Outcomes of Different Reperfusion Strategies of Multivessel Disease Undergoing Newer-Generation Drug-Eluting Stent Implantation in Patients with Non-ST-Elevation Myocardial Infarction and Chronic Kidney Disease
Abstract
:1. Introduction
2. Methods
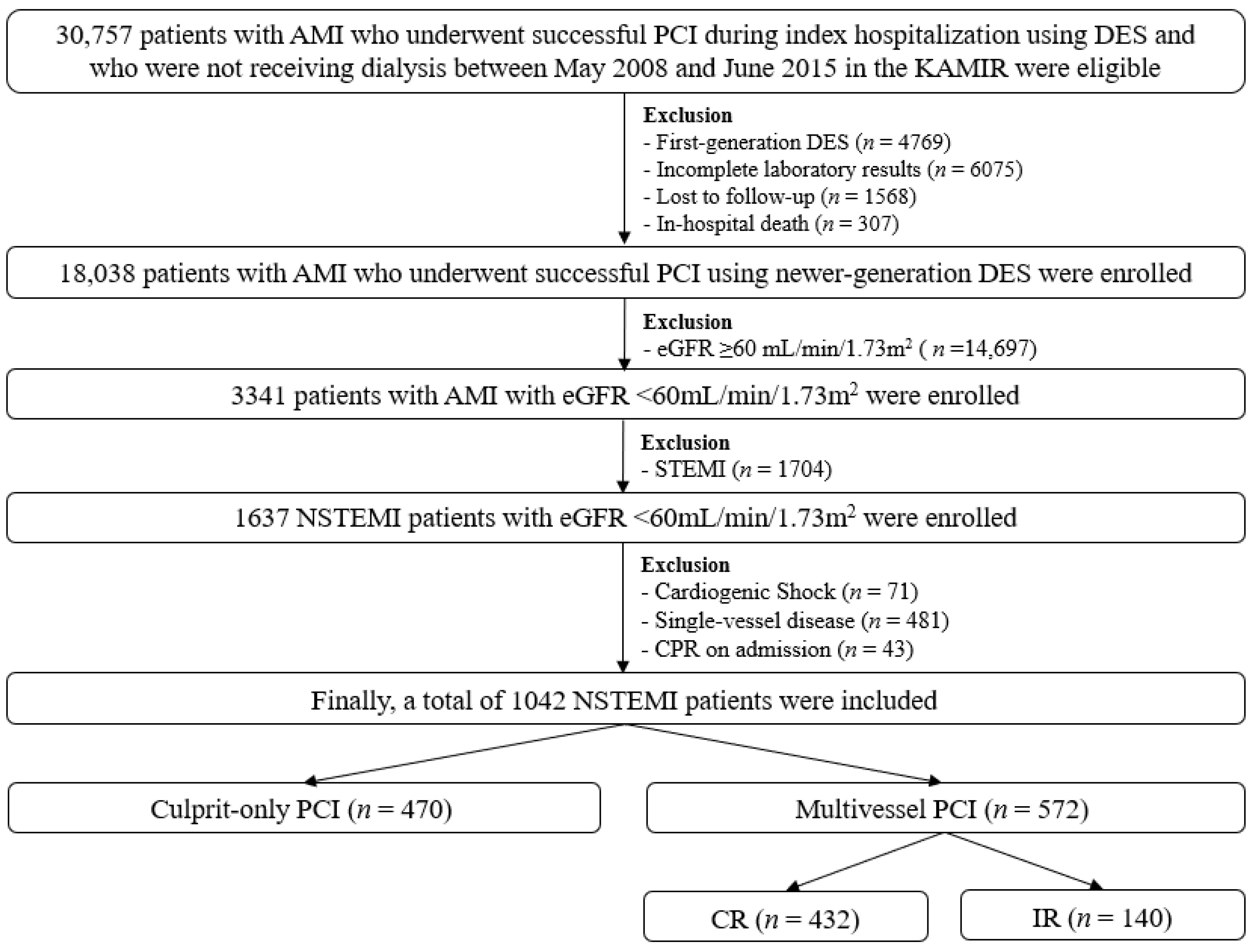
2.1. Study Population
2.2. Percutaneous Coronary Intervention and Medical Treatment
2.3. Study Definitions and Clinical Outcomes
2.4. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Clinical Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sorajja, P.; Gersh, B.J.; Cox, D.A.; McLaughlin, M.G.; Zimetbaum, P.; Costantini, C.; Stuckey, T.; Tcheng, J.E.; Mehran, R.; Lansky, A.J.; et al. Impact of multivessel disease on reperfusion success and clinical outcomes in patients undergoing primary percutaneous coronary intervention for acute myocardial infarction. Eur. Heart J. 2007, 28, 1709–1716. [Google Scholar] [CrossRef] [Green Version]
- Hassanin, A.; Brener, S.J.; Lansky, A.J.; Xu, K.; Stone, G.W. Prognostic impact of multivessel versus culprit vessel only percutaneous intervention for patients with multivessel coronary artery disease presenting with acute coronary syndrome. EuroIntervention 2015, 11, 293–300. [Google Scholar] [CrossRef]
- Ferrara, L.A.; Russo, B.F.; Gente, R.; Esposito, G.; Rapacciuolo, A.; de Simone, G. STEMI and NSTEMI: A mono versus a multivessel disease? Int. J. Cardiol. 2013, 168, 2905–2906. [Google Scholar] [CrossRef]
- Amsterdam, E.A.; Wenger, N.K.; Brindis, R.G.; Casey, D.E., Jr.; Ganiats, T.G.; Holmes, D.R., Jr.; Jaffe, A.S.; Jneid, H.; Kelly, R.F.; Kontos, M.C.; et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 64, e139–e228. [Google Scholar] [CrossRef] [Green Version]
- Roffi, M.; Patrono, C.; Collet, J.P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; Bax, J.J.; Borger, M.A.; Brotons, C.; Chew, D.P.; et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 267–315. [Google Scholar]
- Liu, E.S.; Hung, C.C.; Chiang, C.H.; Chang, C.H.; Cheng, C.C.; Kuo, F.Y.; Mar, G.Y.; Huang, W.C. Comparison of Different Timing of Multivessel Intervention during Index-Hospitalization for Patients with Acute Myocardial Infarction. Front. Cardiovasc. Med. 2021, 8, 639750. [Google Scholar] [CrossRef] [PubMed]
- Hannan, E.L.; Samadashvili, Z.; Walford, G.; Jacobs, A.K.; Stamato, N.J.; Venditti, F.J.; Holmes, D.R., Jr.; Sharma, S.; King, S.B., 3rd. Staged versus one-time complete revascularization with percutaneous coronary intervention for multivessel coronary artery disease patients without ST-elevation myocardial infarction. Cir. Cardiovasc. Interv. 2013, 6, 12–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shishehbor, M.H.; Lauer, M.S.; Singh, I.M.; Chew, D.P.; Karha, J.; Brener, S.J.; Moliterno, D.J.; Ellis, S.G.; Topol, E.J.; Bhatt, D.L. In unstable angina or non-ST-segment acute coronary syndrome, should patients with multivessel coronary artery disease undergo multivessel or culprit-only stenting? J. Am. Coll. Cardiol. 2007, 49, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Rathod, K.S.; Koganti, S.; Jain, A.K.; Astroulakis, Z.; Lim, P.; Rakhit, R.; Kalra, S.S.; Dalby, M.C.; O’Mahony, C.; Malik, I.S.; et al. Complete Versus Culprit-Only Lesion Intervention in Patients with Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2018, 72, 1989–1999. [Google Scholar] [CrossRef] [PubMed]
- McNeice, A.; Nadra, I.J.; Robinson, S.D.; Fretz, E.; Ding, L.; Fung, A.; Aymong, E.; Chan, A.W.; Hodge, S.; Webb, J.; et al. The prognostic impact of revascularization strategy in acute myocardial infarction and cardiogenic shock: Insights from the British Columbia Cardiac Registry. Cath. Cardiovasc. Interv. 2018, 92, E356–E367. [Google Scholar] [CrossRef] [PubMed]
- Mehran, R. Contrast-induced nephropathy remains a serious complication of PCI. J. Interv. Cardiol. 2007, 20, 236–240. [Google Scholar] [CrossRef]
