Impact of the Pre-Transplant Circulatory Supportive Strategy on Post-Transplant Outcome: Double Bridge May Work
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection and Study Population
2.2. Selection of MCS and Procedures
2.3. Follow-Up Protocol
2.4. Statistical Analysis
3. Results
3.1. Characteristics and Post-Transplant Outcomes of MCS and Non-MCS Patients
3.2. Characteristics and Outcomes of Different Types of MCS
3.3. Kaplan–Meier Survival Analysis for 3 Years Follow-Up
4. Discussion
5. Limitation
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Crespo-Leiro, M.G.; Metra, M.; Lund, L.H.; Milicic, D.; Costanzo, M.R.; Filippatos, G.; Gustafsson, F.; Tsui, S.; Barge-Caballero, E.; De Jonge, N.; et al. Advanced heart failure: A position statement of the Heart Failure Association of the European Society of Cardiology. Eur. J. Hear. Fail. 2018, 20, 1505–1535. [Google Scholar] [CrossRef] [PubMed]
- Colvin, M.; Smith, J.M.; Hadley, N.; Skeans, M.A.; Uccellini, K.; Goff, R.; Israni, A.K.; Snyder, J.J.; Kasiske, B.L. OPTN/SRTR 2018 Annual Data Report: Heart. Am. J. Transplant. 2019, 20 (Suppl. 1), 340–426. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, M.S.; Rogers, J.G.; Milano, C.A.; Russell, S.D.; Conte, J.V.; Feldman, D.; Sun, B.; Tatooles, A.J.; Delgado, R.M.; Long, J.W.; et al. Advanced Heart Failure Treated with Continuous-Flow Left Ventricular Assist Device. N. Engl. J. Med. 2009, 361, 2241–2251. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.R.; Uriel, N.; Naka, Y.; Cleveland, J.C.; Yuzefpolskaya, M.; Salerno, C.T.; Walsh, M.N.; Milano, C.A.; Patel, C.B.; Hutchins, S.W.; et al. A Fully Magnetically Levitated Left Ventricular Assist Device—Final Report. N. Engl. J. Med. 2019, 380, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Fried, J.A.; Nair, A.; Takeda, K.; Clerkin, K.; Topkara, V.K.; Masoumi, A.; Yuzefpolskaya, M.; Takayama, H.; Naka, Y.; Burkhoff, D.; et al. Clinical and hemodynamic effects of intra-aortic balloon pump therapy in chronic heart failure patients with cardiogenic shock. J. Hear. Lung Transplant. 