The Role of Uric Acid in Acute and Chronic Coronary Syndromes
Abstract
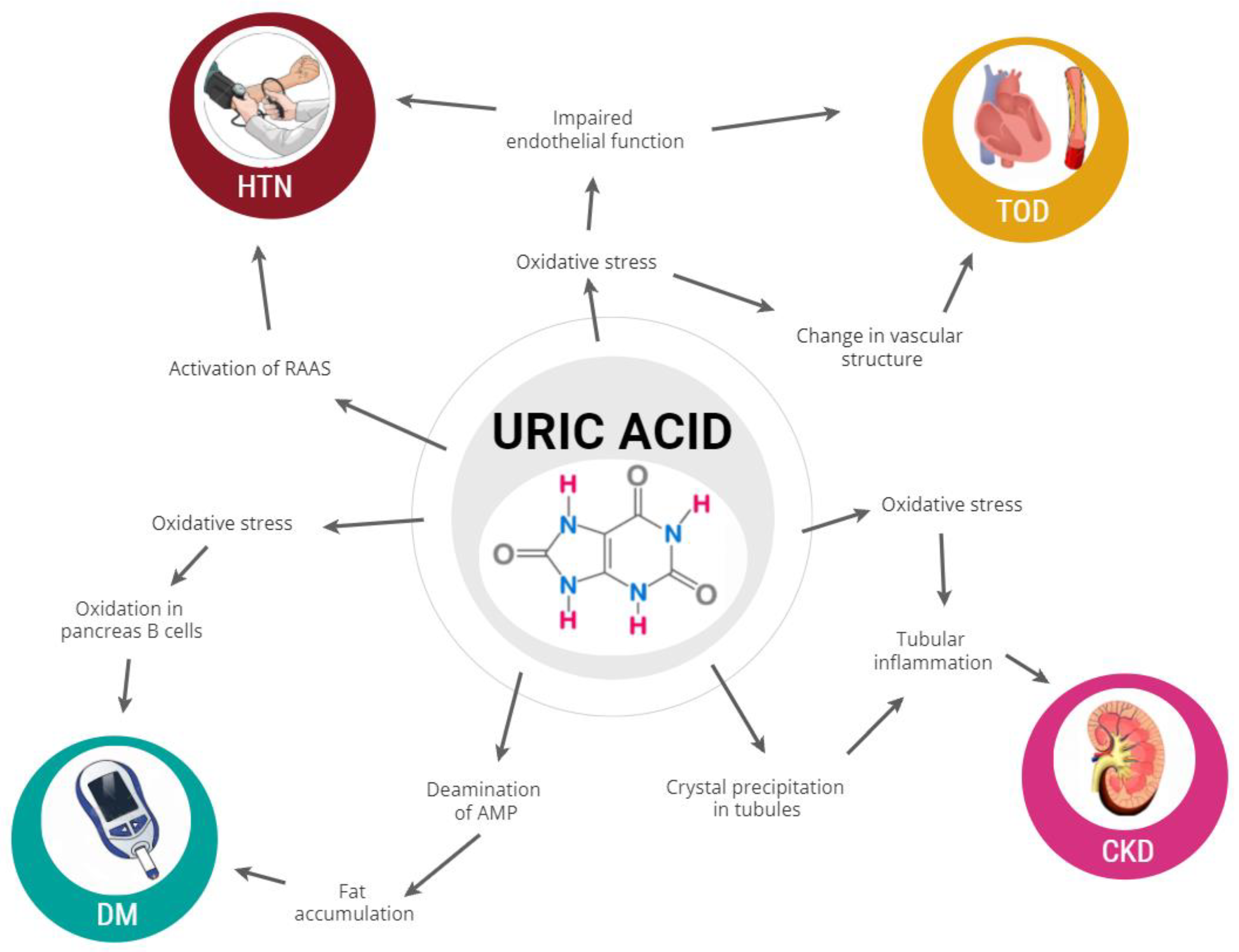
:1. Introduction
2. Uric Acid and Cardiovascular Events
3. Uric Acid and Acute Coronary Syndrome
4. Uric Acid and Chronic Coronary Syndrome
| Study | Type of Study | Supporting Data | Non-Supporting Data | Reference |
|---|---|---|---|---|
| Bos et al. | Prospective cohort study | Significant association between baseline UA and risk of both CAD and stroke, only slightly attenuated by adjustment for other CV risk factors | [4] | |
| Centola et al. | Prospective cohort study | High admission levels of UA are independently associated with in-hospital adverse outcomes and mortality of ACS patients | [11] | |
| Mehmet ed al. | Prospective cohort study | Elevated UA levels on admission are independently associated with impaired coronary flow after primary PCI and both short-term and long-term outcomes in patients who undergo primary PCI for the management of STEMI | [34] | |
| Nadkar et al. | Case control study | UA levels are higher in patients with acute MI and correlate with Killip class. | [36] | |
| Kobayashi et al. | Prospective cohort study | High UA levels are the primary predictor of 2-year cardiac mortality. | [37] | |
| Lazzeri et al. | Prospective cohort study | UA levels are associated to greater risk of acute plaque complications | [38] | |
| Okazaki et al. | Case control study | Plasma XOR activity was extremely high in patients with severely decompensated AHF, in association with a high lactate value and leading eventually to hyperuricaemia | [40] | |
| Maloberti et al. | Prospective cohort study | Diuretic-related hyperuricemia carry a similar risk of CV events and all-cause mortality when compared with individuals that present hyperuricemia in absence of diuretic therapy | [42] |
| Study | Type of Study | Supporting Data | Non-Supporting Data | Reference |
|---|---|---|---|---|
| Okura et al. | Population based cohort study | Elevated UA is an independent predictor of CV events and all-cause mortality combined in patients with CCS | [43] | |
| Tian et al. | Population based cohort study | UA levels were associated with the presence and severity of CAD; UA may be involved in the progression of CCS. | [48] | |
| Duran et al. | Population based cohort study | UA was significantly associated with number of diseased vessels and is an independent risk factor for multivessel disease. | [49] | |
| Karabağ et al. | Population based cohort study | UA was to be associated with high Syntax Score and long-term mortality in patients with MVD | [50] | |
| Tasić et al. | Population based cohort study | Asymptomatic hyperuricemia is not significantly associated with the severity of CAD | [51] | |
| Zand et al. | Case control study | UA is not an independent risk factor for premature CAD but is weakly correlated with the extent of the disease | [52] | |
| Verdoia et al. | Population based cohort study | Among diabetic patients, higher UA is not independently associated with the extent of CAD or with platelet aggregation. | [55] | |
| Maloberti et al. | Population based cohort study | UA do not play a role in determining coronary arteries disease as well as LV diastolic dysfunction in CCS subjects | [12] |