- Carande, E.J.; Brown, K.; Jackson, D.; Maskell, N.; Kouzaris, L.; Greene, G.; Mikhail, A.; Obaid, D.R. Acute Kidney Injury Following Percutaneous Coronary Intervention for Acute Coronary Syndrome: Incidence, Aetiology, Risk Factors and Outcomes. Angiology 2021, 33197211040375. [Google Scholar]
- Hanratty, C.G.; Koyama, Y.; Rasmussen, H.H.; Nelson, G.I.; Hansen, P.S.; Ward, M.R. Exaggeration of nonculprit stenosis severity during acute myocardial infarction: Implications for immediate multivessel revascularization. J. Am. Coll. Cardiol. 2002, 40, 911–916. [Google Scholar] [CrossRef] [Green Version]
- Anavekar, N.S.; McMurray, J.J.; Velazquez, E.J.; Solomon, S.D.; Kober, L.; Rouleau, J.L.; White, H.D.; Nordlander, R.; Maggioni, A.; Dickstein, K.; et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N. Engl. J. Med. 2004, 351, 1285–1295. [Google Scholar] [CrossRef]
- Manjunath, G.; Tighiouart, H.; Ibrahim, H.; MacLeod, B.; Salem, D.N.; Griffith, J.L.; Coresh, J.; Levey, A.S.; Sarnak, M.J. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J. Am. Coll. Cardiol. 2003, 41, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Szummer, K.; Lundman, P.; Jacobson, S.H.; Schön, S.; Lindbäck, J.; Stenestrand, U.; Wallentin, L.; Jernberg, T. Relation between renal function, presentation, use of therapies and in-hospital complications in acute coronary syndrome: Data from the SWEDEHEART register. J. Intern. Med. 2010, 268, 40–49. [Google Scholar] [CrossRef]
- Strippoli, G.F.; Craig, J.C.; Schena, F.P. The number, quality, and coverage of randomized controlled trials in nephrology. J. Am. Soc. Nephrol. 2004, 15, 411–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crimi, G.; Gritti, V.; Galiffa, V.A.; Scotti, V.; Leonardi, S.; Ferrario, M.; Ferlini, M.; De Ferrari, G.M.; Oltrona Visconti, L.; Klersy, C. Drug eluting stents are superior to bare metal stents to reduce clinical outcome and stent-related complications in CKD patients, a systematic review, meta-analysis and network meta-analysis. J. Interv. Cardiol. 2018, 31, 319–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Ahn, Y.; Cho, M.C.; Kim, C.J.; Kim, Y.J.; Jeong, M.H. Current status of acute myocardial infarction in Korea. Korean J. Intern. Med. 2019, 34, 1–10. [Google Scholar] [CrossRef]
- Kim, J.H.; Chae, S.C.; Oh, D.J.; Kim, H.S.; Kim, Y.J.; Ahn, Y.; Cho, M.C.; Kim, C.J.; Yoon, J.H.; Park, H.Y.; et al. Multicenter Cohort Study of Acute Myocardial Infarction in Korea—Interim Analysis of the Korea Acute Myocardial Infarction Registry-National Institutes of Health Registry. Circ. J. 2016, 80, 1427–1436. [Google Scholar] [CrossRef] [Green Version]
- Grech, E.D. ABC of interventional cardiology: Percutaneous coronary intervention. II: The procedure. BMJ 2003, 326, 1137–1140. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.W.; Park, S.W.; Hong, M.K.; Kim, Y.H.; Lee, B.K.; Song, J.M.; Han, K.H.; Lee, C.W.; Kang, D.H.; Song, J.K.; et al. Triple versus dual antiplatelet therapy after coronary stenting: Impact on stent thrombosis. J. Am. Coll. Cardiol. 2005, 46, 1833–1837. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.Y.; Rha, S.W.; Li, Y.J.; Poddar, K.L.; Jin, Z.; Minami, Y.; Wang, L.; Kim, E.J.; Park, C.G.; Seo, H.S.; et al. Triple versus dual antiplatelet therapy in patients with acute ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Circulation 2009, 119, 3207–3214. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Salinero-Fort, M.A.; San Andrés-Rebollo, F.J.; de Burgos-Lunar, C.; Gómez-Campelo, P.; Chico-Moraleja, R.M.; López de Andrés, A.; Jiménez-García, R. Five-year incidence of chronic kidney disease (stage 3-5) and associated risk factors in a Spanish cohort: The MADIABETES Study. PLoS ONE 2015, 10, e0122030. [Google Scholar] [CrossRef] [PubMed]