2018, 37, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Estep, J.D.; Cordero-Reyes, A.M.; Bhimaraj, A.; Trachtenberg, B.; Khalil, N.; Loebe, M.; Bruckner, B.; Orrego, C.M.; Bismuth, J.; Kleiman, N.S.; et al. Percutaneous Placement of an Intra-Aortic Balloon Pump in the Left Axillary/Subclavian Position Provides Safe, Ambulatory Long-Term Support as Bridge to Heart Transplantation. JACC Hear. Fail. 2013, 1, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-S.; Lin, J.-W.; Yu, H.-Y.; Ko, W.-J.; Jerng, J.-S.; Chang, W.-T.; Chen, W.-J.; Huang, S.-C.; Chi, N.-H.; Wang, C.-H.; et al. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: An observational study and propensity analysis. Lancet 2008, 372, 554–561. [Google Scholar] [CrossRef]
- Fukuhara, S.; Takeda, K.; Kurlansky, P.A.; Naka, Y.; Takayama, H. Extracorporeal membrane oxygenation as a direct bridge to heart transplantation in adults. J. Thorac. Cardiovasc. Surg. 2017, 155, 1607–1618.e6. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Montfort, J.A.; Xie, R.; Ton, V.K.; Meyns, B.; Nakatani, T.; Yanase, M.; Pettit, S.; Shaw, S.; Netuka, I.; Kirklin, J.; et al. Longitudinal impact of temporary mechanical circulatory support on durable ventricular assist device outcomes: An IMACS registry propensity matched analysis. J. Hear. Lung Transplant. 2019, 39, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Ramzy, D.; Azarbal, B.; Arabia, F.A.; Esmailian, F.; Czer, L.S.; Kobashigawa, J.A.; Moriguchi, J.D. Device Strategies for Patients in INTERMACS Profiles 1 and 2 Cardiogenic Shock: Double Bridge With Extracorporeal Membrane Oxygenation and Initial Implant of More Durable Devices. Artif. Organs 2016, 41, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Bowen, F.W.; Carboni, A.F.; O’Hara, M.L.; Pochettino, A.; Rosengard, B.R.; Morris, R.J.; Gorman, R.C.; Gorman, J.H.; Acker, M.A. Application of “double bridge mechanical” resuscitation for profound cardiogenic shock leading to cardiac transplantation. Ann. Thorac. Surg. 2001, 72, 86–90. [Google Scholar] [CrossRef]