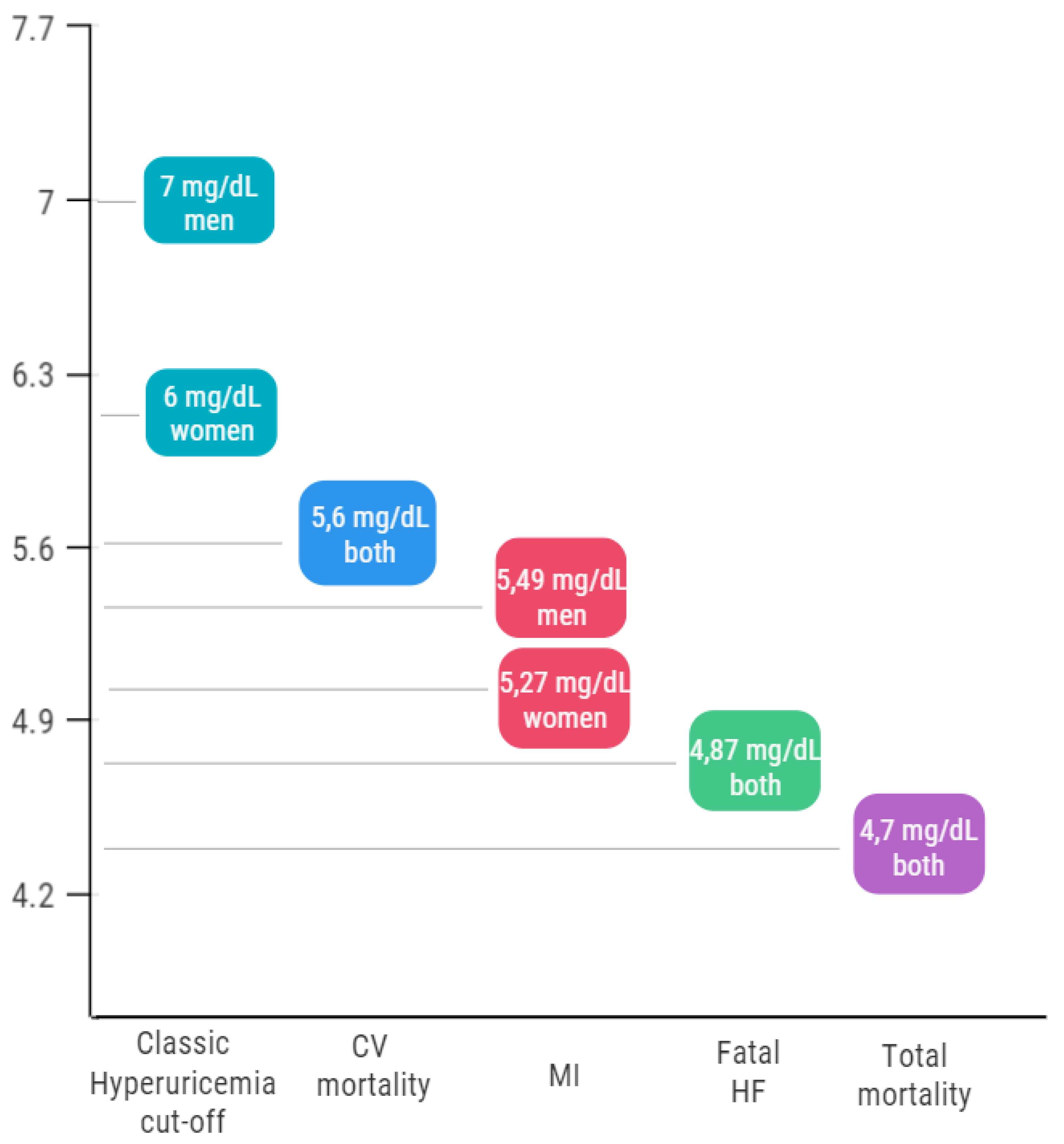
5. The First Open Question: The Cardiovascular Cut-Off
6. The Second Open Question: Are Uric-Acid-Lowering Therapies Effective in Reducing the Risk of Cardiovascular Events?
| Study | No of Participants | Drugs Compared | Outcomes | Results | Reference |
|---|---|---|---|---|---|
| CARES | 6198 | Febuxostat vs. Allopurinol | 4-component MACE (CV death, non-fatal MI, nonfatal stroke and unstable angina with urgent coronary revascularization) | Febuxostat is associated to a greater number of CV events | [73] |
| FAST | 6128 | Febuxostat vs. Allopurinol | Composite of hospitalization for non-fatal MI or biomarker-positive ACS; non-fatal stroke; CV death | Febuxostat is non-inferior to allopurinol | [74] |
| FREED | 1070 | Febuxostat vs. Other treatments | Composite of cerebral or cardiorenovascular events, all deaths | Febuxostat is associated to a redu-ction in renal events. | [76] |
| ALL-HEART | 5938 | Allopurinol vs. placebo | Composite of non-fatal MI, non-fatal stroke or CV death | On going | [77] |
| Huang et al. | 100 | Allopurinol vs. placebo | CV events | Allopurinol reduces inflammatory biomarkers and CV events | [78] |
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Maloberti, A.; Giannattasio, C.; Bombelli, M.; Desideri, G.; Cicero, A.F.G.; Muiesan, M.L.; Rosei, E.A.; Salvetti, M.; Ungar, A.; Rivasi, G.; et al. Hyperuricemia and risk of cardiovascular outcomes: The experience of the URRAH (Uric Acid Right for Heart Health) project. High Blood Press. Cardiovasc. Prev. 2020, 27, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Meisinger, C.; Koenig, W.; Baumert, J.; Doring, A. Uric acid levels are associated with all cause and cardiovascular disease mortality independent of systemic inflammation in men from the general population: The MONICA/KORA cohort study. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1186–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, J.; Alderman, M.H. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971–1992. National Health and nutrition examination survey. JAMA 2000, 283, 2404–2410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bos, M.J.; Koudstaal, P.J.; HofmaN, A.; Witteman, J.C.; Breteler, M.M. Uric acid is a risk factor for myocardial infarction and stroke: The Rotterdam study. Stroke 2006, 37, 1503–1507. [Google Scholar] [CrossRef] [Green Version]
- Muiesan, M.L.; Salvetti, M.; Virdis, A.; Masi, S.; Casiglia, E.; Tikhonoff, V.; Barbagallo, C.M.; Bombelli, M.; Cicero, A.F.G.; Cirillo, M.; et al. Serum uric acid, predicts heart failure in a large Italian cohort: Search for a cut-off value the URic acid Right for heArt Health study. J. Hypertens. 2021, 39, 62–69. [Google Scholar] [CrossRef]
- Tamariz, L.; Harzand, A.; Palacio, A.; Verma, S.; Jones, J.; Hare, J. Uric acid as a predictor of all-cause mortality in heart failure: A meta-analysis. Congest. Heart Fail. 2011, 17, 25–30. [Google Scholar] [CrossRef]
- Tamariz, L.; Agarwal, S.; Soliman, E.Z.; Chamberlain, A.M.; Prineas, R.; Folsom, A.R.; Ambrose, M.; Alonso, A. Association of serum uric acid with incident atrial fibrillation (from the Atherosclerosis Risk in Communities [ARIC] study). Am. J. Cardiol. 2011, 108, 1272–1276. [Google Scholar] [CrossRef] [Green Version]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Maloberti, A.; Qualliu, E.; Occhi, L.; Sun, J.; Grasso, E.; Tognola, C.; Tavecchia, G.; Cartella, I.; Milani, M.; Vallerio, P.; et al. Hyperuricemia prevalence in healthy subjects and its relationship with cardiovascular target organ damage. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 178–185. [Google Scholar] [CrossRef]