- Bangalore, S.; Guo, Y.; Samadashvili, Z.; Blecker, S.; Xu, J.; Hannan, E.L. Revascularization in Patients with Multivessel Coronary Artery Disease and Chronic Kidney Disease: Everolimus-Eluting Stents Versus Coronary Artery Bypass Graft Surgery. J. Am. Coll. Cardiol. 2015, 66, 1209–1220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newby, L.K.; Jesse, R.L.; Babb, J.D.; Christenson, R.H.; De Fer, T.M.; Diamond, G.A.; Fesmire, F.M.; Geraci, S.A.; Gersh, B.J.; Larsen, G.C.; et al. ACCF 2012 expert consensus document on practical clinical considerations in the interpretation of troponin elevations: A report of the American College of Cardiology Foundation task force on Clinical Expert Consensus Documents. J. Am. Coll. Cardiol. 2012, 60, 2427–2463. [Google Scholar] [CrossRef] [PubMed]
- Thiele, H.; Akin, I.; Sandri, M.; de Waha-Thiele, S.; Meyer-Saraei, R.; Fuernau, G.; Eitel, I.; Nordbeck, P.; Geisler, T.; Landmesser, U.; et al. One-Year Outcomes after PCI Strategies in Cardiogenic Shock. N. Engl. J. Med. 2018, 379, 1699–1710. [Google Scholar] [CrossRef]
- Kim, M.C.; Jeong, M.H.; Ahn, Y.; Kim, J.H.; Chae, S.C.; Kim, Y.J.; Hur, S.H.; Seong, I.W.; Hong, T.J.; Choi, D.H.; et al. What is optimal revascularization strategy in patients with multivessel coronary artery disease in non-ST-elevation myocardial infarction? Multivessel or culprit-only revascularization. Int. J. Cardiol. 2011, 153, 148–153. [Google Scholar] [CrossRef]
- Kim, Y.H.; Her, A.Y.; Jeong, M.H.; Kim, B.K.; Hong, S.J.; Kim, J.S.; Ko, Y.G.; Choi, D.; Hong, M.K.; Jang, Y. Impact of stent generation on 2-year clinical outcomes in ST-segment elevation myocardial infarction patients with multivessel disease who underwent culprit-only or multivessel percutaneous coronary intervention. Cath. Cardiovasc. Interv. 2020, 95, E40–E55. [Google Scholar] [CrossRef]
- de Araújo Gonçalves, P.; Ferreira, J.; Aguiar, C.; Seabra-Gomes, R. TIMI, PURSUIT, and GRACE risk scores: Sustained prognostic value and interaction with revascularization in NSTE-ACS. Eur. Heart J. 2005, 26, 865–872. [Google Scholar] [CrossRef]
- Lee, J.M.; Rhee, T.M.; Hahn, J.Y.; Kim, H.K.; Park, J.; Hwang, D.; Choi, K.H.; Kim, J.; Park, T.K.; Yang, J.H.; et al. Multivessel Percutaneous Coronary Intervention in Patients with ST-Segment Elevation Myocardial Infarction with Cardiogenic Shock. J. Am. Coll. Cardiol. 2018, 71, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Her, A.Y.; Jeong, M.H.; Kim, B.K.; Lee, S.Y.; Hong, S.J.; Shin, D.H.; Kim, J.S.; Ko, Y.G.; Choi, D.; et al. Impact of renin-angiotensin system inhibitors on long-term clinical outcomes in patients with acute myocardial infarction treated with successful percutaneous coronary intervention with drug-eluting stents: Comparison between STEMI and NSTEMI. Atherosclerosis 2019, 280, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Her, A.Y.; Jeong, M.H.; Kim, B.K.; Lee, S.Y.; Hong, S.J.; Ahn, C.M.; Kim, J.S.; Ko, Y.G.; Choi, D.; et al. One-year clinical outcomes between biodegradable-polymer-coated biolimus-eluting stent and durable-polymer-coated drug-eluting stents in STEMI patients with multivessel coronary artery disease undergoing culprit-only or multivessel PCI. Atherosclerosis 2019, 284, 102–109. [Google Scholar] [CrossRef]
- Cutlip, D.E.; Windecker, S.; Mehran, R.; Boam, A.; Cohen, D.J.; van Es, G.A.; Steg, P.G.; Morel, M.A.; Mauri, L.; Vranckx, P.; et al. Clinical end points in coronary stent trials: A case for standardized definitions. Circulation 2007, 115, 2344–2351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmud, E.; Ben-Yehuda, O. Percutaneous Coronary Intervention in Acute Coronary Syndrome: Completing the Job Saves Lives. J. Am. Coll. Cardiol. 2018, 72, 2000–2002. [Google Scholar] [CrossRef]
