Gender Differences in the Impact of Plasma Xanthine Oxidoreductase Activity on Coronary Artery Spasm
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. XOR Activity Assay
2.3. Statistical Analysis
3. Results
3.1. Comparisons of Clinical Characteristics between Males and Females
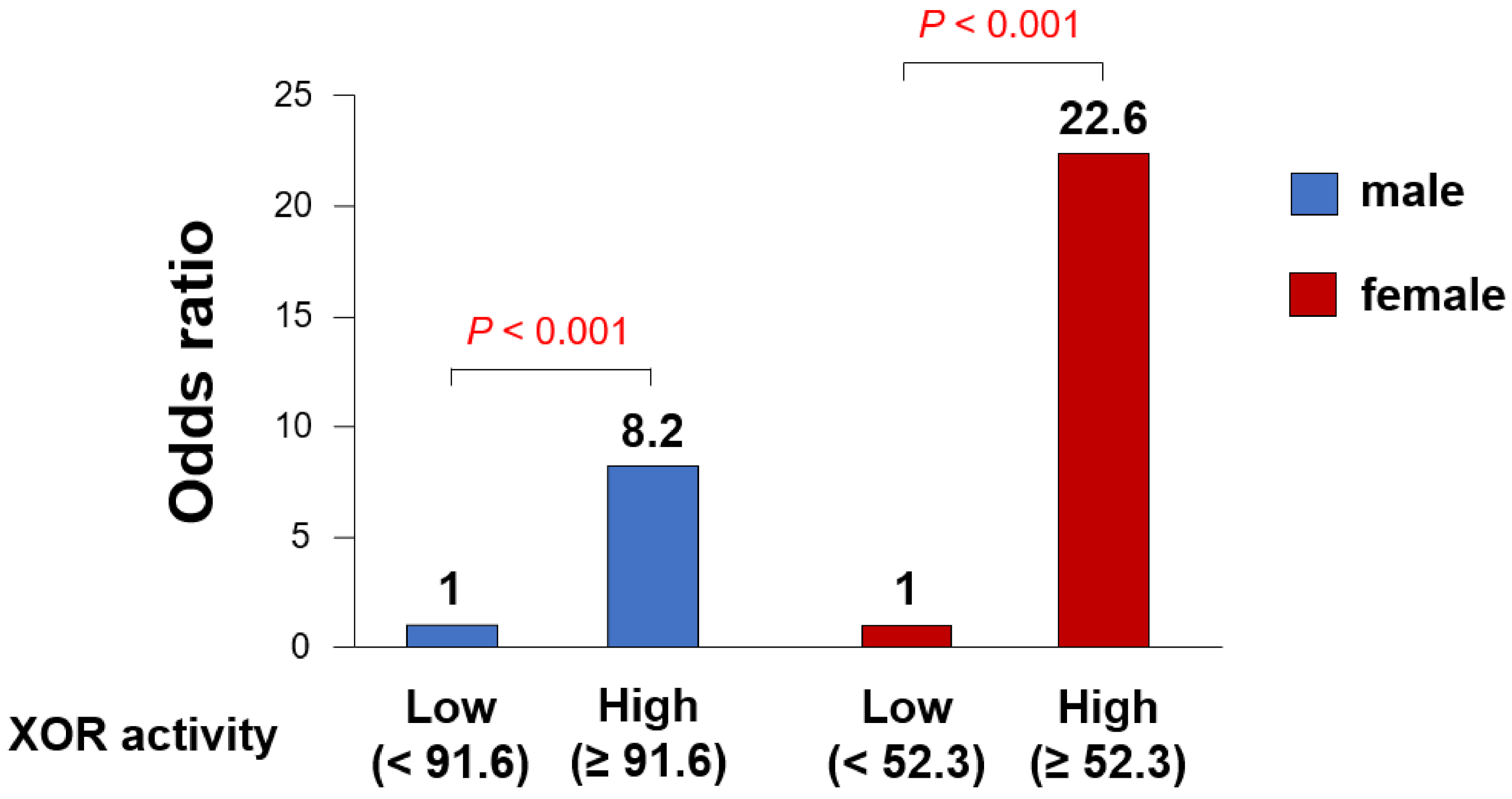
3.2. Gender Differences in the Impact of Plasma XOR Activity on CAS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Satoh, S.; Omura, S.; Inoue, H.; Mori, T.; Takenaka, K.; Numaguchi, K.; Mori, E.; Aso, A.; Nakamura, T.; Hiyamuta, K. Clinical impact of coronary artery spasm in patients with no significant coronary stenosis who are experiencing acute coronary syndrome. J. Cardiol. 2013, 61, 404–409. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wakabayashi, K.; Suzuki, H.; Honda, Y.; Wakatsuki, D.; Kawachi, K.; Ota, K.; Koba, S.; Shimizu, N.; Asano, F.; Sato, T.; et al. Provoked coronary spasm predicts adverse outcome in patients with acute myocardial infarction: A novel predictor of prognosis after acute myocardial infarction. J. Am. Coll. Cardiol. 2008, 52, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Vaccarino, V.; Parsons, L.; Every, N.R.; Barron, H.V.; Krumholz, H.M. Sex-based differences in early mortality after myocardial infarction. National Registry of Myocardial Infarction 2 Participants. N. Engl. J. Med. 1999, 341, 217–225. [Google Scholar] [CrossRef]