- Maloberti, A.; Maggioni, S.; Occhi, L.; Triglione, N.; Panzeri, F.; Nava, S.; Signorini, S.; Falbo, R.; Casati, M.; Grassi, G.; et al. Sex-related relationships between uric acid and target organ damage in hypertension. J. Clin. Hypertens. 2018, 20, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Centola, M.; Maloberti, A.; Castini, D.; Persampieri, S.; Sabatelli, L.; Ferrante, G.; Lucreziotti, S.; Morici, N.; Sacco, A.; Oliva, F.; et al. Impact of admission serum acid uric levels on in-hospital outcomes in patients with acute coronary syndrome. Eur. J. Intern. Med. 2020, 82, 62–67. [Google Scholar] [CrossRef]
- Maloberti, A.; Bossi, I.; Tassistro, E.; Rebora, P.; Racioppi, A.; Nava, S.; Soriano, F.; Piccaluga, E.; Piccalò, G.; Oreglia, J.; et al. Uric acid in chronic coronary syndromes: Relationship with coronary artery disease severity and left ventricular diastolic parameter. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1501–1508. [Google Scholar] [CrossRef]
- Kannel, W.B.; Castelli, W.P.; McNamara, P.M. The coronary profile: 12-year follow-up in the Framingham study. J. Occup. Med. 1967, 9, 611–619. [Google Scholar] [PubMed]
- Li, M.; Hu, X.; Fan, Y.; Li, K.; Zhang, X.; Hou, W.; Tang, Z. Hyperuricemia and the risk for coronary heart disease morbidity and mortality a systematic review and dose-response meta-analysis. Sci. Rep. 2016, 6, 19520. [Google Scholar] [CrossRef] [Green Version]
- Glantzounis, G.K.; Tsimoyiannis, E.C.; Kappas, A.M.; Galaris, D.A. Uric acid and oxidative stress. Curr. Pharm. Des. 2005, 11, 4145–4151. [Google Scholar] [CrossRef] [PubMed]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative stress in atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Berry, C.E.; Hare, J.M. Xanthine oxidoreductase and cardiovascular disease: Molecular mechanisms and pathophysiological implications. J. Physiol. 2004, 555, 589–606. [Google Scholar] [CrossRef]
- Bombelli, M.; Ronchi, I.; Volpe, M.; Facchetti, R.; Carugo, S.; Dell’oro, R.; Cuspidi, C.; Grassi, G.; Mancia, G. Prognostic value of serum uric acid: New-onset in and out-of-office hypertension and long-term mortality. J. Hypertens. 2014, 32, 1237–1244. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Hayashi, T.; Tsumura, K.; Endo, G.; Fujii, S.; Okada, K. Serumuric acid and the risk for hypertension and type 2 diabetes in Japanesemen: The Osaka Health Survey. J. Hypertens. 2001, 19, 1209–1215. [Google Scholar] [CrossRef]
- Pugliese, N.R.; Mengozzi, A.; Virdis, A.; Casiglia, E.; Tikhonoff, V.; Cicero, A.F.G.; Ungar, A.; Rivasi, G.; Salvetti, M.; Barbagallo, C.M.; et al. The importance of including uric acid in the definition of metabolic syndrome when assessing the mortality risk. Clin. Res. Cardiol. 2021, 110, 1073–1082. [Google Scholar] [CrossRef]
- Perlstein, T.S.; Gumieniak, O.; Hopkins, P.N.; Murphey, L.J.; Brown, N.J.; Williams, G.H.; Hollenberg, N.K.; Fisher, N.D. Uric acid and the state of the intrarenal renin-angiotensin system in humans. Kidney Int. 2004, 66, 1465–1470. [Google Scholar] [CrossRef] [Green Version]
- Kato, M.; Hisatome, I.; Tomikura, Y.; Kotani, K.; Kinugawa, T.; Ogino, K.; Ishida, K.; Igawa, O.; Shigemasa, C.; Somers, V.K. Status of endothelial dependent vasodilation in patients with hyperuricemia. Am. J. Cardiol. 2005, 96, 1576–1578. [Google Scholar] [CrossRef]