- Sardella, G.; Lucisano, L.; Garbo, R.; Pennacchi, M.; Cavallo, E.; Stio, R.E.; Calcagno, S.; Ugo, F.; Boccuzzi, G.; Fedele, F.; et al. Single-Staged Compared with Multi-Staged PCI in Multivessel NSTEMI Patients: The SMILE Trial. J. Am. Coll. Cardiol. 2016, 67, 264–272. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.D.; Alam, M.; Hamzeh, I.; Virani, S.; Deswal, A.; Aguilar, D.; Rogers, P.; Kougias, P.; Birnbaum, Y.; Paniagua, D.; et al. Patients with severe chronic kidney disease benefit from early revascularization after acute coronary syndrome. Int. J. Cardiol. 2013, 168, 3741–3746. [Google Scholar] [CrossRef]
- Yong, J.; Tian, J.; Zhao, X.; Yang, X.; Xing, H.; He, Y.; Song, X. Optimal treatment strategies for coronary artery disease in patients with advanced kidney disease: A meta-analysis. Ther. Adv. Chronic Dis. 2021, 12, 20406223211024367. [Google Scholar] [CrossRef]
- Thiele, H.; Akin, I.; Sandri, M.; Fuernau, G.; de Waha, S.; Meyer-Saraei, R.; Nordbeck, P.; Geisler, T.; Landmesser, U.; Skurk, C.; et al. PCI Strategies in Patients with Acute Myocardial Infarction and Cardiogenic Shock. N. Engl. J. Med. 2017, 377, 2419–2432. [Google Scholar] [CrossRef] [Green Version]
- Anderson, M.L.; Peterson, E.D.; Peng, S.A.; Wang, T.Y.; Ohman, E.M.; Bhatt, D.L.; Saucedo, J.F.; Roe, M.T. Differences in the profile, treatment, and prognosis of patients with cardiogenic shock by myocardial infarction classification: A report from NCDR. Cir. Cardiovasc. Qual Outcomes 2013, 6, 708–715. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.H.; Her, A.Y.; Jeong, M.H.; Kim, B.K.; Hong, S.J.; Kim, S.; Ahn, C.M.; Kim, J.S.; Ko, Y.G.; Choi, D.; et al. Culprit-only versus multivessel or complete versus incomplete revascularization in patients with non-ST-segment elevation myocardial infarction and multivessel disease who underwent successful percutaneous coronary intervention using newer-generation drug-eluting stents. Atherosclerosis 2020, 301, 54–64. [Google Scholar] [PubMed]
- Mehta, S.R.; Wood, D.A.; Storey, R.F.; Mehran, R.; Bainey, K.R.; Nguyen, H.; Meeks, B.; Di Pasquale, G.; López-Sendón, J.; Faxon, D.P.; et al. COMPLETE Trial Steering Committee and Investigators. Complete Revascularization with Multivessel PCI for Myocardial Infarction. N. Engl. J. Med. 2019, 381, 1411–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montone, R.A.; Niccoli, G.; Crea, F.; Jang, I.K. Management of non-culprit coronary plaques in patients with acute coronary syndrome. Eur. Heart J. 2020, 41, 3579–3586. [Google Scholar] [CrossRef] [PubMed]
- Chonchol, M.; Whittle, J.; Desbien, A.; Orner, M.B.; Petersen, L.A.; Kressin, N.R. Chronic kidney disease is associated with angiographic coronary artery disease. Am. J. Nephrol. 2008, 28, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Coskun, U.; Orta Kilickesmez, K.; Abaci, O.; Kocas, C.; Bostan, C.; Yildiz, A.; Baskurt, M.; Arat, A.; Ersanli, M.; Gurmen, T. The relationship between chronic kidney disease and SYNTAX score. Angiology 2011, 62, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.C.; Kapoor, R.; Lewandowski, D.; Mason, P.J. Revascularization Strategies in Patients with Chronic Kidney Disease and Acute Coronary Syndromes. Curr. Cardiol. Rep. 2019, 21, 113. [Google Scholar] [CrossRef]
- Charytan, D.M.; Wallentin, L.; Lagerqvist, B.; Spacek, R.; De Winter, R.J.; Stern, N.M.; Braunwald, E.; Cannon, C.P.; Choudhry, N.K. Early angiography in patients with chronic kidney disease: A collaborative systematic review. Clin. J. Am. Soc. Nephrol. 2009, 4, 1032–1043. [Google Scholar] [CrossRef]
- Washam, J.B.; Kaltenbach, L.A.; Wojdyla, D.M.; Patel, M.R.; Klein, A.J.; Abbott, J.D.; Rao, S.V. Anticoagulant Use among Patients with End-Stage Renal Disease Undergoing Percutaneous Coronary Intervention: An Analysis From the National Cardiovascular Data Registry. Circ. Cardiovasc. Interv. 2018, 11, e005628. [Google Scholar] [CrossRef] [PubMed]
| Variables | Culprit-Only PCI (n = 470) | Multivessel PCI (n = 572) | p Value | CR (n = 432) | IR (n = 140) | p Value |
|---|---|---|---|---|---|---|
| Age (years) | 71.7 ± 9.7 | 71.3 ± 9.1 | 0.431 | 71.3 ± 9.2 | 71.2 ± 9.0 | 0.876 |