- Sato, K.; Kaikita, K.; Nakayama, N.; Horio, E.; Yoshimura, H.; Ono, T.; Ohba, K.; Tsujita, K.; Kojima, S.; Tayama, S.; et al. Coronary vasomotor response to intracoronary acet.t.tylcholine injection, clinical features, and long-term prognosis in 873 consecutive patients with coronary spasm: Analysis of a single-center study over 20 years. J. Am. Heart Assoc. 2013, 2, e000227. [Google Scholar] [CrossRef] [PubMed]
- Kawano, H.; Node, K. The role of vascular failure in coronary artery spasm. J. Cardiol. 2011, 57, 2–7. [Google Scholar] [CrossRef]
- Ando, K.; Takahashi, H.; Watanabe, T.; Daidoji, H.; Otaki, Y.; Nishiyama, S.; Arimoto, T.; Shishido, T.; Miyashita, T.; Miyamoto, T.; et al. Impact of Serum Uric Acid Levels on Coronary Plaque Stability Evaluated Using Integrated Backscatter Intravascular Ultrasound in Patients with Coronary Artery Disease. J. Atheroscler. Thromb. 2016, 23, 932–939. [Google Scholar] [CrossRef]
- Saito, Y.; Tanaka, A.; Node, K.; Kobayashi, Y. Uric acid and cardiovascular disease: A clinical review. J. Cardiol. 2021, 78, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Nishino, M.; Mori, N.; Yoshimura, T.; Nakamura, D.; Lee, Y.; Taniike, M.; Makino, N.; Kato, H.; Egami, Y.; Shutta, R.; et al. Higher serum uric acid and lipoprotein(a) are correlated with coronary spasm. Heart Vessel. 2014, 29, 186–190. [Google Scholar] [CrossRef]
- Chen, C.; Lu, J.M.; Yao, Q. Hyperuricemia-Related Diseases and Xanthine Oxidoreductase (XOR) Inhibitors: An Overview. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 2501–2512. [Google Scholar] [CrossRef]