- Lanaspa, M.A.; Sanchez-Lozada, L.G.; Choi, Y.J.; Cicerchi, C.; Kanbay, M.; Roncal-Jimenez, C.A.; Ishimoto, T.; Li, N.; Marek, G.; Duranay, M.; et al. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: Potential role in fructose-dependent and -independent fatty liver. J. Biol. Chem. 2012, 287, 40732–40744. [Google Scholar] [CrossRef] [Green Version]
- Scott, F.W.; Trick, K.D.; Stavric, B.; Braaten, J.T.; Siddiqui, Y. Uric acid-induced decrease in rat insulin secretion. Proc. Soc. Exp. Biol. Med. 1981, 166, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Spencer, H.W.; Yarger, W.E.; Robinson, R.R. Alterations of renal function during dietary-induced hyperuricemia in the rat. Kidney Int. 1976, 9, 489–500. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Lozada, L.G.; Tapia, E.; Lopez-Molina, R.; Nepomuceno, T.; Soto, V.; Avila-Casado, C.; Nakagawa, T.; Johnson, R.J.; Herrera-Acosta, J.; Franco, M. Effects of acute and chronic L-arginine treatment in experimental hyperuricemia. Am. J. Physiol. 2007, 292, 1238–1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, E.; Viazzi, F.; Pontremoli, R.; Barbagallo, C.M.; Bombelli, M.; Casiglia, E.; Cicero, A.F.G.; Cirillo, M.; Cirillo, P.; Desideri, G.; et al. Association of uric acid with kidney function and albuminuria: The Uric Acid Right for heArt Health (URRAH) Project. J. Nephrol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Fathallah-Shaykh, S.A.; Cramer, M.T. Uric acid and the kidney. Pediatr. Nephrol. 2014, 29, 999–1008. [Google Scholar] [CrossRef]
- Rebora, P.; Andreano, A.; Triglione, N.; Piccinelli, E.; Palazzini, M.; Occhi, L.; Grassi, G.; Valsecchi, M.G.; Giannattasio, C.; Maloberti, A. Association between uric acid and pulse wave velocity in hypertensive patients and in the general population: A systematic review and meta-analysis. Blood Press. 2020, 29, 220–231. [Google Scholar] [CrossRef]
- Braga, F.; Pasqualetti, S.; Ferraro, S.; Panteghini, M. Hyperuricemia as risk factor for coronary heart disease incidence and mortality in the general population: A systematic review and meta-analysis. Clin. Chem. Lab. Med. 2016, 54, 7–15. [Google Scholar] [CrossRef]
- Storhaug, H.M.; Norvik, J.V.; Toft, I.; Eriksen, B.O.; Løchen, M.L.; Zykova, S.; Solbu, M.; White, S.; Chadban, S.; Jenssen, T. Uric acid is a risk factor for ischemic stroke and all-cause mortality in the general population: A gender specific analysis from The Tromsø Study. BMC Cardiovasc. Disord. 2013, 13, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolz, M.; Johnson, T.; Sanna, S.; Teumer, A.; Vitart, V.; Perola, M.; Mangino, M.; Albrecht, E.; Wallace, C.; Farrall, M.; et al. Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet. 2009, 5, e1000504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.; Yang, H.; Guo, X.; Zheng, L.; Sun, Y. Hyperuricemia is independently associated with left ventricular hypertrophy in post-menopausal women but not in pre-menopausal women in rural Northeast China. Gynecol. Endocrinol. 2015, 31, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.G.; Uyarel, H.; Akpek, M.; Kalay, N.; Ergelen, M.; Ayhan, E.; Isik, T.; Cicek, G.; Elcik, D.; Sahin, O.; et al. Prognostic value of uric acid in patients with ST-elevated myocardial infarction undergoing primary coronary intervention. Am. J. Cardiol. 2012, 109, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Basar, N.; Sen, N.; Ozcan, F.; Erden, G.; Kanat, S.; Sokmen, E.; Isleyen, A.; Yuzgecer, H.; Ozlu, M.F.; Yildirimkaya, M.; et al. Elevated serum uric acid predicts angiographic impaired reperfusion and 1-year mortality in ST-segment elevation myocardial infarction patients undergoing percutaneous coronary intervention. J. Investig. Med. 2011, 59, 931–937. [Google Scholar] [CrossRef]