| ≥65 years, n (%) | 364 (77.4) | 434 (75.9) | 0.551 | 328 (75.9) | 106 (75.7) | 0.959 |
| Male, n (%) | 278 (59.1) | 298 (52.1) | 0.023 | 217 (50.2) | 81 (57.9) | 0.117 |
| LVEF (%) | 48.3 ± 12.6 | 49.1 ± 12.8 | 0.283 | 49.9 ± 12.7 | 46.1 ± 12.5 | 0.010 |
| <40%, n (%) | 116 (24.7) | 131 (22.9) | 0.502 | 93 (21.5) | 38 (27.1) | 0.169 |
| BMI (kg/m2) | 23.6 ± 3.3 | 23.8 ± 3.3 | 0.354 | 23.9 ± 3.3 | 23.54 ±3.3 | 0.081 |
| SBP (mmHg) | 133.9 ± 30.1 | 134.73 ± 29.7 | 0.664 | 134.5 ± 29.3 | 135.3 ± 31.0 | 0.774 |
| DBP (mmHg) | 78.3 ± 16.6 | 78.1 ± 15.5 | 0.884 | 78.1 ± 15.4 | 78.3 ± 15.6 | 0.864 |
| Killip class III, n (%) | 83 (17.7) | 101 (17.7) | 0.999 | 75 (17.4) | 26 (18.6) | 0.744 |
| Hypertension, n (%) | 363 (77.2) | 439 (76.7) | 0.853 | 323 (74.8) | 116 (82.9) | 0.049 |
| Diabetes mellitus, n (%) | 247 (52.6) | 325 (56.8) | 0.169 | 234 (54.2) | 91 (65.0) | 0.025 |
| Dyslipidemia, n (%) | 294 (62.6) | 378 (66.1) | 0.236 | 294 (68.1) | 84 (60.0) | 0.080 |
| Previous MI, n (%) | 37 (7.9) | 48 (8.4) | 0.761 | 33 (7.6) | 15 (10.7) | 0.254 |
| Previous PCI, n (%) | 71 (15.1) | 68 (11.9) | 0.128 | 42 (9.7) | 26 (18.6) | 0.007 |
| Previous CABG, n (%) | 11 (2.3) | 8 (1.4) | 0.352 | 3 (0.7) | 5 (3.6) | 0.024 |
| Previous HF, n (%) | 22 (4.7) | 25 (4.4) | 0.881 | 18 (4.2) | 7 (5.0) | 0.640 |
| Previous CVA, n (%) | 72 (15.3) | 73 (12.8) | 0.235 | 54 (12.5) | 19 (13.6) | 0.771 |
| Current smokers, n (%) | 98 (20.9) | 103 (18.0) | 0.247 | 80 (18.5) | 23 (16.4) | 0.615 |
| Peak CK-MB (mg/dL) | 61.1 ± 96.8 | 48.8 ± 80.8 | 0.049 | 47.6 ± 75.9 | 52.5 ± 94.5 | 0.578 |
| Peak troponin-I (ng/mL) | 35.6 ± 91.5 | 26.8 ± 97.2 | 0.216 | 24.4 ± 60.0 | 34.3 ± 88.7 | 0.491 |
| NT-ProBNP (pg/mL) | 7027.3 ± 9781.9 | 6152.7 ± 9097.5 | 0.166 | 5825.1 ± 8862.6 | 7015.4 ± 8725.4 | 0.151 |
| Hs-CRP (mg/dL) | 9.4 ± 32.9 | 9.5 ± 40.7 | 0.962 | 10.8 ± 45.8 | 5.5 ± 17.4 | 0.047 |
| Serum creatinine (mg/L) | 2.41 ± 2.45 | 2.36 ± 2.62 | 0.729 | 2.32 ± 2.64 | 2.45 ± 2.58 | 0.634 |
| eGFR, mL/min/1.73 m2 | 39.6 ± 16.7 | 40.2 ± 16.6 | 0.617 | 40.8 ± 16.5 | 38.3 ± 16.8 | 0.121 |
| Blood glucose (mg/dL) | 189.4 ± 100.3 | 198.5 ± 110.0 | 0.162 | 193.6 ± 108.1 | 213.6 ± 114.7 | 0.071 |
| Total cholesterol (mg/dL) | 169.7 ± 56.8 | 173.6 ± 46.3 | 0.224 | 174.1 ± 45.7 | 172.1 ± 48.1 | 0.666 |
| Triglyceride (mg/L) | 118.2 ± 71.0 | 128.6 ± 106.3 | 0.039 | 130.9 ± 116.3 | 121.8 ± 66.0 | 0.253 |
| HDL cholesterol (mg/L) | 42.5 ± 22.2 | 40.6 ± 10.9 | 0.088 | 40.2 ± 10.5 | 42.0 ± 12.0 | 0.097 |
| LDL cholesterol (mg/L) | 103.8 ± 41.9 | 107.2 ± 36.1 | 0.160 | 109.1 ± 35.4 | 101.5 ± 38.0 | 0.038 |
| Discharge medications | ||||||
| Aspirin, n (%) | 451 (96.0) | 554 (96.9) | 0.437 | 418 (96.8) | 136 (97.1) | 0.821 |
| Clopidogrel, n (%) | 435 (92.6) | 530 (92.5) | 0.862 | 405 (93.8) | 125 (89.3) | 0.098 |
| Ticagrelor, n (%) | 23 (4.9) | 31 (5.4) | 0.779 | 19 (4.4) | 12 (8.6) | 0.083 |
| Prasugrel, n (%) | 12 (2.6) | 11 (1.9) | 0.530 | 8 (1.9) | 3 (2.1) | 0.735 |
| Cilostazole, n (%) | 77 (16.4) | 138 (24.1) | 0.002 | 121 (28.0) | 17 (12.1) | <0.001 |
| Beta-blocker, n (%) | 368 (78.3) | 449 (78.5) | 0.938 | 339 (78.5) | 110 (78.6) | 0.980 |
| ACEI, n (%) | 202 (43.0) | 233 (40.7) | 0.465 | 180 (41.7) | 53 (37.9) | 0.489 |
| ARB, n (%) | 167 (35.5) | 214 (37.4) | 0.531 | 158 (36.6) | 56 (40.0) | 0.467 |
| CCB, n (%) | 81 (17.2) | 88 (15.4) | 0.420 | 64 (14.8) | 24 (17.1) | 0.507 |
| Lipid lowering agent, n (%) | 360 (76.6) | 470 (82.2) | 0.028 | 348 (80.6) | 122 (87.1) | 0.077 |
| Angiographic & procedural characteristics | ||||||
| IRA | ||||||
| LM, n (%) | 17 (3.6) | 36 (6.3) | 0.048 | 22 (5.1) | 14 (10.0) | 0.038 |
| LAD, n (%) | 190 (40.4) | 202 (35.3) | 0.104 | 151 (35.0) | 51 (36.4) | 0.751 |
| LCx, n (%) | 105 (22.3) | 143 (25.0) | 0.316 | 114 (26.4) | 29 (20.7) | 0.216 |
| RCA, n (%) | 158 (33.6) | 191 (33.4) | 0.939 | 145 (33.6) | 46 (32.9) | 0.918 |
| Treated vessel | ||||||