- Spiekermann, S.; Landmesser, U.; Dikalov, S.; Bredt, M.; Gamez, G.; Tatge, H.; Reepschläger, N.; Hornig, B.; Drexler, H.; Harrison, D.G. Electron spin resonance characterization of vascular xanthine and NAD(P)H oxidase activity in patients with coronary artery disease: Relation to endothelium-dependent vasodilation. Circulation 2003, 107, 1383–1389. [Google Scholar] [CrossRef]
- Otaki, Y.; Watanabe, T.; Kinoshita, D.; Yokoyama, M.; Takahashi, T.; Toshima, T.; Sugai, T.; Murase, T.; Nakamura, T.; Nishiyama, S.; et al. Association of plasma xanthine oxidoreductase activity with severity and clinical outcome in patients with chronic heart failure. Int. J. Cardiol. 2017, 228, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, H.; Shirakabe, A.; Matsushita, M.; Shibata, Y.; Sawatani, T.; Uchiyama, S.; Tani, K.; Murase, T.; Nakamura, T.; Takayasu, T.; et al. Plasma xanthine oxidoreductase activity in patients with decompensated acute heart failure requiring intensive care. ESC Heart Fail. 2019, 6, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Shishido, T.; Otaki, Y.; Watanabe, T.; Sugai, T.; Toshima, T.; Takahashi, T.; Yokoyama, M.; Kinoshita, D.; Murase, T.; et al. Increased plasma xanthine oxidoreductase activity deteriorates coronary artery spasm. Heart Vessel. 2019, 34, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Alderman, M.H. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971–1992. National Health and Nutrition Examination Survey. JAMA 2000, 283, 2404–2410. [Google Scholar] [CrossRef] [PubMed]