- Nadkar, M.Y.; Jain, V.I. Serum uric acid in acute myocardial infarction. J. Assoc. Physicians India 2008, 56, 759–762. [Google Scholar]
- Kobayashi, N.; Asai, K.; Tsurumi, M.; Shibata, Y.; Okazaki, H.; Shirakabe, A.; Goda, H.; Uchiyama, S.; Tani, K.; Takano, M.; et al. Impact of accumulated serum uric acid on coronary culprit lesion morphology determined by optical coherence tomography and cardiac outcomes in patients with acute coronary syndrome. Cardiology 2018, 141, 190–198. [Google Scholar] [CrossRef]
- Lazzeri, C.; Valente, S.; Chiostri, M.; Picariello, C.; Gensini, G.F. Uric acid in the early risk stratification of ST-elevation myocardial infarction. Intern. Emerg. Med. 2012, 7, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Akpek, M.; Kaya, M.G.; Uyarel, H.; Yarlioglues, M.; Kalay, N.; Gunebakmaz, O.; Dogdu, O.; Ardic, I.; Elcik, D.; Sahin, O.; et al. The association of serum uric acid levels on coronary flow in patients with STEMI undergoing primary PCI. Atherosclerosis 2011, 219, 334–341. [Google Scholar] [CrossRef]
- Okazaki, H.; Shirakabe, A.; Matsushita, M.; Shibata, Y.; Sawatani, T.; Uchiyama, S.; Tani, K.; Murase, T.; Nakamura, T.; Takayasu, T.; et al. Plasma xanthine oxidoreductase activity in patients with decompensated acute heart failure requiring intensive care. ESC Heart Fail. 2019, 6, 336–343. [Google Scholar] [CrossRef]
- Doehner, W.; Jankowska, E.A.; Springer, J.; Lainscak, M.; Anker, S.D. Uric acid and xanthine oxidase in heart failure—Emerging data and therapeutic implications. Int. J. Cardiol. 2016, 213, 15–19. [Google Scholar] [CrossRef]
- Maloberti, A.; Bombelli, M.; Facchetti, R.; Barbagallo, C.M.; Bernardino, B.; Rosei, E.A.; Casiglia, E.; Cicero, A.F.G.; Cirillo, M.; Cirillo, P.; et al. Relationships between diuretic-related hyperuricemia and cardiovascular events: Data from the URic acid Right for heArt Health study. J. Hypertens. 2021, 39, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Okura, T.; Higaki, J.; Kurata, M.; Irita, J.; Miyoshi, K.; Yamazaki, T.; Hayashi, D.; Kohro, T.; Nagai, R. Elevated serum uric acid is an independent predictor for cardiovascular events in patients with severe coronary artery stenosis: Subanalysis of the Japanese Coronary Artery Disease (JCAD) Study. Circ. J. 2009, 73, 885–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spoon, D.B.; Lerman, A.; Rule, A.D.; Prasad, A.; Lennon, R.J.; Holmes, D.R.; Rihal, C.S. The association of serum uric acid levels with outcomes following percutaneous coronary intervention. J. Interv. Cardiol. 2010, 23, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Silbernagel, G.; Hoffmann, M.M.; Grammer, T.B.; Boehm, B.O.; Marz, W. Uric acid is predictive of cardiovascular mortality and sudden cardiac death in subjects referred for coronary angiography. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 46–52. [Google Scholar] [CrossRef]
- Ndrepepa, G.; Braun, S.; King, L.; Fusaro, M.; Tada, T.; Cassese, S.; Hadamitzky, M.; Haase, H.U.; Schömig, A.; Kastrati, A. Uric acid and prognosis in angiography-proven coronary artery disease. Eur. J. Clin. Investig. 2013, 43, 256–266. [Google Scholar] [CrossRef] [Green Version]
