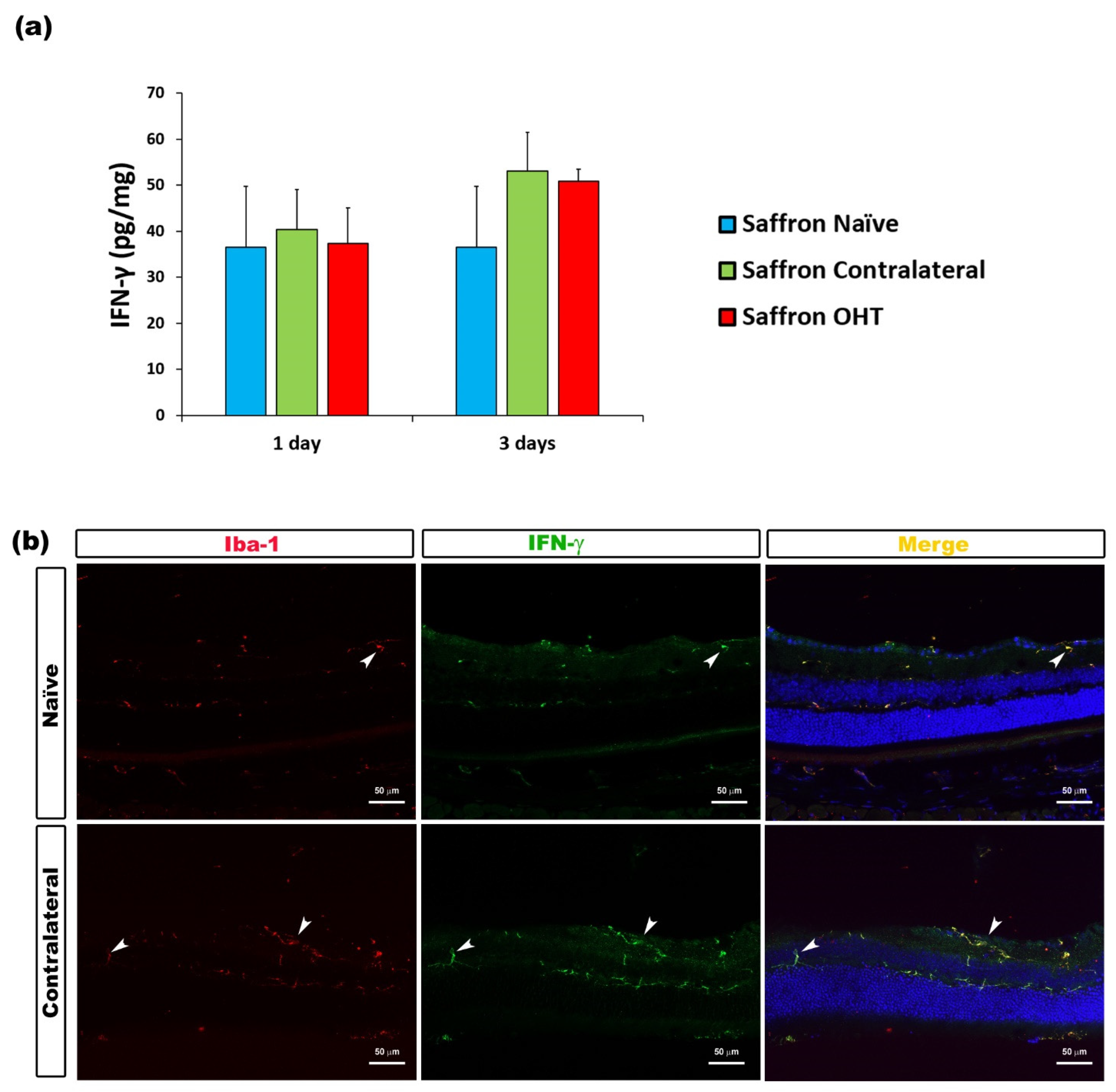
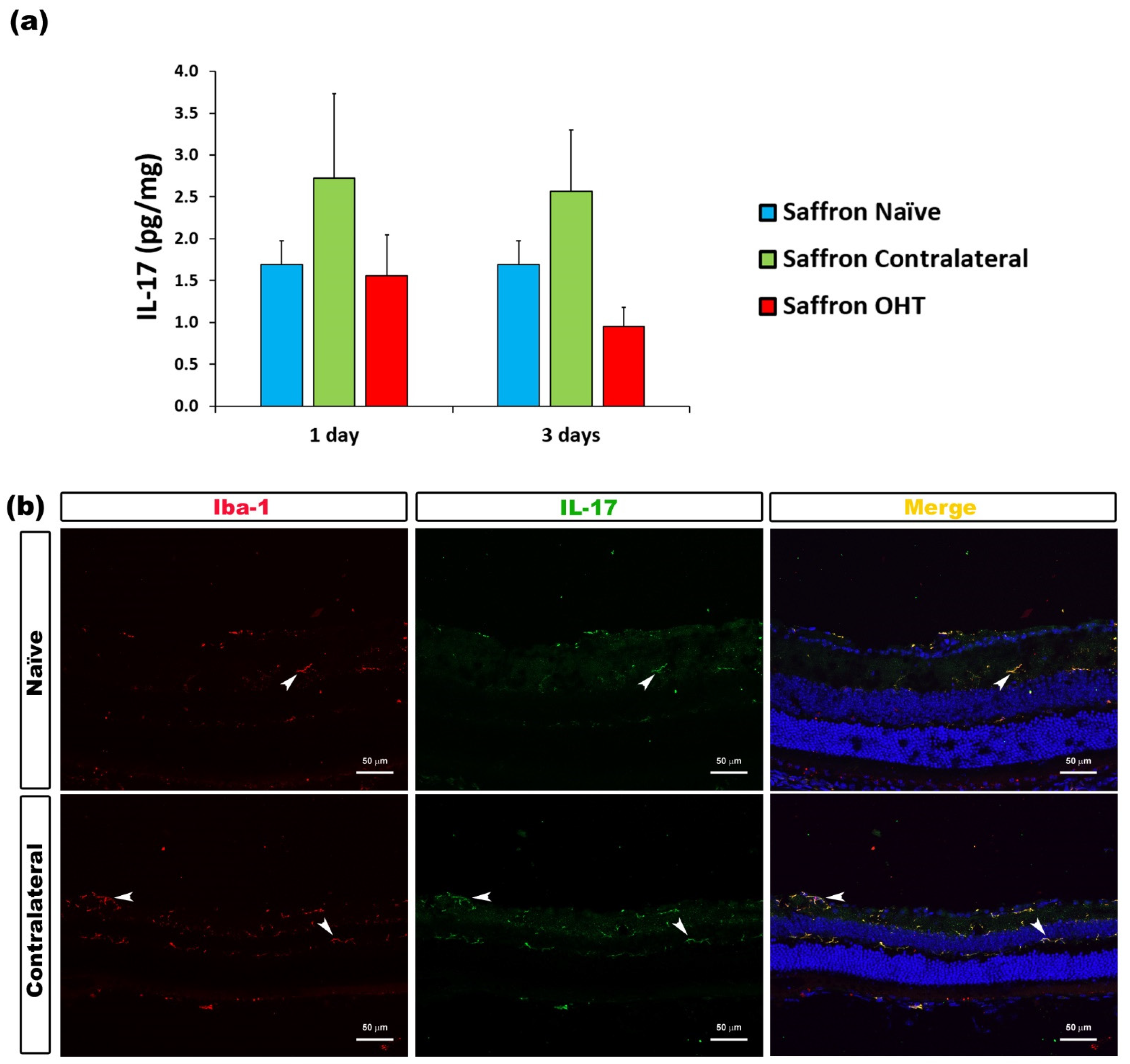
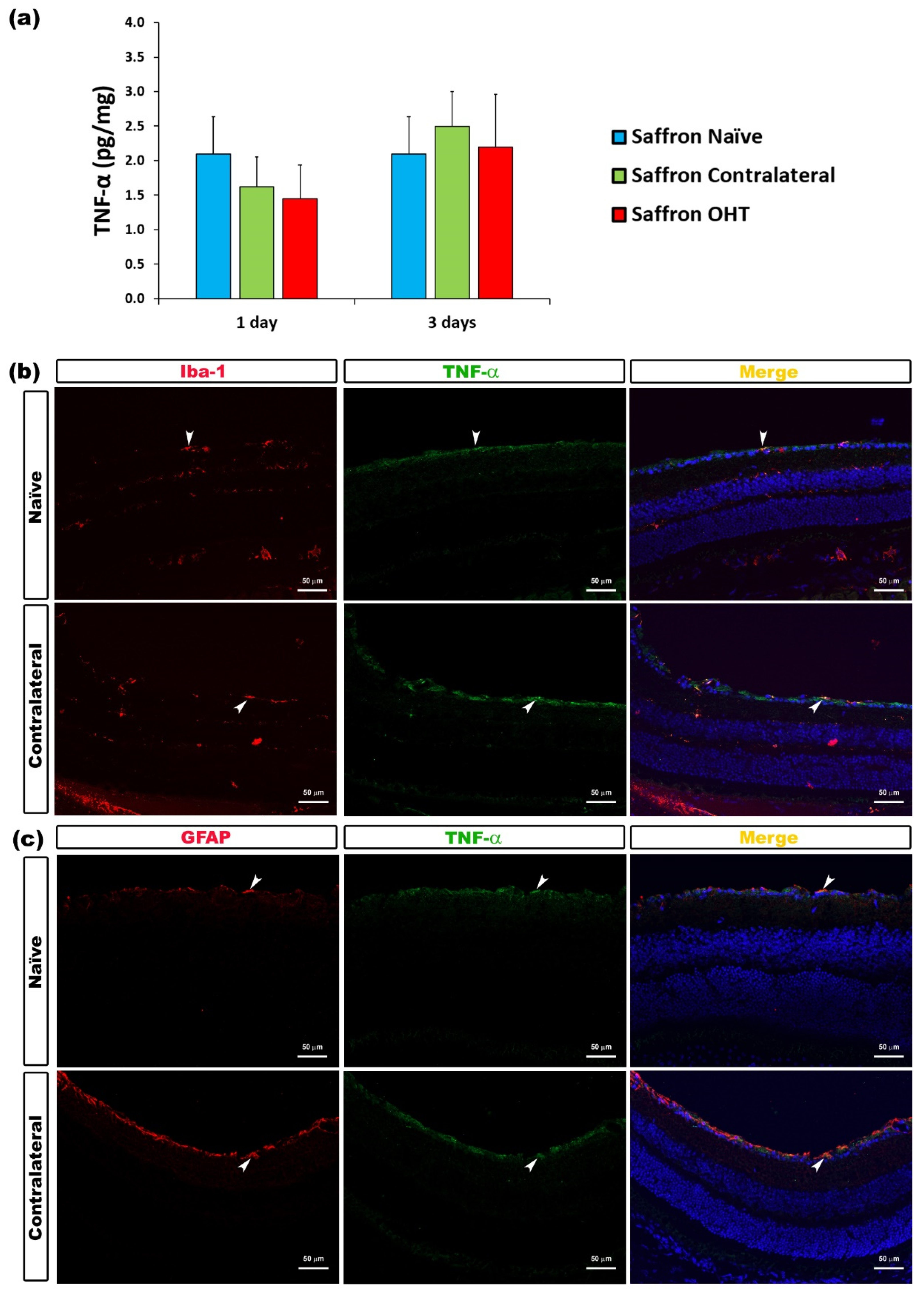
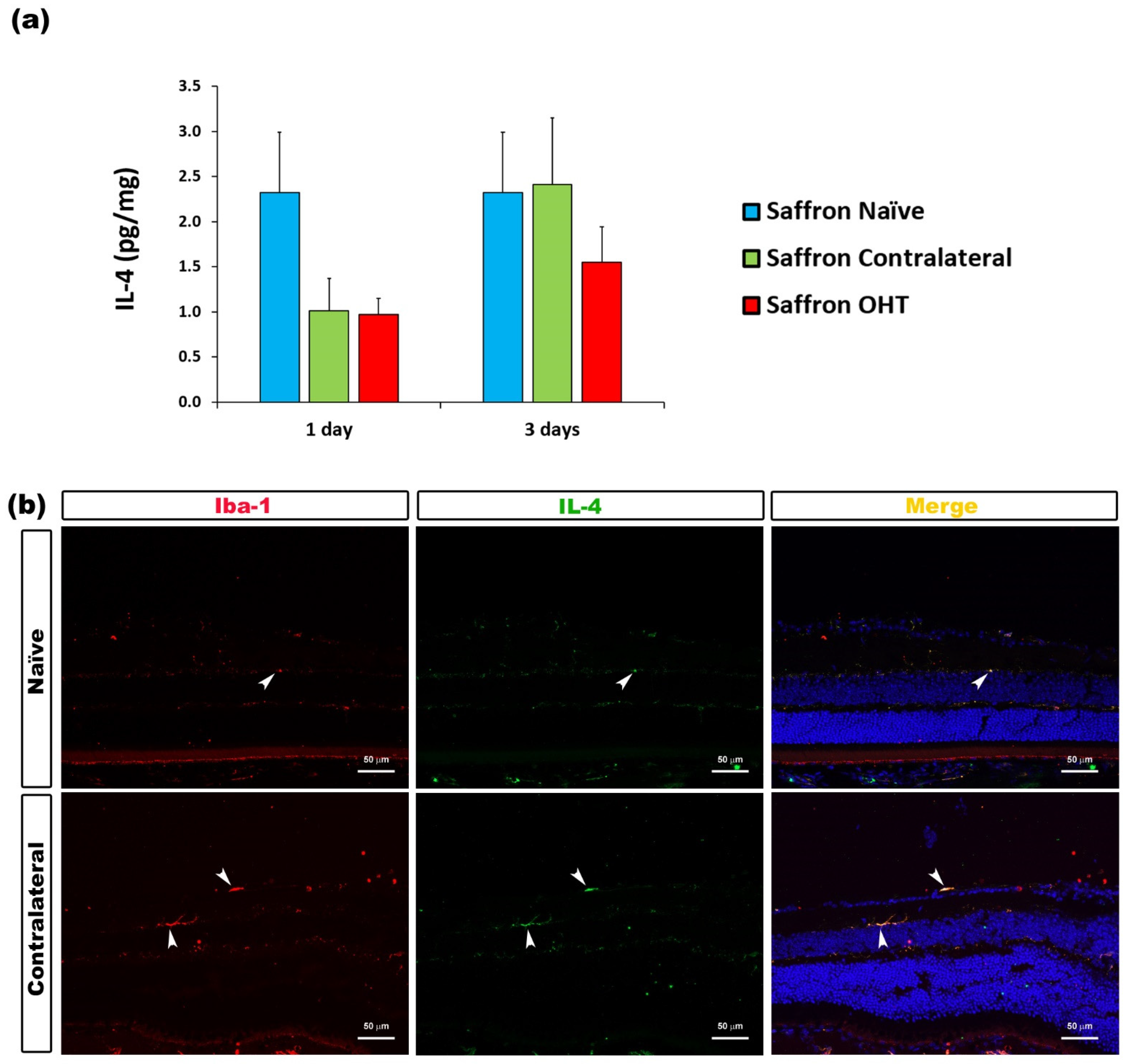
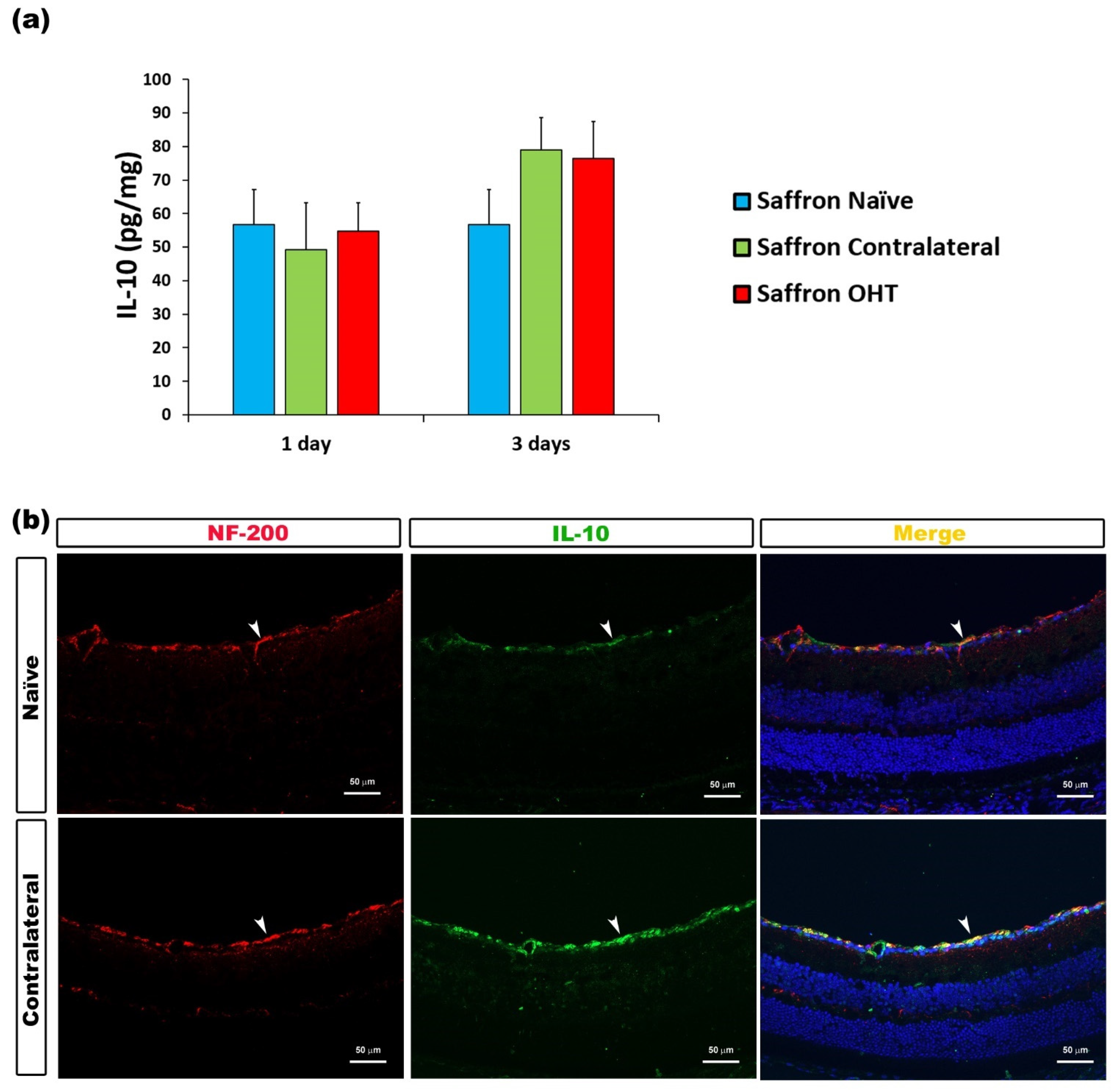
3.2. Multiplex Analysis
3.3. Cytokine Colocalizations with Different Cell Populations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040: A Systematic Review and Meta-Analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Qu, J.; Wang, D.; Grosskreutz, C.L. Mechanisms of retinal ganglion cell injury and defense in glaucoma. Exp. Eye Res. 2010, 91, 48–53. [Google Scholar] [CrossRef] [Green Version]
- Baltmr, A.; Duggan, J.; Nizari, S.; Salt, T.E.; Cordeiro, M.F. Neuroprotection in glaucoma–Is there a future role? Exp. Eye Res. 2010, 91, 554–566. [Google Scholar] [CrossRef]
- Tezel, G.; Ben-Hur, T.; Gibson, G.E.; Stevens, B.; Streit, W.J.; Wekerle, H.; Bhattacharya, S.K.; Borras, T.; Burgoyne, C.F.; Caspi, R.R.; et al. The role of glia, mitochondria, and the immune system in glaucoma. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, A.I.; de Hoz, R.; Salobrar-Garcia, E.; Salazar, J.J.; Rojas, B.; Ajoy, D.; López-Cuenca, I.; Rojas, P.; Triviño, A.; Ramírez, J.M. The Role of Microglia in Retinal Neurodegeneration: Alzheimer’s Disease, Parkinson, and Glaucoma. Front. Aging Neurosci. 2017, 9, 214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Hoz, R.; Rojas, B.; Ramirez, A.I.; Salazar, J.J.; Gallego, B.I.; Trivino, A.; Ramirez, J.M. Retinal Macroglial Responses in Health and Disease. Biomed. Res. Int. 2016, 2016, 2954721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramírez, A.I.; Rojas, B.; de Hoz, R.; Salazar, J.J.; Gallego, B.; Triviño, A.; Ramírez, J.M. Microglia, inflammation, and glaucoma. In Glaucoma; SM Group Open Access eBooks: Dover, DE, USA, 2015; pp. 1–16. [Google Scholar]
- Russo, R.; Varano, G.P.; Adornetto, A.; Nucci, C.; Corasaniti, M.T.; Bagetta, G.; Morrone, L.A. Retinal ganglion cell death in glaucoma: Exploring the role of neuroinflammation. Eur. J. Pharmacol. 2016, 787, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Salobrar-García, E.; Hoyas, I.; Leal, M.; de Hoz, R.; Rojas, B.; Ramirez, A.I.; Salazar, J.J.; Yubero, R.; Gil, P.; Triviño, A.; et al. Analysis of Retinal Peripapillary Segmentation in Early Alzheimer’s Disease Patients. Biomed. Res. Int. 2015, 2015, 636548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallego, B.I.; Salazar, J.J.; de Hoz, R.; Rojas, B.; Ramírez, A.I.; Salinas-Navarro, M.; Ortín-Martínez, A.; Valiente-Soriano, F.J.; Avilés-Trigueros, M.; Villegas-Perez, M.P.; et al. IOP induces upregulation of GFAP and MHC-II and microglia reactivity in mice retina contralateral to experimental glaucoma. J. Neuroinflamm. 2012, 9, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramírez, A.I.; de Hoz, R.; Fernández-Albarral, J.A.; Salobrar-García, E.; Rojas, B.; Valiente-Soriano, F.J.; Avilés-Trigueros, M.; Villegas-Pérez, M.P.; Vidal-Sanz, M.; Triviño, A.; et al. Time course of bilateral microglial activation in a mouse model of laser-induced glaucoma. Sci. Rep. 2020, 10, 4890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojas, B.; Gallego, B.I.; Ramírez, A.I.; Salazar, J.J.; de Hoz, R.; Valiente-Soriano, F.J.; Avilés-Trigueros, M.; Villegas-Perez, M.P.; Vidal-Sanz, M.; Triviño, A.; et al. Microglia in mouse retina contralateral to experimental glaucoma exhibit multiple signs of activation in all retinal layers. J. Neuroinflamm. 