- JCS Joint Working Group. Guidelines for diagnosis and treatment of patients with vasospastic angina (Coronary Spastic Angina) (JCS 2013). Circ. J. Off. J. Jpn. Circ. Soc. 2014, 78, 2779–2801. [Google Scholar]
- Murase, T.; Nampei, M.; Oka, M.; Miyachi, A.; Nakamura, T. A highly sensitive assay of human plasma xanthine oxidoreductase activity using stable isotope-labeled xanthine and LC/TQMS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1039, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2009, 53, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Yasue, H.; Yoshimura, M.; Shimasaki, Y.; Kugiyama, K.; Ogawa, H.; Motoyama, T.; Saito, Y.; Ogawa, Y.; Miyamoto, Y.; et al. T-786-->C mutation in the 5′-flanking region of the endothelial nitric oxide synthase gene is associated with coronary spasm. Circulation 1999, 99, 2864–2870. [Google Scholar] [CrossRef]
- Murase, Y.; Yamada, Y.; Hirashiki, A.; Ichihara, S.; Kanda, H.; Watarai, M.; Takatsu, F.; Murohara, T.; Yokota, M. Genetic risk and gene-environment interaction in coronary artery spasm in Japanese men and women. Eur. Heart J. 2004, 25, 970–977. [Google Scholar] [CrossRef]
- Yang, Y.M.; Huang, A.; Kaley, G.; Sun, D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1829–H1836. [Google Scholar] [CrossRef]
- Gielis, J.F.; Lin, J.Y.; Wingler, K.; Van Schil, P.E.; Schmidt, H.H.; Moens, A.L. Pathogenetic role of eNOS uncoupling in cardiopulmonary disorders. Free Radic. Biol. Med. 2011, 50, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, M.; Matsumoto, M.; Tanaka, M.; Moniwa, N.; Murase, T.; Nakamura, T.; Ohnishi, H.; Saitoh, S.; Shimamoto, K.; Miura, T. Plasma Xanthine Oxidoreductase Activity as a Novel Biomarker of Metabolic Disorders in a General Population. Circ. J. Off. J. Jpn. Circ. Soc. 2018, 82, 1892–1899. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, M.; Matsumoto, M.; Murase, T.; Nakamura, T.; Higashiura, Y.; Koyama, M.; Tanaka, M.; Moniwa, N.; Ohnishi, H.; Saitoh, S.; et al. Independent links between plasma xanthine oxidoreductase activity and levels of adipokines. J. Diabetes Investig. 2019, 10, 1059–1067. [Google Scholar] [CrossRef]
- Tsushima, Y.; Nishizawa, H.; Tochino, Y.; Nakatsuji, H.; Sekimoto, R.; Nagao, H.; Shirakura, T.; Kato, K.; Imaizumi, K.; Takahashi, H.; et al. Uric acid secretion from adipose tissue and its increase in obesity. J. Biol. Chem. 2013, 288, 27138–27149. [Google Scholar] [CrossRef] [PubMed]
- Geer, E.B.; Shen, W. Gender differences in insulin resistance, body composition, and energy balance. Gend. Med. 2009, 6 (Suppl. 1), 60–75. [Google Scholar] [CrossRef]
- Adamopoulos, D.; Vlassopoulos, C.; Seitanides, B.; Contoyiannis, P.; Vassilopoulos, P. The relationship of sex steroids to uric acid levels in plasma and urine. Acta Endocrinol. 1977, 85, 198–208. [Google Scholar] [CrossRef]
- Feig, D.I.; Kang, D.H.; Johnson, R.J. Uric acid and cardiovascular risk. N. Engl. J. Med. 2008, 359, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