- Bickel, C.; Rupprecht, H.J.; Blankenberg, S.; Rippin, G.; Hafner, G.; Daunhauer, A.; Hofmann, K.P.; Meyer, J. Serum uric acid as an independent predictor of mortality in patients with angiographically proven coronary artery disease. Am. J. Cardiol. 2002, 89, 12–17. [Google Scholar] [CrossRef]
- Tian, T.T.; Li, H.; Chen, S.J.; Wang, Q.; Tian, Q.W.; Zhang, B.B.; Zhu, J.; He, G.W.; Lun, L.M.; Xuan, C. Serum uric acid as an independent risk factor for the presence and severity of early-onset coronary artery disease: A case-control study. Dis. Markers 2018, 2018, 1236837. [Google Scholar] [CrossRef] [PubMed]
- Duran, M.; Kalay, N.; Akpek, M.; Orscelik, O.; Elcik, D.; Ocak, A.; Inanc, M.T.; Kasapkara, H.A.; Oguzhan, A.; Eryol, N.K.; et al. High levels of serum uric acid predict severity of coronary artery disease in patients with acute coronary syndrome. Angiology 2012, 63, 448–452. [Google Scholar] [CrossRef]
- Karabağ, Y.; Rencuzogullari, I.; Çağdaş, M.; Karakoyun, S.; Yesin, M.; Atalay, E.; Çağdaş, Ö.S.; Gürsoy, M.O.; Burak, C.; Tanboğa, H.I. Association of serum uric acid levels with SYNTAX score II and long term mortality in the patients with stable angina pectoris who undergo percutaneous coronary interventions due to multivessel and/or unprotected left main disease. Int. J. Cardiovasc. Imaging 2019, 35, 1–7. [Google Scholar] [CrossRef]
- Tasić, I.; Kostić, S.; Stojanović, N.M.; Skakić, V.; Cvetković, J.; Djordjević, A.; Karadzić, M.; Djordjević, D.; Andonov, S.; Stoičkov, V.; et al. Significance of asymptomatic hyperuricemia in patients after coronary events. Scand. J. Clin. Lab. Investig. 2018, 78, 312–317. [Google Scholar] [CrossRef]
- Zand, S.; Shafiee, A.; Boroumand, M.; Jalali, A.; Nozari, Y. Serum uric Acid is not an independent risk factor for premature coronary artery disease. Cardiorenal Med. 2013, 3, 246–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaubert, M.; Marlinge, M.; Alessandrini, M.; Laine, M.; Bonello, L.; Fromonot, J.; Cautela, J.; Thuny, F.; Barraud, J.; Mottola, G.; et al. Uric acid levels are associated with endothelial dysfunction and severity of coronary atherosclerosis during a first episode of acute coronary syndrome. Purinergic Signal. 2018, 14, 191–199. [Google Scholar] [CrossRef]
- Barbieri, L.; Verdoia, M.; Schaffer, A.; Marino, P.; Suryapranata, H.; De Luca, G. Impact of sex on uric acid levels and its relationship with the extent of coronary artery disease: A single-centre study. Atherosclerosis 2015, 241, 241–248. [Google Scholar] [CrossRef]
- Verdoia, M.; Barbieri, L.; Schaffer, A.; Cassetti, E.; Nardin, M.; Bellomo, G.; Aimaretti, G.; Marino, P.; Sinigaglia, F.; De Luca, G. Impact of diabetes on uric acid and its relationship with the extent of coronary artery disease and platelet aggregation: A single-centre cohort study. Metabolism 2014, 63, 640–646. [Google Scholar] [CrossRef]
- Zhang, J.W.; He, L.J.; Cao, S.J.; Yang, Q.; Yang, S.W.; Zhou, Y.J. Association of serum uric acid and coronary artery disease in premenopausal women. PLoS ONE 2014, 9, e106130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demir, Ş.; Karakoyun, G.; Kanadasi, M. Elevated high sensitivity C-reactive protein and uric acid levels in coronary artery ectasia. Acta Biochim. Pol. 2014, 61, 687–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virdis, A.; Masi, S.; Casiglia, E.; Tikhonoff, V.; Cicero, A.F.G.; Ungar, A.; Rivasi, G.; Salvetti, M.; Barbagallo, C.M.; Bombelli, M.; et al. Identification of the uric acid thresholds predicting an increased total and cardiovascular mortality over 20 years. Hypertension 2020, 75, 302–308. [Google Scholar] [CrossRef]