2014, 11, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Hoz, R.; Gallego, B.I.; Ramírez, A.I.; Rojas, B.; Salazar, J.J.; Valiente-Soriano, F.J.; Avilés-Trigueros, M.; Villegas-Perez, M.P.; Vidal-Sanz, M.; Triviño, A.; et al. Rod-like microglia are restricted to eyes with laser-induced ocular hypertension but absent from the microglial changes in the contralateral untreated eye. PLoS ONE 2013, 8, e83733. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Albarral, J.A.; Salazar, J.J.; de Hoz, R.; Marco, E.M.; Martín-Sánchez, B.; Flores-Salguero, E.; Salobrar-García, E.; López-Cuenca, I.; Barrios-Sabador, V.; Avilés-Trigueros, M.; et al. Retinal Molecular Changes Are Associated with Neuroinflammation and Loss of RGCs in an Experimental Model of Glaucoma. Int. J. Mol. Sci. 2021, 22, 2066. [Google Scholar] [CrossRef] [PubMed]
- Haynes, S.E.; Hollopeter, G.; Yang, G.; Kurpius, D.; Dailey, M.E.; Gan, W.-B.; Julius, D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat. Neurosci. 2006, 9, 1512–1519. [Google Scholar] [CrossRef]
- Salinas-Navarro, M.; Alarcón-Martínez, L.; Valiente-Soriano, F.J.; Ortín-Martínez, A.; Jiménez-López, M.; Avilés-Trigueros, M.; Villegas-Pérez, M.P.; de la Villa, P.; Vidal-Sanz, M. Functional and morphological effects of laser-induced ocular hypertension in retinas of adult albino Swiss mice. Mol. Vis. 2009, 15, 2578–2598. [Google Scholar]
- Poma, A.; Fontecchio, G.; Carlucci, G.; Chichiriccò, G.; Chichiricco, G. Anti-inflammatory properties of drugs from saffron crocus. Antiinflamm. Antiallergy. Agents Med. Chem. 2012, 11, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, M.; Tsuruma, K.; Imai, S.; Nakanishi, T.; Umigai, N.; Shimazawa, M.; Hara, H. Crocetin prevents retinal degeneration induced by oxidative and endoplasmic reticulum stresses via inhibition of caspase activity. Eur. J. Pharmacol. 2011, 650, 110–119. [Google Scholar] [CrossRef]
- Ohno, Y.; Nakanishi, T.; Umigai, N.; Tsuruma, K.; Shimazawa, M.; Hara, H. Oral administration of crocetin prevents inner retinal damage induced by N-methyl-D-aspartate in mice. Eur. J. Pharmacol. 2012, 690, 84–89. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Younesi, H.M. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2002, 2, 7. [Google Scholar] [CrossRef] [Green Version]
- Bathaie, S.Z.; Farajzade, A.; Hoshyar, R. A review of the chemistry and uses of crocins and crocetin, the carotenoid natural dyes in saffron, with particular emphasis on applications as colorants including their use as biological stains. Biotech. Histochem. 2014, 89, 401–411. [Google Scholar] [CrossRef]
- Rameshrad, M.; Razavi, B.M.; Hosseinzadeh, H. Saffron and its derivatives, crocin, crocetin and safranal: A patent review. Expert Opin. Ther. Pat. 2018, 28, 147–165. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.; Hosseinzadeh, H. A comprehensive review on biological activities and toxicology of crocetin. Food Chem. Toxicol. 2019, 130, 44–60. [Google Scholar] [CrossRef]
- Fernández-Albarral, J.A.; Hoz, R.; de Ramírez, A.I.; López-Cuenca, I.; Salobrar-García, E.; Pinazo-Durán, M.D.; Ramírez, J.M.; Salazar, J.J. Beneficial effects of saffron (Crocus sativus L.) in ocular pathologies, particularly neurodegenerative retinal diseases. Neural Regen. Res. 2020, 15, 1408. [Google Scholar] [CrossRef]