- Pagani, F.D.; Lynch, W.; Swaniker, F.; Dyke, D.B.; Bartlett, R.; Koelling, T.; Moscucci, M.; Deeb, G.M.; Bolling, S.; Monaghan, H.; et al. Extracorporeal Life Support to Left Ventricular Assist Device Bridge to Heart Transplant: A Strategy to Optimize Survival and Resource Utilization. Circulation 1999, 100, II206. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yu, H.-Y.; Wang, C.-H.; Chi, N.-H.; Huang, S.-C.; Chou, H.-W.; Chou, N.-K.; Chen, Y.-S. Effect of interplay between age and low-flow duration on neurologic outcomes of extracorporeal cardiopulmonary resuscitation. Intensiv. Care Med. 2018, 45, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, B.; Stephenson, E.R.; Pae, W.E. Technique for Insertion of HeartMate II Left Ventricular Assist Device Inflow Cannula. Ann. Thorac. Surg. 2011, 91, 2001–2002. [Google Scholar] [CrossRef] [PubMed]
- Chiu, P.; Schaffer, J.M.; Oyer, P.E.; Pham, M.; Banerjee, D.; Woo, Y.J.; Ha, R. Influence of durable mechanical circulatory support and allosensitization on mortality after heart transplantation. J. Hear. Lung Transplant. 2016, 35, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Billingham, M.E.; Cary, N.R.; Hammond, M.E.; Kemnitz, J.; Marboe, C.; McCallister, H.A.; Snovar, D.C.; Winters, G.L.; Zerbe, A. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Heart Rejection Study Group. The International Society for Heart Transplantation. J. Hear. Transplant. 1990, 9, 593–601. [Google Scholar]
- Ton, V.-K.; Xie, R.; Hernandez-Montfort, J.A.; Meyns, B.; Nakatani, T.; Yanase, M.; Shaw, S.; Pettit, S.; Netuka, I.; Kirklin, J.; et al. Short- and long-term adverse events in patients on temporary circulatory support before durable ventricular assist device: An IMACS registry analysis. J. Hear. Lung Transplant. 2020, 39, 342–352. [Google Scholar] [CrossRef] [PubMed]
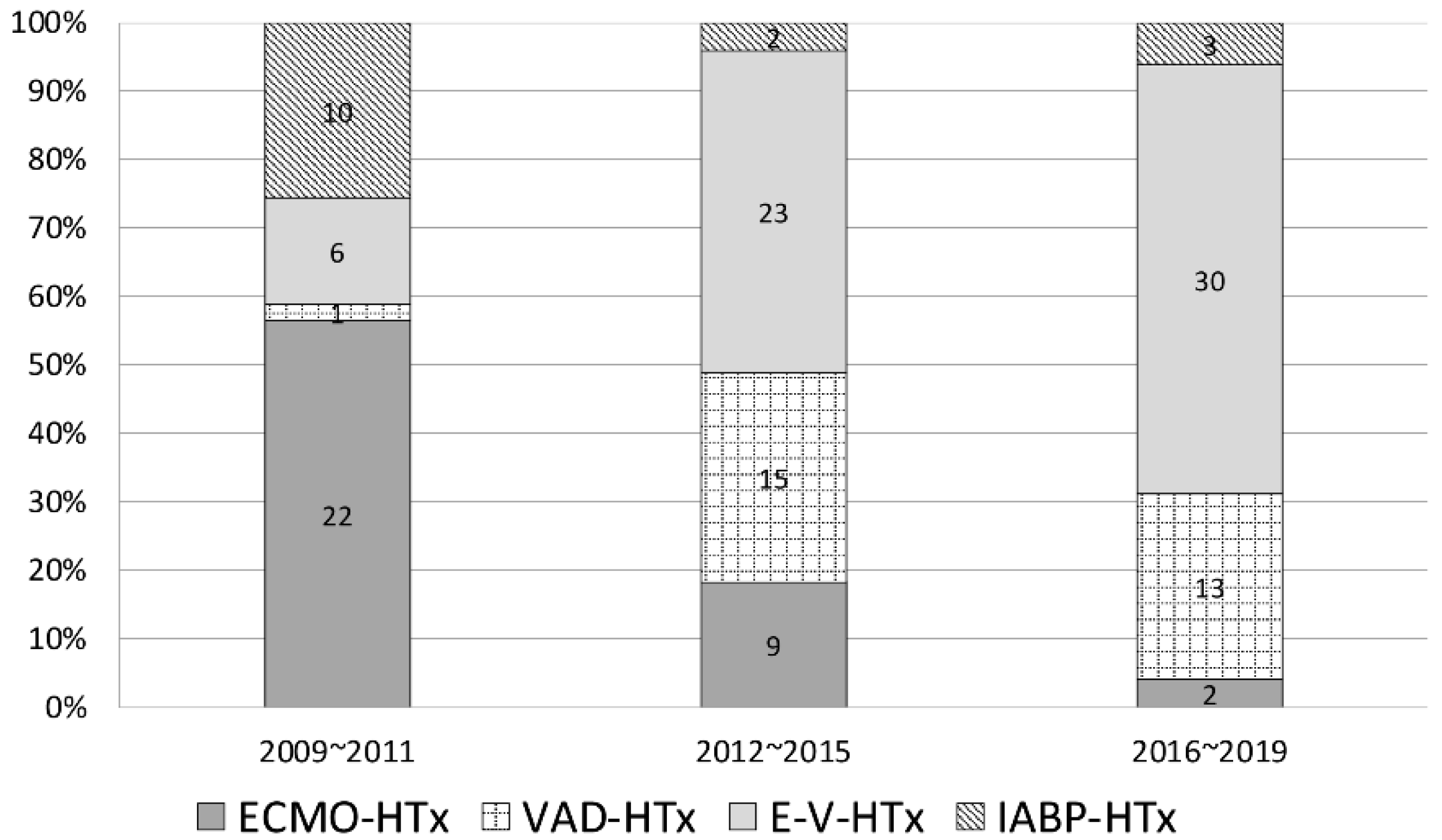
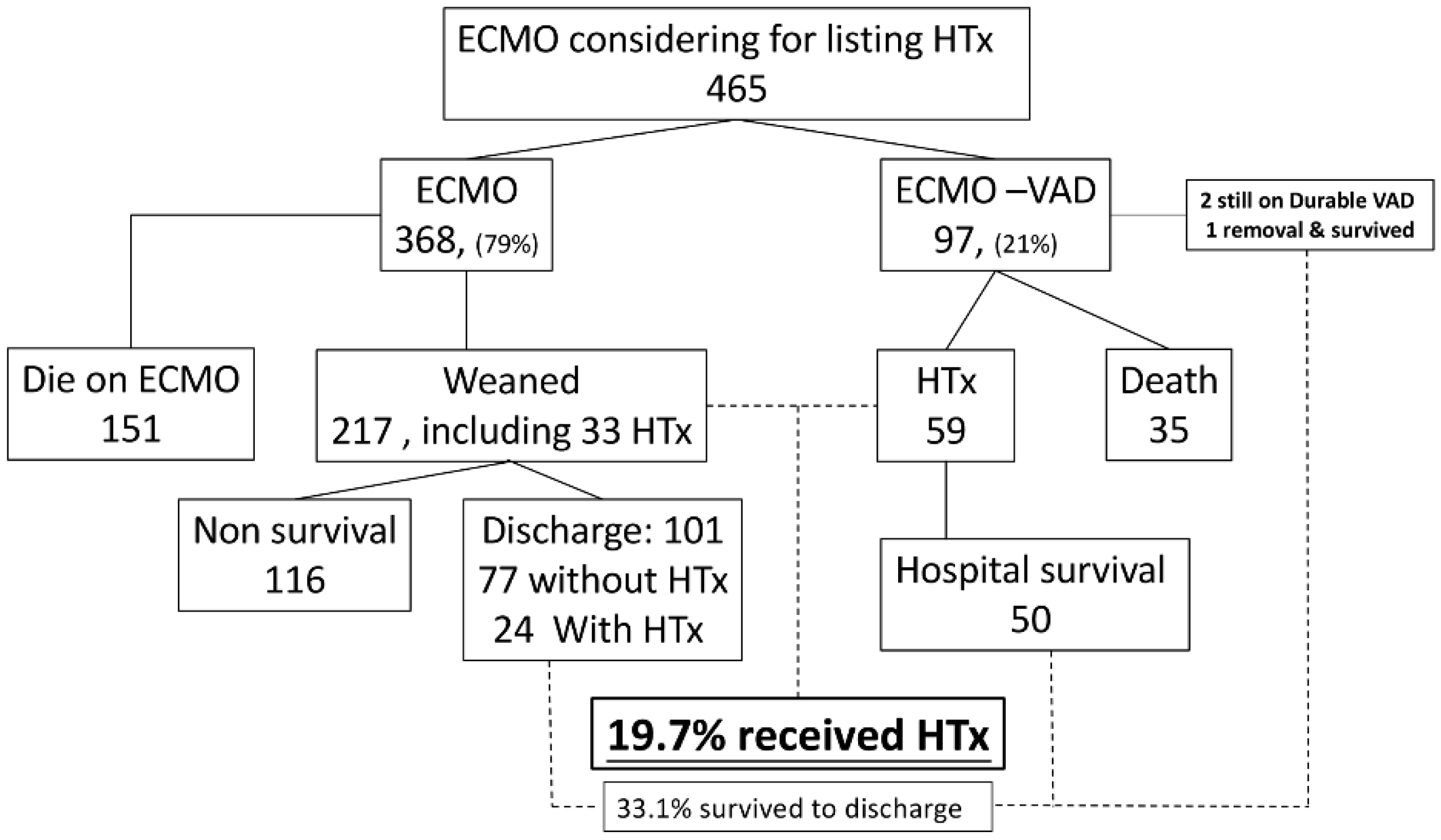
| Without MCS | With MCS | p | Total | |
|---|---|---|---|---|
| Patients, n (%) | 115 (45.8) | 136 (54.2) | 251 | |
| Gender, male n (%) | 95 (82.6) | 116 (85.3) | 0.342 | 211 (84.1) |
| Age, years (mean ± SD) | 44.1 ± 18.3 | 47.4 ± 14.3 | 0.113 | 45.9 ± 16.3 |