- Casiglia, E.; Tikhonoff, V.; Virdis, A.; Masi, S.; Barbagallo, C.M.; Bombelli, M.; Bruno, B.; Cicero, A.F.G.; Cirillo, M.; Cirillo, P.; et al. Serum uric acid and fatal myocardial infarction: Detection of prognostic cut-off values: The URRAH (Uric Acid Right for Heart Health) study. J. Hypertens. 2020, 38, 412–419. [Google Scholar] [CrossRef]
- Kojima, S.; Sakamoto, T.; Ishihara, M.; Kimura, K.; Miyazaki, S.; Yamagishi, M.; Tei, C.; Hiraoka, H.; Sonoda, M.; Tsuchihashi, K.; et al. Prognostic usefulness of serum uric acid after acute myocardial infarction (the Japanese Acute Coronary Syndrome Study). Am. J. Cardiol. 2005, 96, 489–495. [Google Scholar] [CrossRef]
- Bae, E.; Cho, H.J.; Shin, N.; Kim, S.M.; Yang, S.H.; Kim, D.K.; Kim, Y.L.; Kang, S.W.; Yang, C.W.; Kim, N.H.; et al. Lower serum uric acid level predicts mortality in dialysis patients. Medicine 2016, 95, e3701. [Google Scholar] [CrossRef] [PubMed]
- Browne, L.D.; Jaouimaa, F.Z.; Walsh, C.; Perez-Ruiz, F.; Richette, P.; Burke, K.; Stack, A.G. Serum uric acid and mortality thresholds among men and women in the Irish health system: A cohort study. Eur. J. Intern. Med. 2021, 84, 46–55. [Google Scholar] [CrossRef]
- Hink, H.U.; Santanam, N.; Dikalov, S.; McCann, L.; Nguyen, A.D.; Parthasarathy, S.; Harrison, D.G.; Fukai, T. Peroxidase properties of extracellular superoxide dismutase role of uric acid in modulating in vivo activity. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1402–1408. [Google Scholar] [CrossRef] [Green Version]
- Stamp, L.K.; Merriman, T.R.; Singh, J.A. Expert opinion on emerging urate-lowering therapies. Expert Opin. Emerg. Drugs 2018, 23, 201–209. [Google Scholar] [CrossRef]
- Clebak, K.T.; Morrison, A.; Croad, J.R. Gout: Rapid evidence review. Am. Fam. Physician 2020, 102, 533–538. [Google Scholar]
- Beattie, C.J.; Fulton, R.L.; Higgins, P.; Padmanabhan, S.; McCallum, L.; Walters, M.R.; Dominiczak, A.F.; Touyz, R.M.; Dawson, J. Allopurinol initiation and change in blood pressure in older adults with hypertension. Hypertension 2014, 64, 1102–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, P.; Walters, M.R.; Murray, H.M.; McArthur, K.; McConnachie, A.; Lees, K.R.; Dawson, J. Allopurinol reduces brachial and central blood pressure, and carotid intima-media thickness progression after ischaemic stroke and transient ischaemic attack: A randomised controlled trial. Heart 2014, 100, 1085–1092. [Google Scholar] [CrossRef]
- Kao, M.P.; Ang, D.S.; Gandy, S.J.; Nadir, M.A.; Houston, J.G.; Lang, C.C.; Struthers, A.D. Allopurinol benefits left ventricular mass and endothelial dysfunction in chronic kidney disease. J. Am. Soc. Nephrol. 2011, 22, 1382–1389. [Google Scholar] [CrossRef] [Green Version]
- Cappola, T.P.; Kass, D.A.; Nelson, G.S.; Berger, R.D.; Rosas, G.O.; Kobeissi, Z.A.; Marbán, E.; Hare, J.M. Allopurinol improves myocardial efficiency in patients with idiopathic dilated cardiomyopathy. Circulation 2001, 104, 2407–2411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noman, A.; Ang, D.S.; Ogston, S.; Lang, C.C.; Struthers, A.D. Effect of high-dose allopurinol on exercise in patients with chronic stable angina: A randomised, placebo controlled crossover trial. Lancet 2010, 375, 2161–2167. [Google Scholar] [CrossRef] [Green Version]