- Falsini, B.; Piccardi, M.; Minnella, A.; Savastano, M.C.; Capoluongo, E.; Fadda, A.; Balestrazzi, E.; Maccarone, R.; Bisti, S. Influence of Saffron Supplementation on Retinal Flicker Sensitivity in Early Age-Related Macular Degeneration. Investig. Opthalmology Vis. Sci. 2010, 51, 6118–6124. [Google Scholar] [CrossRef]
- Korani, S.; Korani, M.; Sathyapalan, T.; Sahebkar, A. Therapeutic effects of Crocin in autoimmune diseases: A review. BioFactors 2019, 45, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.N.; Park, Y.M.; Jung, H.J.; Lee, J.Y.; Min, B.D.; Park, S.U.; Jung, W.S.; Cho, K.H.; Park, J.H.; Kang, I.; et al. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur. J. Pharmacol. 2010, 648, 110–116. [Google Scholar] [CrossRef]
- Natoli, R.; Zhu, Y.; Valter, K.; Bisti, S.; Eells, J.; Stone, J. Gene and noncoding RNA regulation underlying photoreceptor protection: Microarray study of dietary antioxidant saffron and photobiomodulation in rat retina. Mol. Vis. 2010, 16, 1801–1822. [Google Scholar]
- Soeda, S.; Ochiai, T.; Paopong, L.; Tanaka, H.; Shoyama, Y.; Shimeno, H. Crocin suppresses tumor necrosis factor-α-induced cell death of neuronally differentiated PC-12 cells. Life Sci. 2001, 69, 2887–2898. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, L.; Rao, W.; Su, N.; Hui, H.; Wang, L.; Peng, C.; Tu, Y.; Zhang, S.; Fei, Z. Neuroprotective effects of crocin against traumatic brain injury in mice: Involvement of notch signaling pathway. Neurosci. Lett. 2015, 591, 53–58. [Google Scholar] [CrossRef]
- Zeinali, M.; Zirak, M.R.; Rezaee, S.A.; Karimi, G.; Hosseinzadeh, H. Immunoregulatory and anti-inflammatory properties of Crocus sativus (Saffron) and its main active constituents: A review. Iran. J. Basic Med. Sci. 2019, 22, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.I.; Manzoor, M.; Dhar, M.K. A comprehensive review of the pharmacological potential of Crocus sativus and its bioactive apocarotenoids. Biomed. Pharmacother. 2018, 98, 733–745. [Google Scholar] [CrossRef]
- Cerdá-Bernad, D.; Valero-Cases, E.; Pastor, J.-J.; Frutos, M.J. Saffron bioactives crocin, crocetin and safranal: Effect on oxidative stress and mechanisms of action. Crit. Rev. Food Sci. Nutr. 2020, 1–18. [Google Scholar] [CrossRef]
- Assimopoulou, A.N.; Sinakos, Z.; Papageorgiou, V.P. Radical scavenging activity of Crocus sativus L. extract and its bioactive constituents. Phyther. Res. 2005, 19, 997–1000. [Google Scholar] [CrossRef]
- Urbani, E.; Blasi, F.; Simonetti, M.S.; Chiesi, C.; Cossignani, L. Investigation on secondary metabolite content and antioxidant activity of commercial saffron powder. Eur. Food Res. Technol. 2016, 242, 987–993. [Google Scholar] [CrossRef]
- Fernández-Albarral, J.A.; Ramírez, A.I.; de Hoz, R.; López-Villarín, N.; Salobrar-García, E.; López-Cuenca, I.; Licastro, E.; Inarejos-García, A.M.; Almodóvar, P.; Pinazo-Durán, M.D.; et al. Neuroprotective and anti-inflammatory effects of a hydrophilic saffron extract in a model of glaucoma. Int. J. Mol. Sci. 2019, 20, 4110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almodóvar, P.; Prodanov, M.; Arruñada, O.; Inarejos-García, A.M. affron®eye, a natural extract of saffron (Crocus sativus L.) with colorant properties as novel replacer of saffron stigmas in culinary and food applications. Int. J. Gastron. Food Sci. 2018, 12, 1–5. [Google Scholar] [CrossRef]