| LM, n (%) | 21 (4.5) | 56 (9.8) | 0.001 | 38 (8.8) | 18 (12.9) | 0.160 |
| LAD, n (%) | 217 (46.2) | 420 (73.4) | <0.001 | 321 (74.3) | 99 (70.7) | 0.403 |
| LCx, n (%) | 131 (27.9) | 352 (61.5) | <0.001 | 284 (65.7) | 68 (48.6) | <0.001 |
| RCA, n (%) | 180 (38.3) | 317 (55.4) | <0.001 | 247 (57.2) | 70 (50.0) | 0.138 |
| Extent of CAD | ||||||
| 2-vessel disease, n (%) | 229 (48.7) | 270 (47.2) | 0.663 | 227 (52.5) | 43 (30.7) | <0.001 |
| ≥3-vessel disease, n (%) | 241 (51.3) | 302 (52.8) | 0.663 | 205 (47.5) | 97 (69.3) | <0.001 |
| ACC/AHA lesion type | ||||||
| Type B1, n (%) | 62 (13.2) | 78 (13.6) | 0.856 | 54 (12.5) | 24 (17.1) | 0.164 |
| Type B2, n (%) | 154 (32.8) | 180 (31.5) | 0.655 | 154 (35.6) | 26 (18.6) | <0.001 |
| Type C, n (%) | 224 (47.7) | 272 (47.6) | 0.973 | 196 (45.4) | 76 (54.3) | 0.066 |
| Pre-PCI TIMI flow grade 0/1, n (%) | 185 (39.4) | 228 (39.9) | 0.870 | 179 (41.4) | 49 (35.0) | 0.197 |
| In-hospital GP IIb/IIIa, n (%) | 28 (6.0) | 25 (4.4) | 0.260 | 17 (3.9) | 8 (5.7) | 0.371 |
| Drug-eluting stents a | ||||||
| ZES, n (%) | 168 (35.7) | 203 (35.5) | 0.932 | 164 (38.0) | 39 (27.9) | 0.033 |
| EES, n (%) | 248 (52.8) | 321 (56.1) | 0.279 | 233 (53.9) | 88 (62.9) | 0.064 |
| BES, n (%) | 54 (11.5) | 66 (11.5) | 0.980 | 47 (10.9) | 19 (13.6) | 0.386 |
| Others, n (%) | 6 (1.3) | 7 (1.2) | 0.939 | 6 (1.4) | 1 (0.7) | 0.528 |
| IVUS, n (%) | 68 (14.5) | 138 (24.1) | <0.001 | 99 (22.9) | 39 (27.9) | 0.235 |
| OCT, n (%) | 1 (0.2) | 2 (0.3) | 0.682 | 1 (0.2) | 1 (0.7) | 0.430 |
| FFR, n (%) | 3 (0.6) | 2 (0.3) | 0.502 | 1 (0.2) | 1 (0.7) | 0.430 |
| Completeness of multivessel PCI | ||||||
| CR, n (%) | - | 432 (75.5) | - | 432 (100.0) | - | - |
| IR, n (%) | 140 (24.5) | - | - | 140 (100.0) | - | |
| PCI for non-IRA | - | |||||
| During index PCI, n (%) | - | 402 (70.3) | - | 315 (72.9) | 87 (62.1) | 0.015 |
| Staged PCI before discharge, n (%) | - | 170 (29.7) | - | 117 (27.1) | 53 (37.9) | 0.015 |
| Time from admission to PCI (hours) | 18.1 ± 54.6 | 22.6 ± 56.7 | 0.008 | 22.6 ± 57.3 | 22.9 ± 55.4 | 0.928 |
| Stent diameter (mm) | 3.03 ± 0.41 | 3.04 ± 0.40 | 0.689 | 3.02 ± 0.38 | 3.11 ± 0.45 | 0.028 |
| Stent length (mm) | 28.8 ± 13.4 | 29.1 ± 14.6 | 0.735 | 28.6 ± 14.6 | 30.5 ± 14.6 | 0.192 |
| Number of stent | 1.42 ± 0.70 | 2.31 ± 0.99 | <0.001 | 2.40 ± 1.00 | 2.03 ± 0.92 | <0.001 |
| GRACE risk score | 150.9 ± 27.3 | 150.1 ± 26.7. | 0.640 | 149.7 ± 26.8 | 151.4 ± 26.7. | 0.509 |
| >140, n (%) | 294 (62.6) | 343 (60.0) | 0.394 | 255 (59.0) | 88 (62.9) | 0.422 |
| Outcomes | Cumulative Events (%) | Unadjusted | Adjusted a | ||||
|---|---|---|---|---|---|---|---|
| Culprit-Only (n = 470) | Multivessel (n = 572) | Log-Rank | HR (95% CI) | p Value | HR (95% CI) | p Value | |
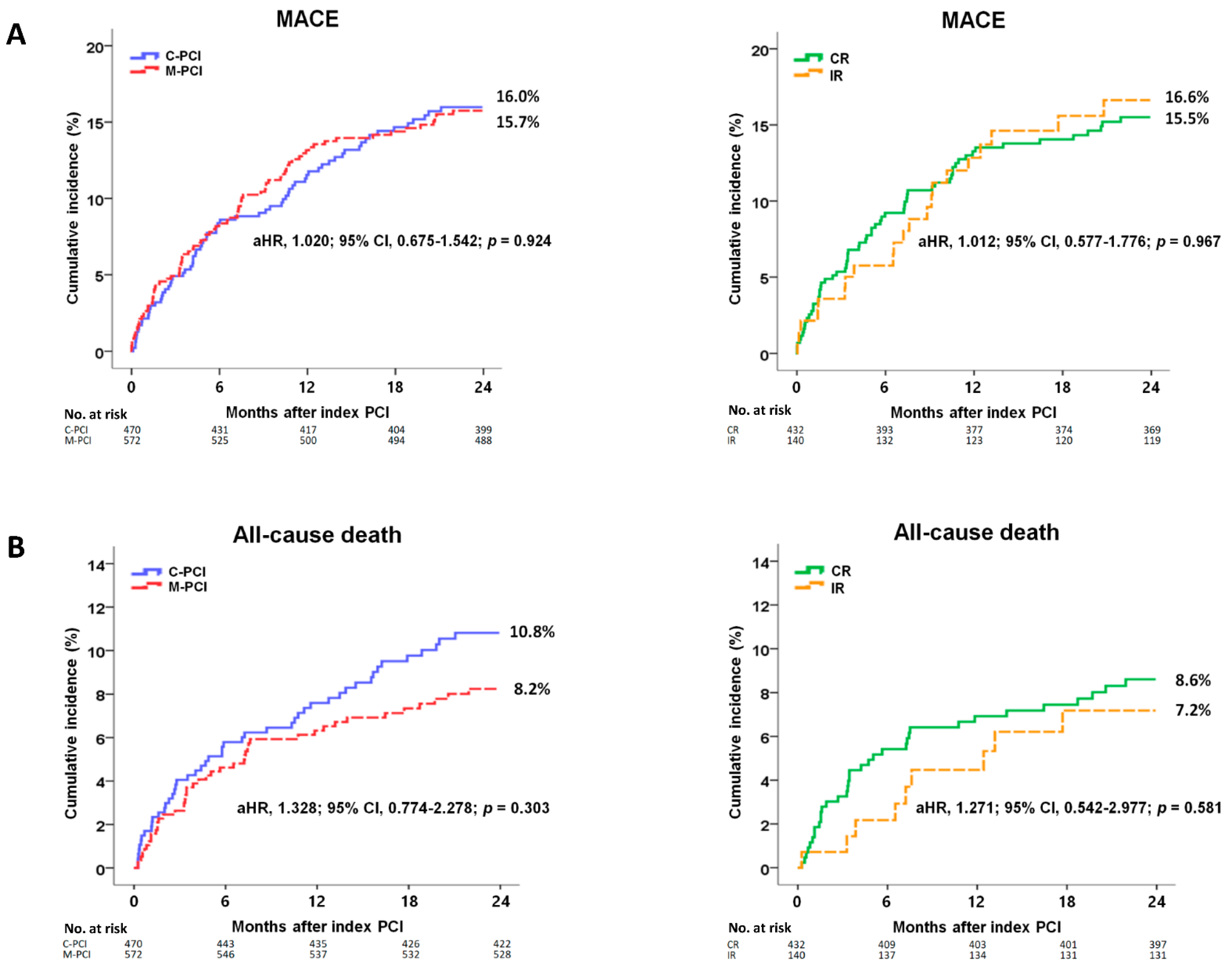
| MACE | 71 (16.0) | 84 (15.7) | 0.985 | 1.003 (0.731–1.376) | 0.985 | 1.020 (0.675–1.542) | 0.924 |
| All-cause death | 48 (10.8) | 44 (8.2) | 0.187 | 1.316 (0.874–1.981) | 0.188 | 1.328 (0.774–2.278) | 0.303 |
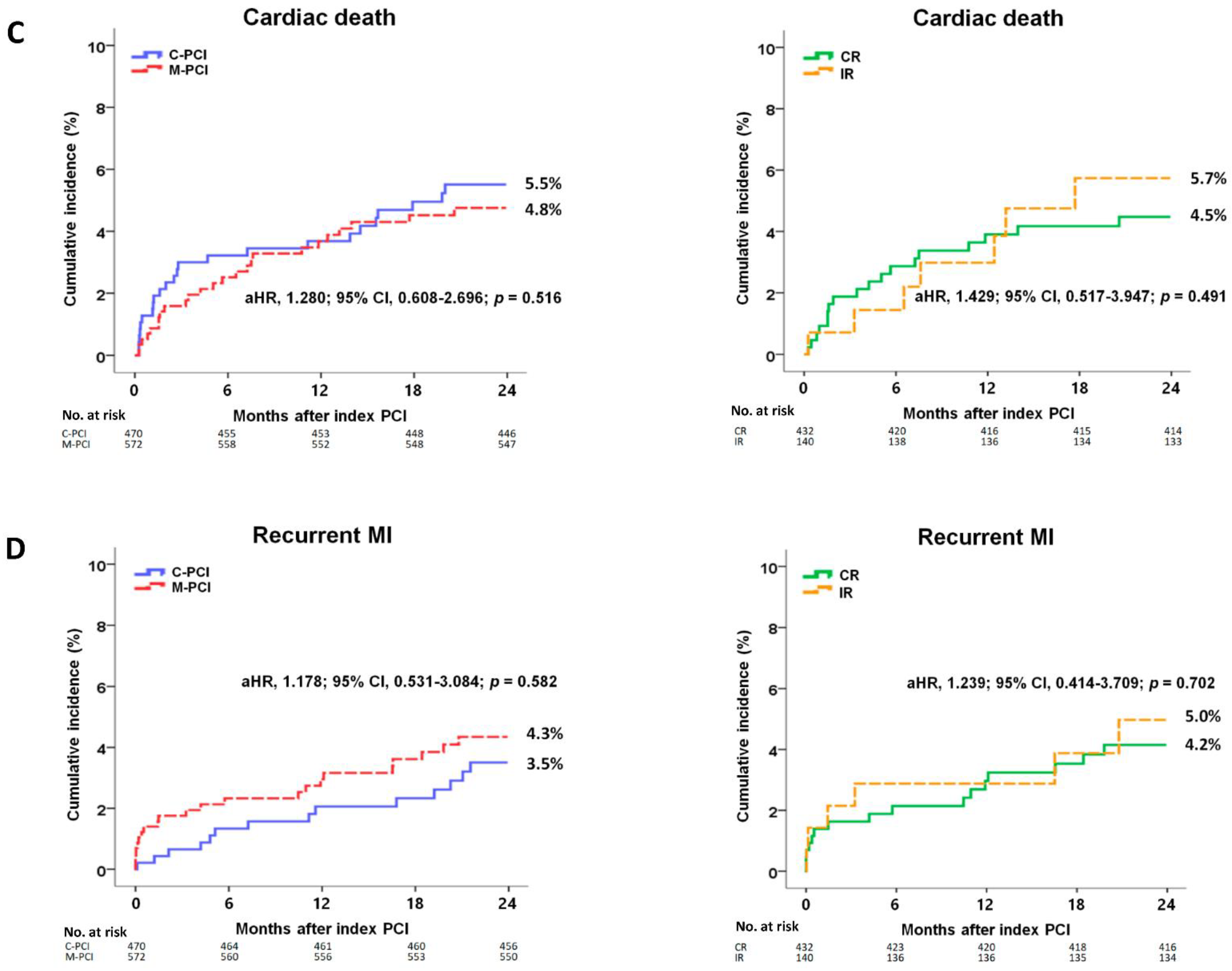
| Cardiac death | 24 (5.5) | 25 (4.8) | 0.606 | 1.159 (0.662–2.029) | 0.606 | 1.280 (0.608–2.696) | 0.516 |
| Re-MI | 14 (3.5) | 22 (4.3) | 0.410 | 0.755 (0.386–1.476) | 0.412 | 1.178 (0.531–3.084) | 0.582 |
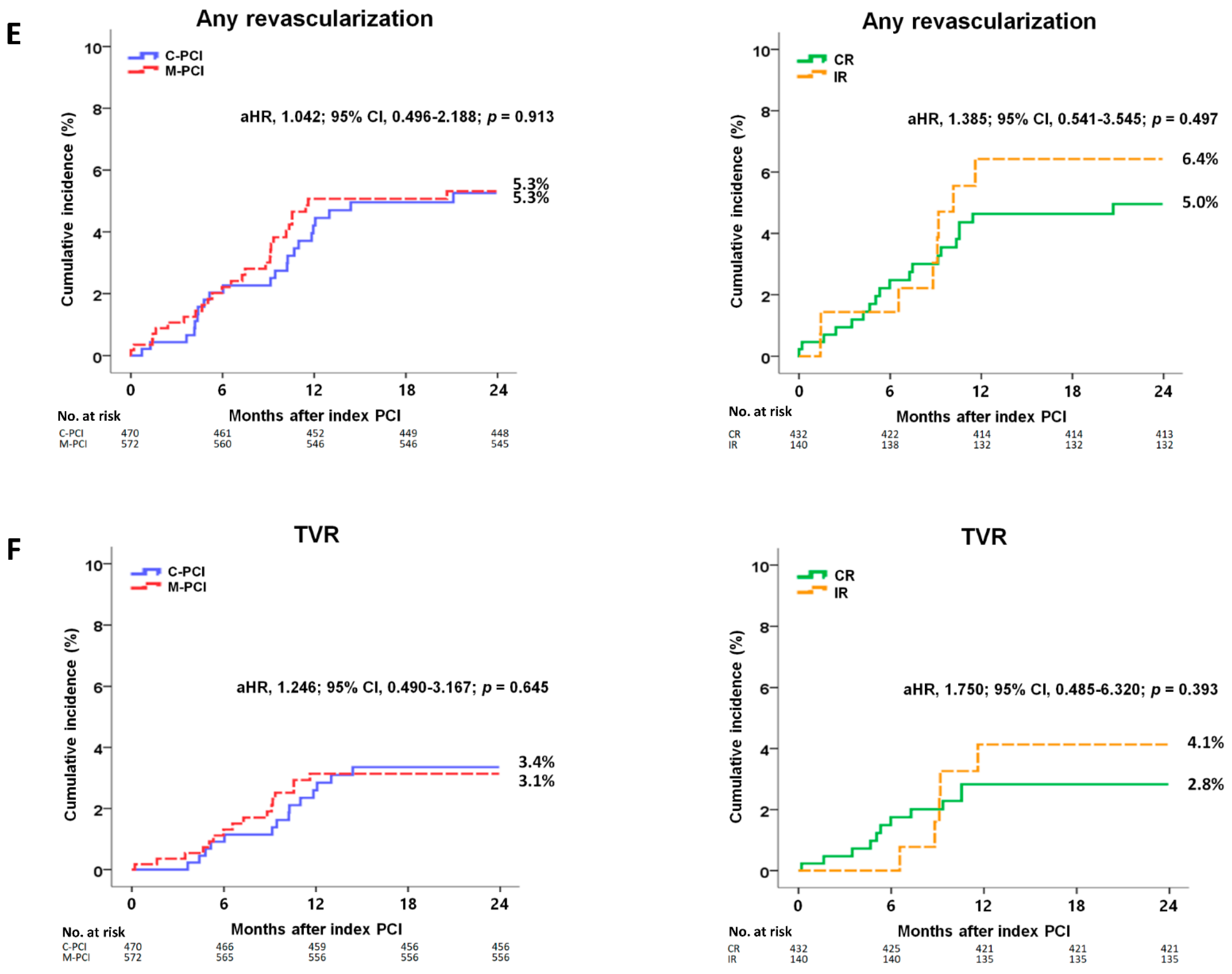
| Any revascularization | 22 (5.3) | 27 (5.3) | 0.899 | 0.964 (0.549–1.693) | 0.899 | 1.042 (0.496–2.188) | 0.913 |
| TVR | 14 (3.4) | 16 (3.1) | 0.927 | 1.034 (0.505–2.119) | 0.927 | 1.246 (0.490–3.167) | 0.645 |
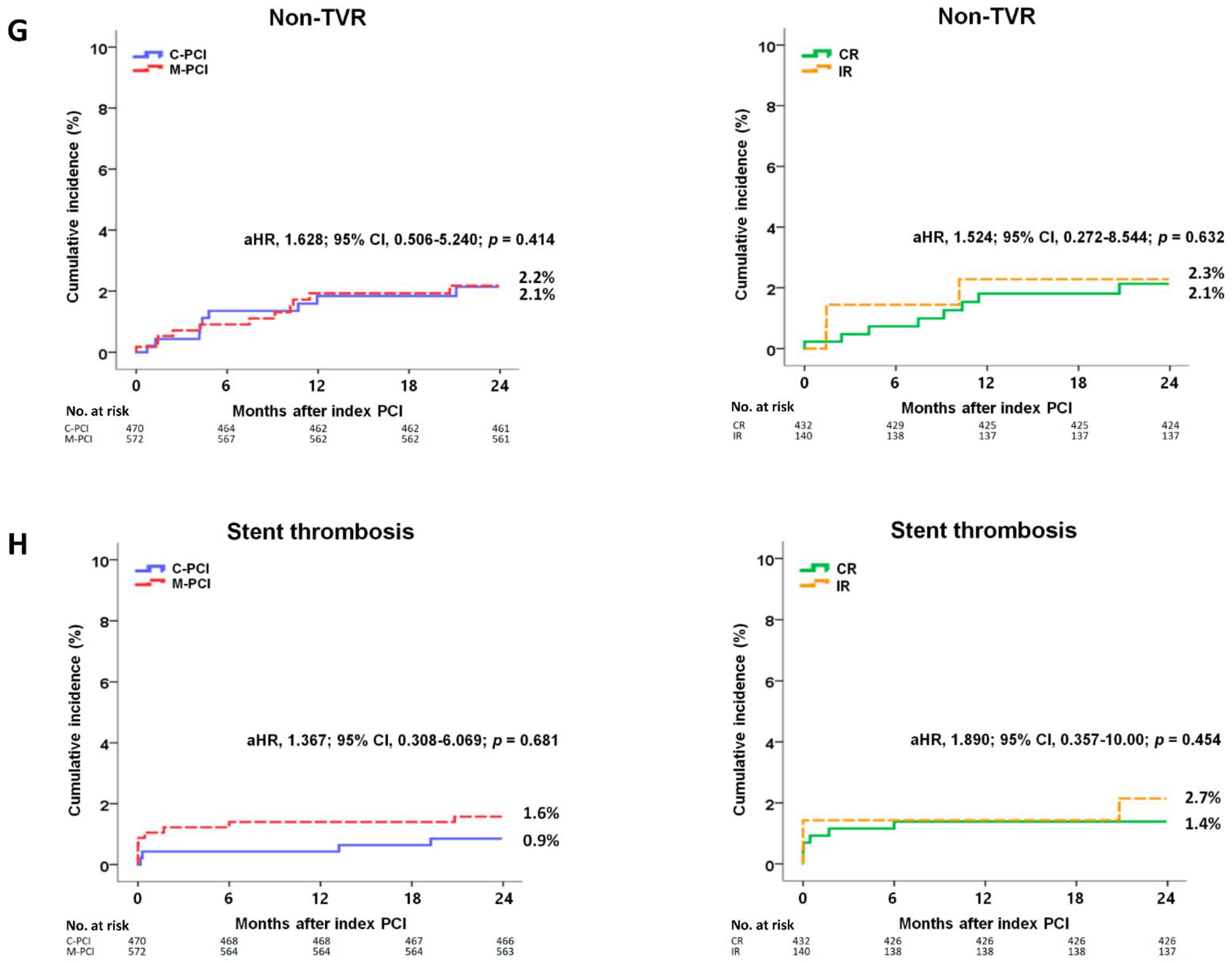
| Non-TVR | 9 (2.1) | 11 (2.2) | 0.968 | 0.982 (0.407–2.370) | 0.968 | 1.628 (0.506–5.240) | 0.414 |
| ST (definite or probable) | 4 (0.9) | 9 (1.6) | 0.295 | 0.538 (0.166–1.748) | 0.303 | 1.367 (0.308–6.069) | 0.681 |
| Outcomes | Cumulative Events (%) | Unadjusted | Adjusted b | ||||