- Kozakowski, J.; Gietka-Czernel, M.; Leszczynska, D.; Majos, A. Obesity in menopause—Our negligence or an unfortunate inevitability? Prz. Menopauzalny Menopause Rev. 2017, 16, 61–65. [Google Scholar] [CrossRef] [PubMed]
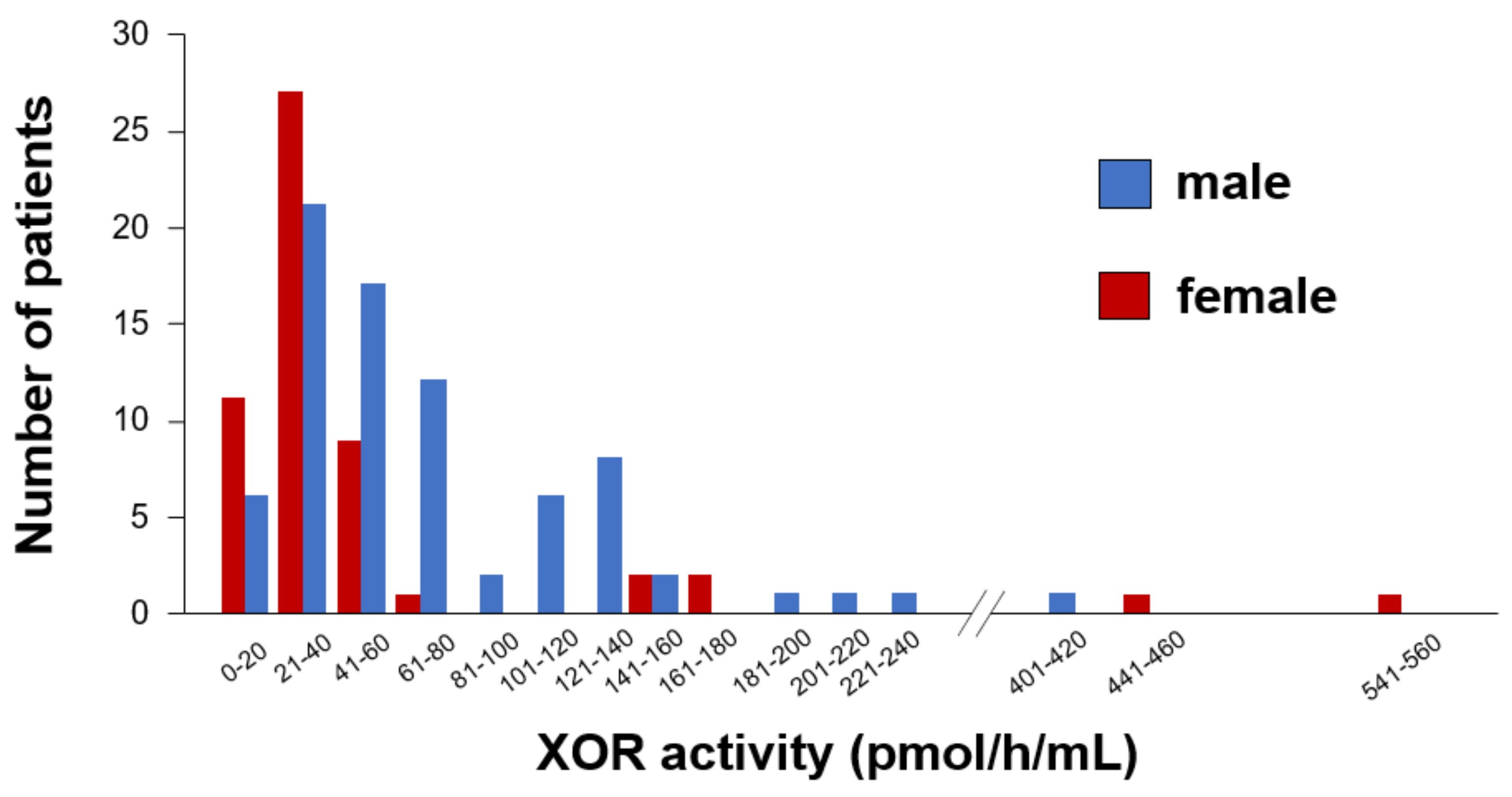
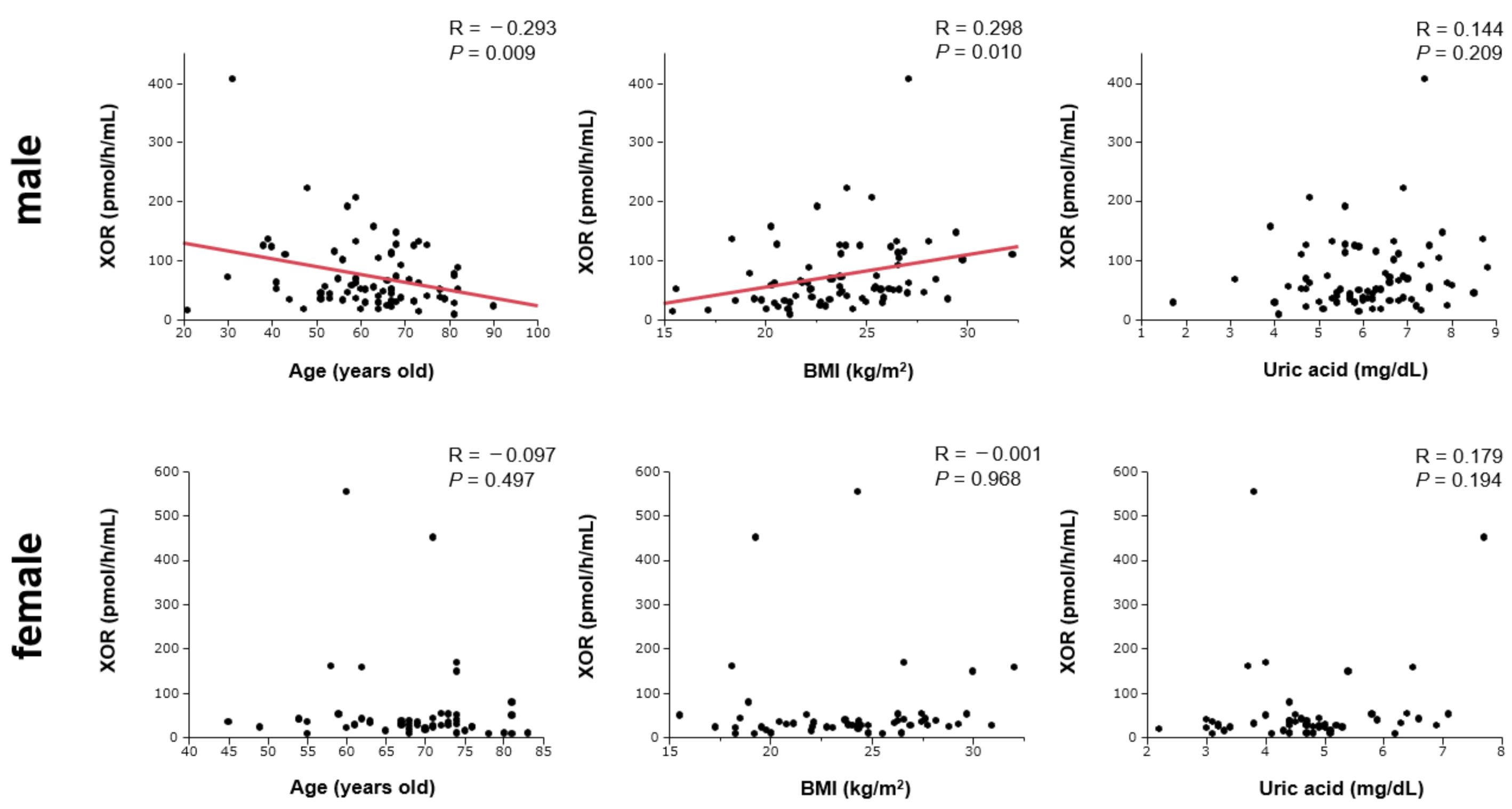
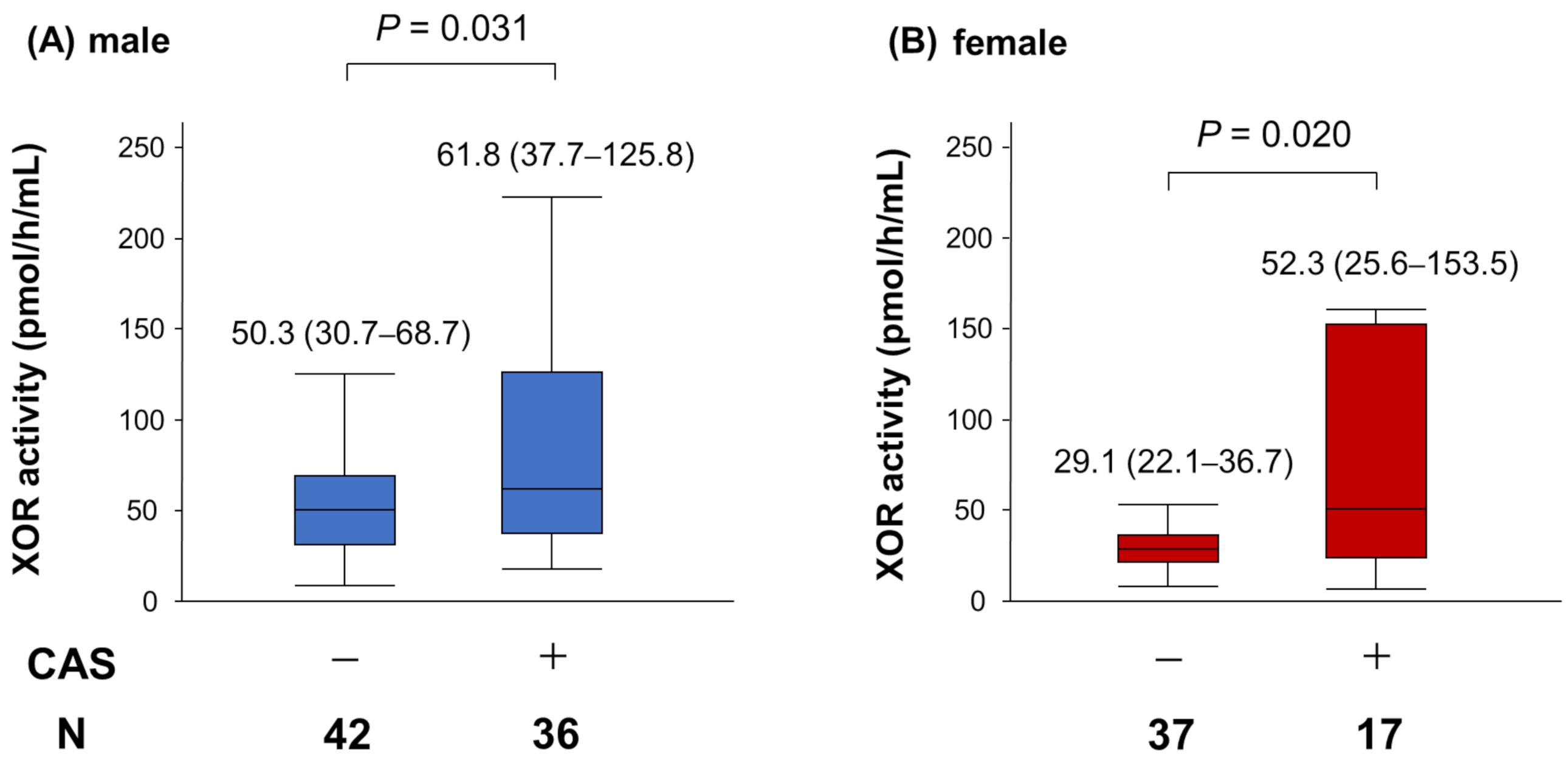
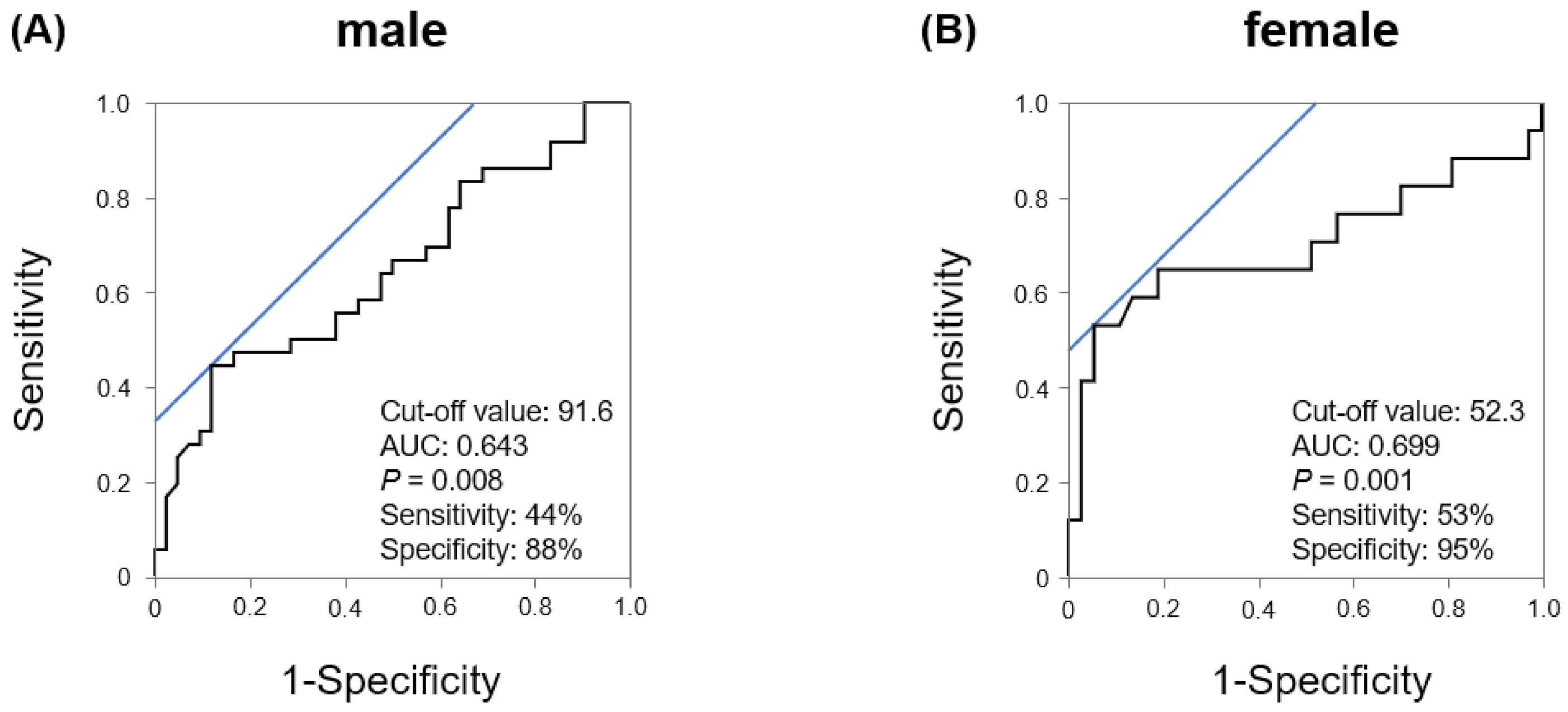
| Variables | Male n = 78 | Female n = 54 | p Value |
|---|---|---|---|
| Age (years old) | 62 ± 13 | 68 ± 8 | 0.003 |
| BMI (kg/m2) | 23.6 ± 3.3 | 23.8 ± 3.9 | 0.728 |
| Hypertension, n (%) | 50 (64) | 31 (57) | 0.438 |
| Dyslipidemia, n (%) | 32 (41) | 31 (57) | 0.064 |
| Diabetes mellitus, n (%) | 12 (15) | 7 (13) | 0.695 |
| Smoking, n (%) | 43 (55) | 18 (33) | 0.013 |
| Blood examination | |||
| Triglycerides (mg/dL) | 128 (93–188) | 100 (76–132) | 0.006 |
| LDL-C (mg/dL) | 102 ± 28 | 107 ± 26 | 0.239 |
| HDL-C (mg/dL) | 50 ± 9 | 62 ± 18 | <0.001 |
| HbA1c (%) | 5.7 ± 0.8 | 5.7 ± 0.6 | 0.729 |
| eGFR (mL/min/1.73 m2) | 79 ± 22 | 72 ± 17 | 0.045 |
| UA (mg/dL) | 6.1 ± 1.3 | 4.7 ± 1.1 | <0.001 |
| XOR (pmol/h/mL) | 51.7 (34.7–101.8) | 30.3 (22.8–42.7) | <0.001 |
| hs-CRP (mg/dL) | 0.053 (0.021–0.133) | 0.032 (0.018–0.087) | 0.052 |
| Medications | |||
| ACEIs and/or ARBs, n (%) | 37 (47) | 18 (33) | 0.104 |
| CCBs, n (%) | 52 (67) | 40 (74) | 0.360 |
| Statins, n (%) | 33 (42) | 24 (44) | 0.808 |
| Antiplatelet drugs, n (%) | 41 (53) | 25 (46) | 0.479 |
| Nitrates, n (%) | 27 (35) | 12 (22) | 0.121 |
| Nicorandils, n (%) | 27 (35) | 15 (28) | 0.446 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variables | OR | 95% CI | p-Value | OR | 95% CI | p-Value |