- Kell, G.; Rao, A.; Beccaria, G.; Clayton, P.; Inarejos-García, A.M.; Prodanov, M. affron® a novel saffron extract (Crocus sativus L.) improves mood in healthy adults over 4 weeks in a double-blind, parallel, randomized, placebo-controlled clinical trial. Complement. Ther. Med. 2017, 33, 58–64. [Google Scholar] [CrossRef]
- Cuenca, N.; Pinilla, I.; Fernández-Sánchez, L.; Salinas-Navarro, M.; Alarcón-Martínez, L.; Avilés-Trigueros, M.; de la Villa, P.; Miralles de Imperial, J.; Villegas-Pérez, M.P.; Vidal-Sanz, M. Changes in the inner and outer retinal layers after acute increase of the intraocular pressure in adult albino Swiss mice. Exp. Eye Res. 2010, 91, 273–285. [Google Scholar] [CrossRef]
- Salinas-Navarro, M.; Alarcón-Martínez, L.; Valiente-Soriano, F.J.; Jiménez-López, M.; Mayor-Torroglosa, S.; Avilés-Trigueros, M.; Villegas-Pérez, M.P.; Vidal-Sanz, M. Ocular hypertension impairs optic nerve axonal transport leading to progressive retinal ganglion cell degeneration. Exp. Eye Res. 2010, 90, 168–183. [Google Scholar] [CrossRef]
- Naskar, R.; Wissing, M.; Thanos, S. Detection of early neuron degeneration and accompanying microglial responses in the retina of a rat model of glaucoma. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2962–2968. [Google Scholar]
- Quigley, H.A.; Hohman, R.M. Laser energy levels for trabecular meshwork damage in the primate eye. Investig. Ophthalmol. Vis. Sci. 1983, 24, 1305–1307. [Google Scholar]
- Neufeld, A.H. Microglia in the optic nerve head and the region of parapapillary chorioretinal atrophy in glaucoma. Arch. Ophthalmol. 1999, 117, 1050–1056. [Google Scholar] [CrossRef] [Green Version]
- Ding, H.; Chen, J.; Su, M.; Lin, Z.; Zhan, H.; Yang, F.; Li, W.; Xie, J.; Huang, Y.; Liu, X.; et al. BDNF promotes activation of astrocytes and microglia contributing to neuroinflammation and mechanical allodynia in cyclophosphamide-induced cystitis. J. Neuroinflammation 2020, 17, 19. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Wang, X.; Dong, J.; Lv, W.; Zhao, S.; Jin, L.; Guo, J.; Wang, M.; Cai, C.; Sun, J.; et al. Effects of Ginkgo biloba on Early Decompression after Spinal Cord Injury. Evid. Based Complement. Altern. Med. 2020, 2020, 6958246. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, H.; Wang, W.; Chen, Z.; Wang, S.; Li, J.; Li, G.; Gao, C.; Sun, X. Astragaloside IV Exerts Cognitive Benefits and Promotes Hippocampal Neurogenesis in Stroke Mice by Downregulating Interleukin-17 Expression via Wnt Pathway. Front. Pharmacol. 2020, 11, 421. [Google Scholar] [CrossRef] [Green Version]
- Johnson, S.; Duncan, J.; Hussain, S.A.; Chen, G.; Luo, J.; Mclaurin, C.; May, W.; Rajkowska, G.; Ou, X.M.; Stockmeier, C.A.; et al. The IFNγ-PKR pathway in the prefrontal cortex reactions to chronic excessive alcohol use. Alcohol. Clin. Exp. Res. 2015, 39, 476–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaquié, A.; Sauvain, A.; Duman, M.; Nocera, G.; Egger, B.; Meyenhofer, F.; Falquet, L.; Bartesaghi, L.; Chrast, R.; Lamy, C.M.; et al. Injured Axons Instruct Schwann Cells to Build Constricting Actin Spheres to Accelerate Axonal Disintegration. Cell Rep. 2019, 27, 3152–3166.e7. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Zhou, Z.; Sha, W.; Wang, L.; Yan, F.; Yang, X.; Qin, X.; Wu, M.; Li, D.; Tian, S.; et al. A novel CX3CR1 inhibitor AZD8797 facilitates early recovery of rat acute spinal cord injury by inhibiting inflammation and apoptosis. Int. J. Mol. Med. 2020, 45, 1373–1384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.Z.; Wang, Q.Q.; Yang, Q.Q.; Gu, H.Y.; Yin, Y.Q.; Li, Y.D.; Hou, J.C.; Chen, R.; Sun, Q.Q.; Sun, Y.F.; et al. NG2 glia regulate brain innate immunity via TGF-β2/TGFBR2 axis. BMC Med. 2019, 17, 204. [Google Scholar] [CrossRef]
- Honjoh, K.; Nakajima, H.; Hirai, T.; Watanabe, S.; Matsumine, A. Relationship of Inflammatory Cytokines from M1-Type Microglia/Macrophages at the Injured Site and Lumbar Enlargement with Neuropathic Pain After Spinal Cord Injury in the CCL21 Knockout (plt) Mouse. Front. Cell. Neurosci. 2019, 13, 525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Liu, J.; Gu, G.; Han, X.; Zhang, Q.; Zhang, W. Impact of neural stem cell-derived extracellular vesicles on mitochondrial dysfunction, sirtuin 1 level, and synaptic deficits in Alzheimer’s disease. J. Neurochem. 2020, 154, 502–518. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kim, M.; Imai, H.; Itakura, Y.; Ohtsuki, G. Microglia-Triggered Plasticity of Intrinsic Excitability Modulates Psychomotor Behaviors in Acute Cerebellar Inflammation. Cell Rep. 2019, 28, 2923–2938.e8. [Google Scholar] [CrossRef]
- Niesman, I.R.; Schilling, J.M.; Shapiro, L.A.; Kellerhals, S.E.; Bonds, J.A.; Kleschevnikov, A.M.; Cui, W.; Voong, A.; Krajewski, S.; Ali, S.S.; et al. Traumatic brain injury enhances neuroinflammation and lesion volume in caveolin deficient mice. J. Neuroinflammation 2014, 11, 39. [Google Scholar] [CrossRef] [Green Version]
- Bocquet, A.; Berges, R.; Frank, R.; Robert, P.; Peterson, A.C.; Eyer, J. Neurofilaments bind tubulin and modulate its polymerization. J. Neurosci. 2009, 29, 11043–11054. [Google Scholar] [CrossRef] [PubMed]
- Hoaglin, D.C.; Iglewicz, B. Fine-tuning some resistant rules for outlier labeling. J. Am. Stat. Assoc. 1987, 82, 1147–1149. [Google Scholar] [CrossRef]
- Wilson, G.N.; Inman, D.M.; Denger-Crish, C.M.; Smith, M.A.; Crish, S.D. Early pro-inflammatory cytokine elevations in the DBA/2J mouse model of glaucoma. J. Neuroinflammation 2015, 12, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Wu, L.; Guo, X.; Wang, D.; Li, Y. Improved retinal ganglion cell survival through retinal microglia suppression by a chinese herb extract, triptolide, in the dba/2j mouse model of glaucoma. Ocul. Immunol. Inflamm. 2013, 21, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, L.; Abnous, K.; Razavi, B.M.; Hosseinzadeh, H. Crocin-protected malathion-induced spatial memory deficits by inhibiting TAU protein hyperphosphorylation and antiapoptotic effects. Nutr. Neurosci. 2020, 23, 221–236. [Google Scholar] [CrossRef]
- Mozaffari, S.; Yasuj, S.R.; Motaghinejad, M.; Motevalian, M.; Kheiri, R. Crocin acting as a neuroprotective agent against methamphetamine-induced neurodegeneration via CREB-BDNF signaling pathway. Iran. J. Pharm. Res. 2019, 18, 745–758. [Google Scholar] [CrossRef]
- Sadoughi, D. The effect of crocin on apoptotic, inflammatory, BDNF, Pt, and Aβ40 indicators and neuronal density of CA1, CA2, and CA3 regions of hippocampus in the model of Alzheimer suffering rats induced with trimethyltin chloride. Comp. Clin. Path. 2019, 28, 1403–1413. [Google Scholar] [CrossRef]