| CR (n = 432) | IR (n = 140) | Log-Rank | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| MACE | 63 (15.5) | 21 (16.6) | 0.871 | 0.960 (0.586–1.573) | 0.871 | 1.012 (0.577–1.776) | 0.967 |
| All-cause death | 35 (8.6) | 9 (7.2) | 0.543 | 1.254 (0.603–2.610) | 0.544 | 1.271 (0.542–2.977) | 0.581 |
| Cardiac death | 18 (4.5) | 7 (5.7) | 0.673 | 0.829 (0.346–1.985) | 0.674 | 1.429 (0.517–3.947) | 0.491 |
| Re-MI | 16 (4.2) | 6 (5.0) | 0.719 | 0.842 (0.329–2.153) | 0.720 | 1.239 (0.414–3.709) | 0.702 |
| Any revascularization | 19 (5.0) | 8 (6.4) | 0.538 | 0.772 (0.338–1.764) | 0.539 | 1.385 (0.541–3.545) | 0.497 |
| TVR | 11 (2.8) | 5 (4.1) | 0.550 | 0.726 (0.252–2.089) | 0.552 | 1.750 (0.485–6.320) | 0.393 |
| Non-TVR | 8 (2.1) | 3 (2.3) | 0.823 | 0.860 (0.228–3.240) | 0.823 | 1.524 (0.272–8.544) | 0.632 |
| ST (definite or probable) | 6 (1.4) | 3 (2.7) | 0.533 | 0.646 (0.162–2.584) | 0.537 | 1.890 (0.357–10.00) | 0.454 |
| Outcomes | Cumulative Events (%) | Unadjusted | Adjusted c | ||||
| Culprit-Only (n = 470) | CR (n = 432) | Log-Rank | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| MACE | 71 (16.0) | 63 (15.5) | 0.956 | 1.010 (0.719–1.417) | 0.956 | 1.042 (0.656–1.654) | 0.863 |
| All-cause death | 48 (10.8) | 35 (8.6) | 0.308 | 1.254 (0.811–1.938) | 0.309 | 1.223 (0.673–2.223) | 0.509 |
| Cardiac death | 24 (5.5) | 18 (4.5) | 0.522 | 1.220 (0.662–2.248) | 0.523 | 1.305 (0.569–2.993) | 0.529 |
| Re-MI | 14 (3.5) | 16 (4.2) | 0.515 | 0.789 (0.385–1.616) | 0.516 | 1.107 (0.434–2.823) | 0.832 |
| Any revascularization | 22 (5.3) | 19 (5.0) | 0.907 | 1.037 (0.562–1.917) | 0.907 | 1.096 (0.461–2.605) | 0.836 |
| TVR | 14 (3.4) | 11 (2.8) | 0.750 | 1.137 (0.516–2.504) | 0.751 | 1.906 (0.703–5.171) | 0.205 |
| Non-TVR | 9 (2.1) | 8 (2.1) | 0.966 | 1.021 (0.394–2.646) | 0.966 | 2.958 (0.683–12.81) | 0.147 |
| ST (definite or probable) | 4 (0.9) | 6 (1.4) | 0.439 | 0.610 (0.172–2.161) | 0.443 | 1.344 (0.276–6.654) | 0.715 |
| Outcomes | Cumulative Events (%) | Unadjusted | Adjusted d | ||||
| Culprit-Only (n = 470) | IR (n = 140) | Log-Rank | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| MACE | 71 (16.0) | 21 (16.6) | 0.853 | 0.955 (0.587–1.554) | 0.853 | 1.060 (0.594–1.891) | 0.844 |
| All-cause death | 48 (10.8) | 9 (7.2) | 0.222 | 1.553 (0.762–3.165) | 0.226 | 2.007 (0.882–4.569) | 0.097 |
| Cardiac death | 24 (5.5) | 7 (5.7) | 0.993 | 0.996 (0.429–2.313) | 0.993 | 1.057 (0.381–2.929) | 0.916 |
| Re-MI | 14 (3.5) | 6 (5.0) | 0.393 | 0.661 (0.254–1.721) | 0.396 | 1.807 (0.517–6.312) | 0.354 |
| Any revascularization | 22 (5.3) | 8 (6.4) | 0.562 | 0.788 (0.351–1.769) | 0.563 | 1.524 (0.542–4.280) | 0.424 |
| TVR | 14 (3.4) | 5 (4.1) | 0.672 | 0.802 (0.289–2.228) | 0.672 | 1.592 (0.405–6.264) | 0.506 |
| Non-TVR | 9 (2.1) | 3 (2.3) | 0.850 | 0.882 (0.239–3.257) | 0.850 | 1.043 (0.183–5.931) | 0.962 |
| ST (definite or probable) | 4 (0.9) | 3 (2.7) | 0.207 | 0.394 (0.088–1.762) | 0.223 | 1.446 (0.172–12.16) | 0.735 |
| Variables | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| C-PCI vs. M-PCI | 1.003 (0.731–1.376) | 0.985 | 1.090 (0.706–1.684) | 0.696 |
| CR vs. IR | 0.960 (0.586–1.573) | 0.871 | 1.021 (0.552–1.888) | 0.947 |
| C-PCI vs. CR | 1.010 (0.719–1.417) | 0.956 | 1.192 (0.741–1.920) | 0.469 |
| C-PCI vs. IR | 0.955 (0.587–1.554) | 0.853 | 1.143 (0.592–2.206) | 0.691 |
| Age, ≥65 years | 1.438 (1.019–2.030) | 0.039 | 1.422 (0.951–2.010) | 0.083 |
| Male | 1.317 (0.954–1.820) | 0.095 | 1.282 (0.894–1.838) | 0.177 |
| LVEF, <40% | 1.993 (1.438–2.763) | <0.001 | 1.482 (1.026–2.235) | 0.029 |
| Killip class III | 1.691 (1.180–2.423) | 0.004 | 1.349 (0.836–2.177) | 0.220 |
| Hypertension | 1.169 (0.792–1.724) | 0.432 | 1.146 (0.756–1.737) | 0.520 |
| Diabetes mellitus | 1.471 (1.060–2.041) | 0.021 | 1.387 (0.948–2.028) | 0.092 |
| Previous PCI | 1.105 (0.704–1.735) | 0.665 | 1.080 (0.667–1.748) | 0.756 |
| Previous CABG | 1.409 (0.522–3.803) | 0.499 | 1.451 (0.520–4.050) | 0.477 |