- Rajendra, N.S.; Ireland, S.; George, J.; Belch, J.J.; Lang, C.C.; Struthers, A.D. Mechanistic insights into the therapeutic use of high-dose allopurinol in angina pectoris. J. Am. Coll. Cardiol. 2011, 58, 820–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimaldi-Bensouda, L.; Alpérovitch, A.; Aubrun, E.; Danchin, N.; Rossignol, M.; Abenhaim, L.; Richette, P. Impact of allopurinol on risk of myocardial infarction. Ann. Rheum. Dis. 2015, 74, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Pontremoli, R. The role of urate-lowering treatment on cardiovascular and renal disease: Evidence from CARES, FAST, ALL-HEART, and FEATHER studies. Curr. Med. Res. Opin. 2017, 33, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Mackenzie, I.S.; Ford, I.; Nuki, G.; Hallas, J.; Hawkey, C.J.; Webster, J.; Ralston, S.H.; Walters, M.; Robertson, M.; De Caterina, R.; et al. Long-term cardiovascular safety of febuxostat compared with allopurinol in patients with gout (FAST): A multicentre, prospective, randomised, open-label, non-inferiority trial. Lancet 2020, 396, 1745–1757. [Google Scholar] [CrossRef]
- Katsiki, N.; Borghi, C. The future of febuxostat after the Cardiovascular Safety of Febuxostat and Allopurinol in Patients with Gout and Cardiovascular Morbidities (CARES) trial: Who CARES? Expert Opin. Pharmacother. 2018, 19, 1853–1856. [Google Scholar] [CrossRef] [Green Version]
- Kojima, S.; Matsui, K.; Ogawa, H.; Jinnouchi, H.; Hiramitsu, S.; Hayashi, T.; Yokota, N.; Kawai, N.; Tokutake, E.; Uchiyama, K.; et al. Rationale, design, and baseline characteristics of a study to evaluate the effect of febuxostat in preventing cerebral, cardiovascular, and renal events in patients with hyperuricemia. J. Cardiol. 2017, 69, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Mackenzie, I.S.; Ford, I.; Walker, A.; Hawkey, C.; Begg, A.; Avery, A.; Taggar, J.; Wei, L.; Struthers, A.D.; MacDonald, T.M. Multicentre, prospective, randomised, open-label, blinded end point trial of the efficacy of allopurinol therapy in improving cardiovascular outcomes in patients with ischaemic heart disease: Protocol of the ALL-HEART study. BMJ Open 2016, 6, e013774. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, C.; Xu, Z.; Shen, J.; Zhang, X.; Du, H.; Zhang, K.; Zhang, D. Clinical Study on efficacy of allopurinol in patients with acute coronary syndrome and its functional mechanism. Hellenic J. Cardiol. 2017, 58, 360–365. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maloberti, A.; Biolcati, M.; Ruzzenenti, G.; Giani, V.; Leidi, F.; Monticelli, M.; Algeri, M.; Scarpellini, S.; Nava, S.; Soriano, F.; et al. The Role of Uric Acid in Acute and Chronic Coronary Syndromes. J. Clin. Med. 2021, 10, 4750. https://doi.org/10.3390/jcm10204750
Maloberti A, Biolcati M, Ruzzenenti G, Giani V, Leidi F, Monticelli M, Algeri M, Scarpellini S, Nava S, Soriano F, et al. The Role of Uric Acid in Acute and Chronic Coronary Syndromes. Journal of Clinical Medicine. 2021; 10(20):4750. https://doi.org/10.3390/jcm10204750
Chicago/Turabian StyleMaloberti, Alessandro, Marco Biolcati, Giacomo Ruzzenenti, Valentina Giani, Filippo Leidi, Massimiliano Monticelli, Michela Algeri, Sara Scarpellini, Stefano Nava, Francesco Soriano, and et al. 2021. "The Role of Uric Acid in Acute and Chronic Coronary Syndromes" Journal of Clinical Medicine 10, no. 20: 4750. https://doi.org/10.3390/jcm10204750
APA StyleMaloberti, A., Biolcati, M., Ruzzenenti, G., Giani, V., Leidi, F., Monticelli, M., Algeri, M., Scarpellini, S., Nava, S., Soriano, F., Oreglia, J., Sacco, A., Morici, N., Oliva, F., Piani, F., Borghi, C., & Giannattasio, C. (2021). The Role of Uric Acid in Acute and Chronic Coronary Syndromes. Journal of Clinical Medicine, 10(20), 4750. https://doi.org/10.3390/jcm10204750