- Lv, B.; Huo, F.; Zhu, Z.; Xu, Z.; Dang, X.; Chen, T.; Zhang, T.; Yang, X. Crocin Upregulates CX3CR1 Expression by Suppressing NF-κB/YY1 Signaling and Inhibiting Lipopolysaccharide-Induced Microglial Activation. Neurochem. Res. 2016, 41, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sánchez, L.; Lax, P.; Esquiva, G.; Martín-Nieto, J.; Pinilla, I.; Cuenca, N. Safranal, a saffron constituent, attenuates retinal degeneration in P23H rats. PLoS ONE 2012, 7, e43074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; He, Q.; Chen, H.; Lin, Z.; Xu, Y.; Yang, C. Crocin ameliorates chronic obstructive pulmonary disease-induced depression via PI3K/Akt mediated suppression of inflammation. Eur. J. Pharmacol. 2019, 862, 172640. [Google Scholar] [CrossRef]
- Du, J.; Chi, Y.; Song, Z.; Di, Q.; Mai, Z.; Shi, J.; Li, M. Crocin reduces Aspergillus fumigatus-induced airway inflammation and NF-κB signal activation. J. Cell. Biochem. 2018, 119, 1746–1754. [Google Scholar] [CrossRef]
- Rezaee-Khorasany, A.; Razavi, B.M.; Taghiabadi, E.; Yazdi, A.T.; Hosseinzadeh, H. Effect of crocin, an active saffron constituent, on ethanol toxicity in the rat: Histopathological and biochemical studies. Iran. J. Basic Med. Sci. 2020, 23, 51–62. [Google Scholar] [CrossRef]
- Kalantar, M.; Kalantari, H.; Goudarzi, M.; Khorsandi, L.; Bakhit, S.; Kalantar, H. Crocin ameliorates methotrexate-induced liver injury via inhibition of oxidative stress and inflammation in rats. Pharmacol. Rep. 2019, 71, 746–752. [Google Scholar] [CrossRef]
- Elsherbiny, N.M.; Salama, M.F.; Said, E.; El-Sherbiny, M.; Al-Gayyar, M.M.H. Crocin protects against doxorubicin-induced myocardial toxicity in rats through down-regulation of inflammatory and apoptic pathways. Chem. Biol. Interact. 2016, 247, 39–48. [Google Scholar] [CrossRef]
- Baradaran Rahim, V.; Khammar, M.T.; Rakhshandeh, H.; Samzadeh-Kermani, A.; Hosseini, A.; Askari, V.R. Crocin protects cardiomyocytes against LPS-Induced inflammation. Pharmacol. Rep. 2019, 71, 1228–1234. [Google Scholar] [CrossRef]
- Adali, F.; Gonul, Y.; Aldemir, M.; Hazman, O.; Ahsen, A.; Bozkurt, M.F.; Sen, O.G.; Keles, I.; Keles, H. Investigation of the effect of crocin pretreatment on renal injury induced by infrarenal aortic occlusion. J. Surg. Res. 2016, 203, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qin, X.; Lu, X. Crocin improves endometriosis by inhibiting cell proliferation and the release of inflammatory factors. Biomed. Pharmacother. 2018, 106, 1678–1685. [Google Scholar] [CrossRef] [PubMed]
- Chidlow, G.; Wood, J.P.M.; Ebneter, A.; Casson, R.J. Interleukin-6 is an efficacious marker of axonal transport disruption during experimental glaucoma and stimulates neuritogenesis in cultured retinal ganglion cells. Neurobiol. Dis. 2012, 48, 568–581. [Google Scholar] [CrossRef]
- Koeberle, P.D.; Gauldie, J.; Ball, A.K. Effects of adenoviral-mediated gene transfer of interleukin-10, interleukin-4, and transforming growth factor-β on the survival of axotomized retinal ganglion cells. Neuroscience 2004, 125, 903–920. [Google Scholar] [CrossRef]