| Peak CK-MB | 1.001 (1.000–1.002) | 0.186 | 1.001 (1.000–1.002) | 0.192 |
| Peak troponin-I | 1.001 (1.001–1.002) | <0.001 | 1.002 (1.001–1.002) | 0.002 |
| NT-ProBNP | 1.000 (0.999–1.001) | 0.001 | 1.001 (1.000–1.002) | 0.026 |
| Hs-CRP | 0.998 (0.993–1.004) | 0.550 | 0.997 (0.990–1.003) | 0.325 |
| Blood glucose | 1.000 (0.999–1.002) | 0.790 | 0.999 (0.997–1.001) | 0.214 |
| Total cholesterol | 0.999 (0.995–1.002) | 0.425 | 0.999 (0.995–1.004) | 0.833 |
| Triglyceride | 0.999 (0.997–1.001) | 0.445 | 0.999 (0.976–1.002) | 0.527 |
| HDL-cholesterol | 0.979 (0.964–0.995) | 0.008 | 0.980 (0.970–1.001) | 0.051 |
| LDL-cholesterol | 1.002 (0.998–1.005) | 0.397 | 1.002 (0.998–1.007) | 0.353 |
| Ticagrelor | 1.228 (0.574–2.628) | 0.596 | 1.436 (0.650–3.174) | 0.371 |
| Prasugrel | 1.422 (0.527–3.839) | 0.488 | 1.144 (0.398–3.288) | 0.802 |
| Cilostazole | 1.238 (0.862–1.780) | 0.248 | 1.293 (0.874–1.911) | 0.198 |
| ACEI | 1.098 (0.796–1.514) | 0.569 | 1.014 (0.660–1.559) | 0.949 |
| ARB | 1.067 (0.766–1.484) | 0.702 | 1.066 (0.691–1.645) | 0.722 |
| Beta-blocker | 1.166 (0.783–1.737) | 0.449 | 1.132 (0.731–1.753) | 0.578 |
| Lipid lowering agent | 1.057 (0.722–1.546) | 0.775 | 1.168 (0.768–1.776) | 0.467 |
| LM-IRA | 1.451 (0.786–2.679) | 0.234 | 1.004 (0.304–3.312) | 0.995 |
| LM-treated vessel | 1.398 (0.821–2.381) | 0.218 | 1.444 (0.522–3.993) | 0.179 |
| LAD-treated vessel | 1.036 (0.752–1.429) | 0.827 | 1.069 (0.743–1.538) | 0.719 |
| LCx-treated vessel | 1.076 (0.784–1.478) | 0.649 | 1.133 (0.791–1.622) | 0.497 |
| RCA-treated vessel | 1.153 (0.842–1.580) | 0.375 | 1.184 (0.812–1.725) | 0.369 |
| ACC/AHA type B2/C lesion | 1.263 (0.834–1.913) | 0.270 | 1.105 (0.709–1.721) | 0.680 |
| IVUS | 1.041 (0.695–1.559) | 0.844 | 1.038 (0.681–1.582) | 0.862 |
| Time from admission to PCI | 1.001 (0.998–1.003) | 0.193 | 1.091 (1.000–1.271) | 0.080 |
| Stent diameter <3.0 mm | 0.900 (0.647–1.253) | 0.533 | 1.171 (0.820–1.671) | 0.385 |
| Stent length ≥30 mm | 1.417 (1.026–1.957) | 0.034 | 1.379 (0.968–1.966) | 0.075 |
| GRACE risk score | 1.071 (1.012–1.031) | 0.037 | 1.001 (0.993–1.010) | 0.778 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.H.; Her, A.-Y.; Jeong, M.H.; Kim, B.-K.; Hong, S.-J.; Lee, S.-J.; Ahn, C.-M.; Kim, J.-S.; Ko, Y.-G.; Choi, D.; et al. Outcomes of Different Reperfusion Strategies of Multivessel Disease Undergoing Newer-Generation Drug-Eluting Stent Implantation in Patients with Non-ST-Elevation Myocardial Infarction and Chronic Kidney Disease. J. Clin. Med. 2021, 10, 4629. https://doi.org/10.3390/jcm10204629
Kim YH, Her A-Y, Jeong MH, Kim B-K, Hong S-J, Lee S-J, Ahn C-M, Kim J-S, Ko Y-G, Choi D, et al. Outcomes of Different Reperfusion Strategies of Multivessel Disease Undergoing Newer-Generation Drug-Eluting Stent Implantation in Patients with Non-ST-Elevation Myocardial Infarction and Chronic Kidney Disease. Journal of Clinical Medicine. 2021; 10(20):4629. https://doi.org/10.3390/jcm10204629
Chicago/Turabian StyleKim, Yong Hoon, Ae-Young Her, Myung Ho Jeong, Byeong-Keuk Kim, Sung-Jin Hong, Seung-Jun Lee, Chul-Min Ahn, Jung-Sun Kim, Young-Guk Ko, Donghoon Choi, and et al. 2021. "Outcomes of Different Reperfusion Strategies of Multivessel Disease Undergoing Newer-Generation Drug-Eluting Stent Implantation in Patients with Non-ST-Elevation Myocardial Infarction and Chronic Kidney Disease" Journal of Clinical Medicine 10, no. 20: 4629. https://doi.org/10.3390/jcm10204629
APA StyleKim, Y. H., Her, A.-Y., Jeong, M. H., Kim, B.-K., Hong, S.-J., Lee, S.-J., Ahn, C.-M., Kim, J.-S., Ko, Y.-G., Choi, D., Hong, M.-K., & Jang, Y. (2021). Outcomes of Different Reperfusion Strategies of Multivessel Disease Undergoing Newer-Generation Drug-Eluting Stent Implantation in Patients with Non-ST-Elevation Myocardial Infarction and Chronic Kidney Disease. Journal of Clinical Medicine, 10(20), 4629. https://doi.org/10.3390/jcm10204629