| Age † | 0.903 | 0.570–1.418 | 0.656 | |||
| BMI † | 1.236 | 0.778–2.008 | 0.371 | |||
| Hypertension | 1.231 | 0.486–3.167 | 0.662 | |||
| Dyslipidemia | 2.000 | 0.806–5.076 | 0.135 | |||
| Diabetes mellitus | 0.333 | 0.069–1.231 | 0.102 | |||
| Smoking | 0.680 | 0.274–1.666 | 0.399 | |||
| Triglycerides † | 1.480 | 0.931–2.515 | 0.099 | |||
| LDL-C † | 1.215 | 0.775–1.931 | 0.396 | |||
| HDL-C † | 0.642 | 0.384–1.024 | 0.063 | 0.495 | 0.264–0.849 | 0.010 |
| HbA1c † | 0.799 | 0.473–1.263 | 0.344 | |||
| eGFR † | 0.940 | 0.589–1.478 | 0.788 | |||
| UA † | 0.886 | 0.557–1.390 | 0.596 | |||
| XOR † | 2.125 | 1.194–4.286 | 0.008 | 2.821 | 1.426–6.616 | 0.001 |
| hs-CRP † | 1.654 | 0.997–3.246 | 0.052 | 1.742 | 1.012–3.523 | 0.049 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variables | OR | 95% CI | p Value | OR | 95% CI | p Value |
| Age † | 1.745 | 0.945–3.570 | 0.076 | 1.742 | 0.989–5.522 | 0.054 |
| BMI † | 0.886 | 0.485–1.598 | 0.687 | |||
| Hypertension | 0.952 | 0.305–3.018 | 0.933 | |||
| Dyslipidemia | 1.336 | 0.428–4.351 | 0.620 | |||
| Diabetes mellitus | 0.304 | 0.015–1.987 | 0.236 | |||
| Smoking | 2.160 | 0.660–7.140 | 0.201 | 3.493 | 0.880–15.151 | 0.075 |
| Triglycerides † | 1.155 | 0.638–2.047 | 0.620 | |||
| LDL-C † | 1.144 | 0.634–2.050 | 0.646 | |||
| HDL-C † | 0.797 | 0.421–1.430 | 0.452 | |||
| HbA1c † | 0.977 | 0.521–1.728 | 0.939 | |||
| eGFR † | 0.967 | 0.527–1.725 | 0.910 | |||
| UA † | 1.416 | 0.801–2.598 | 0.232 | |||