- Echevarria, F.D.; Formichella, C.R.; Sappington, R.M. Interleukin-6 deficiency attenuates retinal ganglion cell axonopathy and glaucoma-related vision loss. Front. Neurosci. 2017, 11, 318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sappington, R.M.; Chan, M.; Calkins, D.J. Interleukin-6 protects retinal ganglion cells from pressure-induced death. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2932–2942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, Y.; Chiba, K. Role of microglial M1/M2 polarization in relapse and remission of psychiatric disorders and diseases. Pharmaceuticals 2014, 7, 1028–1048. [Google Scholar] [CrossRef] [Green Version]
- Gaffen, S.L. Structure and signalling in the IL-17 receptor family. Nat. Rev. Immunol. 2009, 9, 556–567. [Google Scholar] [CrossRef] [Green Version]
- Valiente-Soriano, F.J.; Nadal-Nicolás, F.M.; Salinas-Navarro, M.; Jiménez-López, M.; Bernal-Garro, J.M.; Villegas-Pérez, M.P.; Agudo-Barriuso, M.; Vidal-Sanz, M. BDNF rescues RGCs but not intrinsically photosensitive RGCs in ocular hypertensive albino rat retinas. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1924–1936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barleon, B.; Sozzani, S.; Zhou, D.; Weich, H.A.; Mantovani, A.; Marmé, D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood 1996, 87, 3336–3343. [Google Scholar] [CrossRef] [Green Version]
- Luna, J.D.; Chan, C.-C.; Derevjanik, N.L.; Mahlow, J.; Chiu, C.; Peng, B.; Tobe, T.; Campochiaro, P.A.; Vinores, S.A. Blood-Retinal Barrier (BRB) Breakdown in Experimental Autoimmune Uveoretinitis: Comparison with Vascular Endothelial Growth Factor, Tumor Necrosis Factor a, and Interleukin-1b-Mediated Breakdown. J. Neurosci. Res. 1997, 49, 268–280. [Google Scholar] [CrossRef]
- Hatori, K.; Nagai, A.; Heisel, R.; Ryu, J.K.; Kim, S.U. Fractalkine and fractalkine receptors in human neurons and glial cells. J. Neurosci. Res. 2002, 69, 418–426. [Google Scholar] [CrossRef]
- Zujovic, V.; Benavides, J.; Vigé, X.; Carter, C.; Taupin, V. Fractalkine modulates TNF-α secretion and neurotoxicity induced by microglial activation. Glia 2000, 29, 305–315. [Google Scholar] [CrossRef]
- Sokolowski, J.D.; Chabanon-Hicks, C.N.; Han, C.Z.; Heffron, D.S.; Mandell, J.W. Fractalkine is a “find-me” signal released by neurons undergoing ethanol-induced apoptosis. Front. Cell. Neurosci. 2014, 8, 360. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawabata, K.; Tung, N.H.; Shoyama, Y.; Sugie, S.; Mori, T.; Tanaka, T. Dietary crocin inhibits colitis and colitis-associated colorectal carcinogenesis in male ICR mice. Evid. Based Complement. Altern. Med. 2012, 2012, 820415. [Google Scholar] [CrossRef]
- Li, K.; Li, Y.; Ma, Z.; Zhao, J. Crocin exerts anti-inflammatory and anti-catabolic effects on rat intervertebral discs by suppressing the activation of JNK. Int. J. Mol. Med. 2015, 36, 1291–1299. [Google Scholar] [CrossRef] [Green Version]
- Dong, N.; Dong, Z.; Chen, Y.; Gu, X. Crocetin Alleviates Inflammation in MPTP-Induced Parkinson’s Disease Models through Improving Mitochondrial Functions. Parkinsons. Dis. 2020, 2020, 9864370. [Google Scholar] [CrossRef] [PubMed]
| Colour | Primary Antibody | Conc. | Secondary Antibody | Conc. |
|---|---|---|---|---|
| GREEN | Rabbit polyclonal anti-IL-1β (ref. ab9722, Abcam plc) [44] | 1:250 | Goat anti-rabbit Alexa Fluor 488 (ref. ab150077, Abcam plc) | 1:150 |
| Rabbit polyclonal anti-IL-6 (ref. ab208113, Abcam plc) [45] | 1:200 | |||
| Rabbit polyclonal anti-IL-17 (ref. ab79056, Abcam plc) [46] | 1:300 | |||
| Rabbit polyclonal anti-IFNγ (ref. ab9657, Abcam plc) [47] | 1:300 | |||
| Rabbit monoclonal anti-BDNF (ref. ab213323, Abcam plc) [44] | 1:250 | |||
| Rabbit monoclonal anti-VEGF receptor 1 (ref. ab32152, Abcam plc) [48] | 1:200 | |||
| Rabbit polyclonal anti-CX3CL1 (ref. ab25088, Abcam plc) [49] | 1:500 | |||
| Rabbit polyclonal anti-TNFα (ref. ab9739, Abcam plc) [50] | 1:300 | |||
| Rat monoclonal anti-IL-4 (ref. ab11524, Abcam plc) [51] | 1:250 | Goat anti-rat Alexa Fluor 488 (ref. ab150165, Abcam plc) | 1:150 | |
| Rat monoclonal anti-IL-10 (ref. ab189392, Abcam plc) [52] | 1:200 | |||
| RED | Rabbit polyclonal anti-Iba1 Red Fluorochrome 635 conjugated (ref. 5100756, Wako Chemicals GmbH) [53] | 1:200 | ||
| Chicken polyclonal anti-GFAP (ref. AB5541, Sigma-Aldrich) [54] | 1:200 | Goat anti-chicken IgY (H+L) Alexa Fluor 594 (ref. A-11042, Invitrogen) | 1:300 | |
| Rabbit polyclonal anti-NF-200 (ref. N4142, Sigma-Aldrich) [55] | 1:150 | Donkey anti-rabbit IgG1 Alexa Fluor 594 (ref. A21207, Invitrogen) | 1:800 |
| Cytokine | Co-Expression Cell Type | Figures |
|---|---|---|
| IL-1β | Microglia and Macroglia (astrocytes and Müller cells) | Figure 2 |
| IL-6 | Microglia | Figure 3 |
| INF-γ | Microglia | Figure 4 |
| IL-17 | Microglia | Figure 5 |
| TNF-α | Microglia and Astrocytes | Figure 6 |
| IL-4 | Microglia | Figure 7 |
| IL-10 | Axons of retinal ganglion cells | Figure 8 |
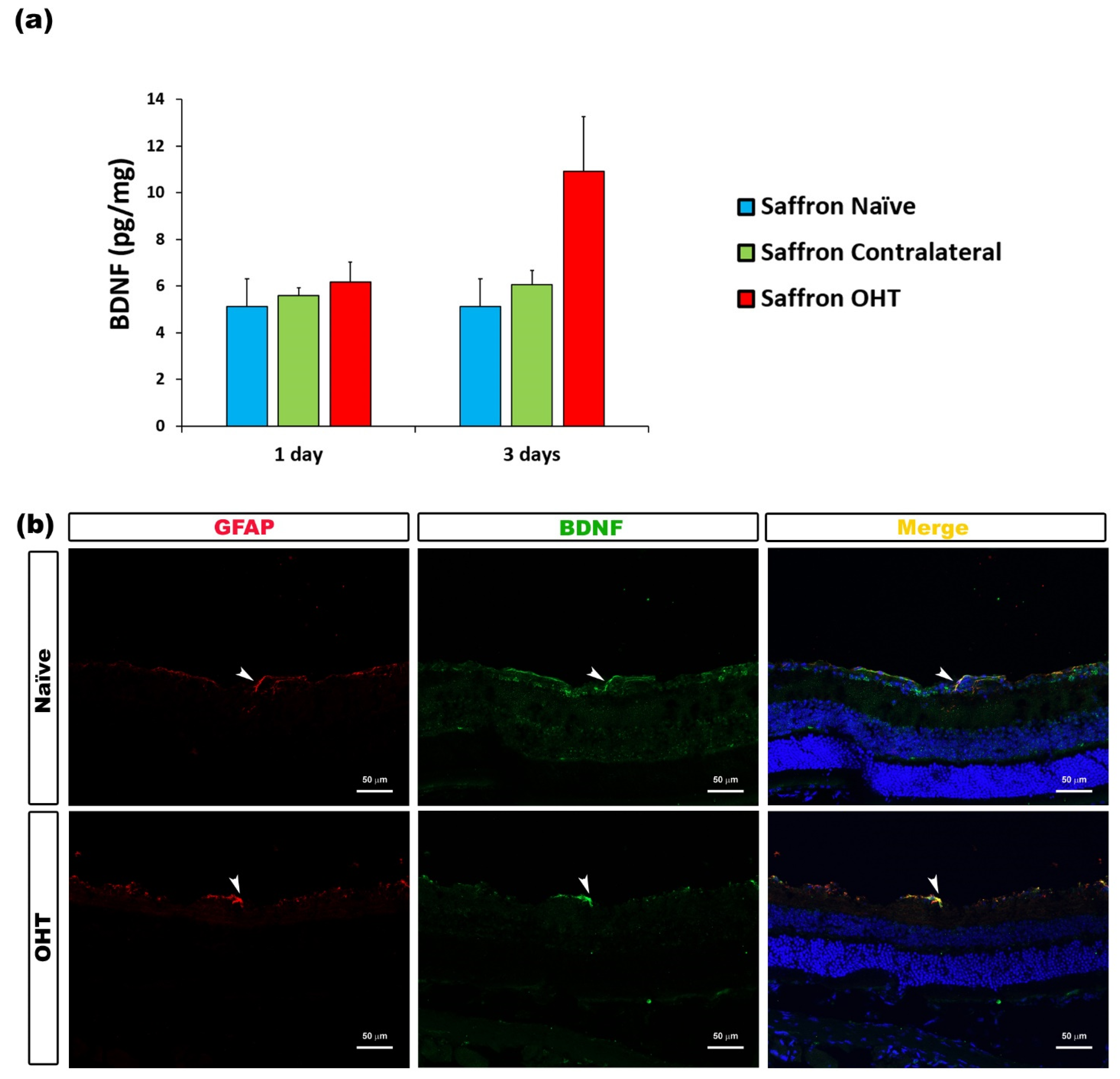
| BDNF | Macroglia (astrocytes and Müller cells) | Figure 9 |
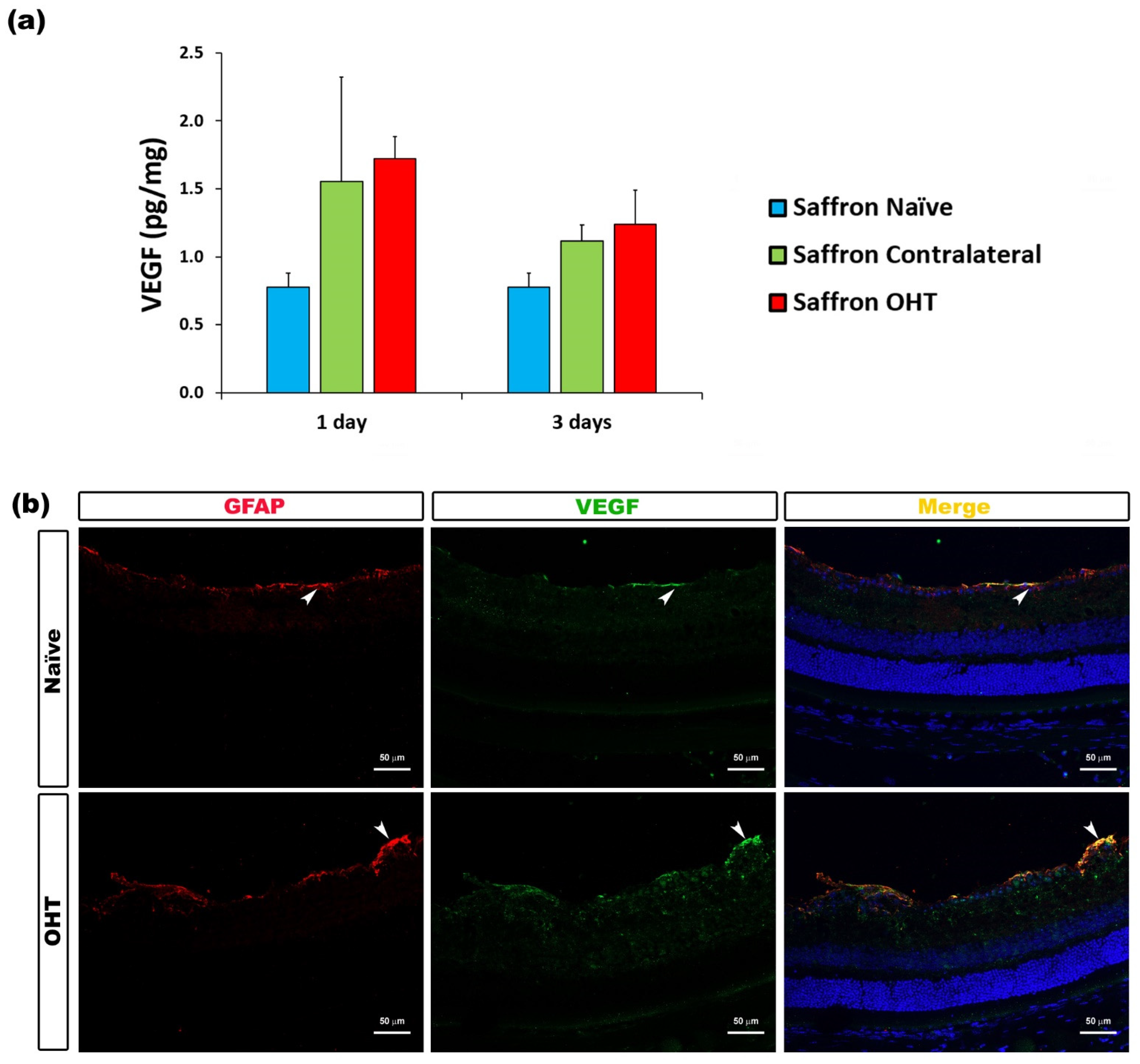
| VEGF | Macroglia (astrocytes and Müller cells) | Figure 10 |
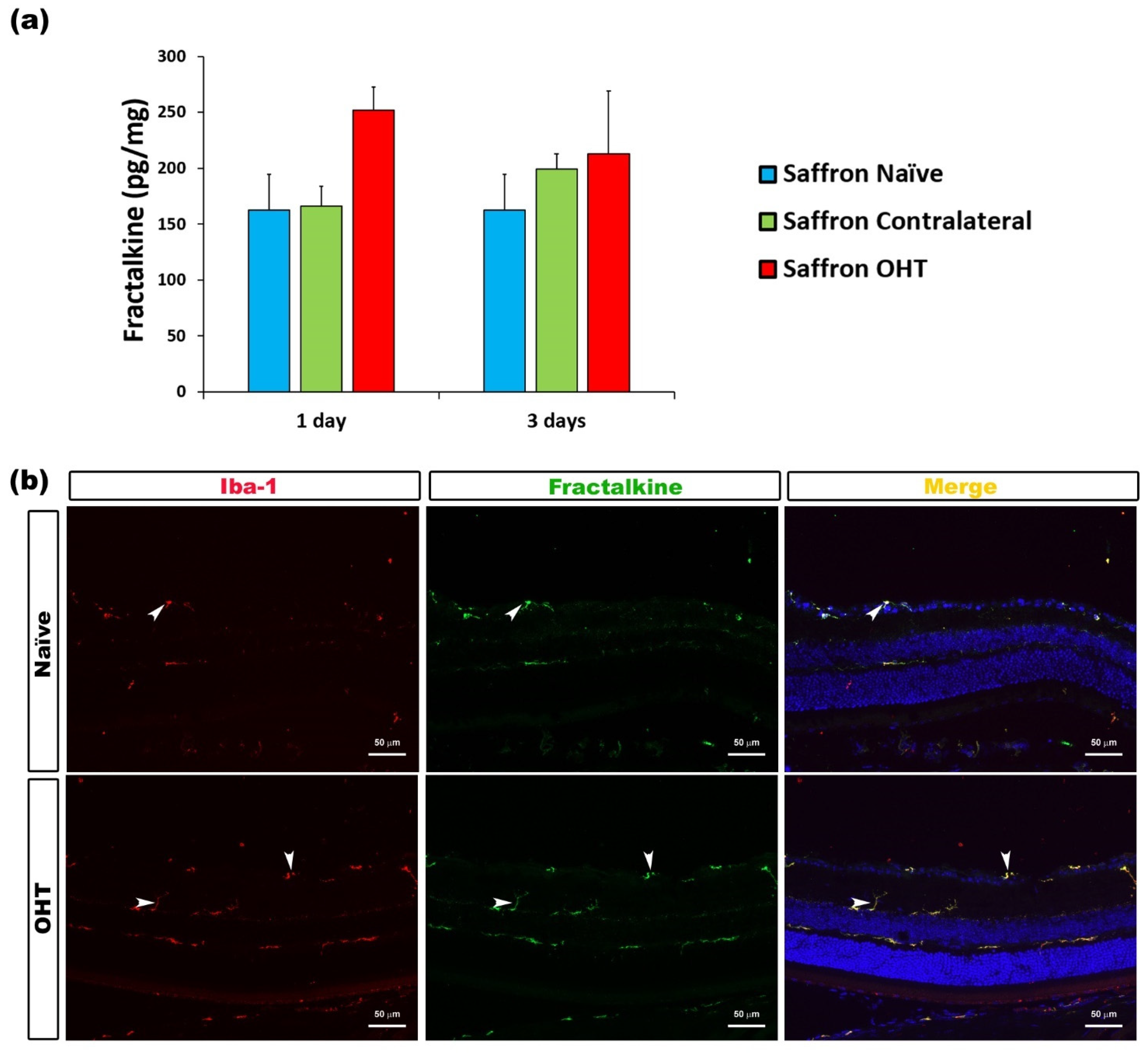
| Fractalkine | Microglia | Figure 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Albarral, J.A.; Martínez-López, M.A.; Marco, E.M.; de Hoz, R.; Martín-Sánchez, B.; San Felipe, D.; Salobrar-García, E.; López-Cuenca, I.; Pinazo-Durán, M.D.; Salazar, J.J.; et al. Is Saffron Able to Prevent the Dysregulation of Retinal Cytokines Induced by Ocular Hypertension in Mice? J. Clin. Med. 2021, 10, 4801. https://doi.org/10.3390/jcm10214801
Fernández-Albarral JA, Martínez-López MA, Marco EM, de Hoz R, Martín-Sánchez B, San Felipe D, Salobrar-García E, López-Cuenca I, Pinazo-Durán MD, Salazar JJ, et al. Is Saffron Able to Prevent the Dysregulation of Retinal Cytokines Induced by Ocular Hypertension in Mice? Journal of Clinical Medicine. 2021; 10(21):4801. https://doi.org/10.3390/jcm10214801
Chicago/Turabian StyleFernández-Albarral, José A., Miguel A. Martínez-López, Eva M. Marco, Rosa de Hoz, Beatriz Martín-Sánchez, Diego San Felipe, Elena Salobrar-García, Inés López-Cuenca, María D. Pinazo-Durán, Juan J. Salazar, and et al. 2021. "Is Saffron Able to Prevent the Dysregulation of Retinal Cytokines Induced by Ocular Hypertension in Mice?" Journal of Clinical Medicine 10, no. 21: 4801. https://doi.org/10.3390/jcm10214801
APA StyleFernández-Albarral, J. A., Martínez-López, M. A., Marco, E. M., de Hoz, R., Martín-Sánchez, B., San Felipe, D., Salobrar-García, E., López-Cuenca, I., Pinazo-Durán, M. D., Salazar, J. J., Ramírez, J. M., López-Gallardo, M., & Ramírez, A. I. (2021). Is Saffron Able to Prevent the Dysregulation of Retinal Cytokines Induced by Ocular Hypertension in Mice? Journal of Clinical Medicine, 10(21), 4801. https://doi.org/10.3390/jcm10214801