| XOR † | 6.365 | 1.613–54.975 | 0.001 | 9.251 | 1.974–85.363 | <0.001 |
| hs-CRP † | 0.995 | 0.496–1.742 | 0.986 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, K.; Watanabe, T.; Otaki, Y.; Murase, T.; Nakamura, T.; Kato, S.; Tamura, H.; Nishiyama, S.; Takahashi, H.; Arimoto, T.; et al. Gender Differences in the Impact of Plasma Xanthine Oxidoreductase Activity on Coronary Artery Spasm. J. Clin. Med. 2021, 10, 5550. https://doi.org/10.3390/jcm10235550
Watanabe K, Watanabe T, Otaki Y, Murase T, Nakamura T, Kato S, Tamura H, Nishiyama S, Takahashi H, Arimoto T, et al. Gender Differences in the Impact of Plasma Xanthine Oxidoreductase Activity on Coronary Artery Spasm. Journal of Clinical Medicine. 2021; 10(23):5550. https://doi.org/10.3390/jcm10235550
Chicago/Turabian StyleWatanabe, Ken, Tetsu Watanabe, Yoichiro Otaki, Takayo Murase, Takashi Nakamura, Shigehiko Kato, Harutoshi Tamura, Satoshi Nishiyama, Hiroki Takahashi, Takanori Arimoto, and et al. 2021. "Gender Differences in the Impact of Plasma Xanthine Oxidoreductase Activity on Coronary Artery Spasm" Journal of Clinical Medicine 10, no. 23: 5550. https://doi.org/10.3390/jcm10235550
APA StyleWatanabe, K., Watanabe, T., Otaki, Y., Murase, T., Nakamura, T., Kato, S., Tamura, H., Nishiyama, S., Takahashi, H., Arimoto, T., & Watanabe, M. (2021). Gender Differences in the Impact of Plasma Xanthine Oxidoreductase Activity on Coronary Artery Spasm. Journal of Clinical Medicine, 10(23), 5550. https://doi.org/10.3390/jcm10235550