| Blood type | 0.006 | |||
| Type O, n (%) | 23 (20) | 52 (38.2) | 75 (29.9) | |
| Non-type O, n (%) | 92 (80) | 84 (61.8) | 176 (70.1) | |
| Hypertension, n (%) | 28 (24.3) | 44 (32.4) | 0.104 | 72 (28.7) |
| Diabetes mellitus, n (%) | 27 (23.5) | 37 (27.2) | 0.299 | 64 (25.5) |
| Hyperlipidemia, n (%) | 19 (16.5) | 30 (22.1) | 0.173 | 49 (19.5) |
| Smoking, n (%) | 27 (23.5%) | 41 (30.1) | 0.149 | 68 (27.1) |
| Primary etiology | 0.064 | |||
| ICMP, n (%) | 39 (33.9) | 60 (44.1) | 99 (39.4) | |
| Non-ICMP, n (%) | 76 (66.1) | 76 (55.9) | 152 (60.6) | |
| Previous cardiac surgery, n (%) | 40 (34.8) | 49 (36.0) | 0.471 | 89 (35.5) |
| Re-Tx, n (%) | 3 (2.6) | 5 (3.7) | 0.457 | 8 (3.2) |
| UNOS status | 0.000 | |||
| 1A, n (%) | 4 (3.5) | 136 (100) | 140 (55.6) | |
| 1B, n (%) | 45 (39.1) | 0 (0) | 45 (17.9) | |
| CPR *, n (%) | 6 (5.2%) | 73 (53.2%) | 0.000 | 79 (32.2%) |
| Listing duration # Days, median(IQR) | 253 (67~546) | 54 (16–134) | 0.005 | 89 (25–366) |
| Donor data | ||||
| Gender, male n (%) | 84 (73) | 94 (69.1) | 0.495 | 178 (70.9) |
| Age, years (mean ± SD) | 34.9 ± 15.0 | 37.7 ± 12.8 | 0.113 | 36.4 ± 13.9 |
| Blood type | 0.000 | |||
| Type O, n (%) | 29 (25.2) | 81 (59.6) | 100 (43.8) | |
| Non-type O, n (%) | 86 (74.8) | 55 (40.4) | 141 (56.2) | |
| Etiologies in brain death | 0.064 | |||
| Head trauma, n (%) | 51 (44.3) | 66(48.5) | 117(46.6) | |
| Cerebrovascular/stroke, n (%) | 38 (33.0) | 46 (33.8) | 84 (33.5) | |
| Brain tumor, n (%) | 2 (1.7) | 4 (2.9) | 6 (2.4) | |
| Hypoxia, n (%) | 16 (13.9) | 14 (10.3) | 30 (12.0) | |
| Other, n (%) | 8 (7.0) | 6 (4.4) | 14 (5.6) | |
| CPR before donation, n (%) | 34 (29.6) | 38 (27.9) | 0.952 | 72 (28.5) |
| Hypotension episode, n (%) | 42 (36.5) | 54 (39.7) | 0.862 | 96 (37.9) |
| LVEF, % (mean ± SD) | 65.3 ± 9.3 | 64.4 ± 10.1 | 0.434 | 64.8 ± 9.7 |
| Without MCS Group 1 | With MCS Group 2–5 | p | Total | |
|---|---|---|---|---|
| Patients, n (%) | 115 (45.8) | 136 (54.2) | 251 | |
| H/D before HTx, n (%) | 9 (8) | 66 (49.3) | 0.000 | 75 (30.4) |
| Ischemic time, min (mean ± SD) | 148.1 ± 63.3 | 175.0 ± 68.6 | 0.002 | 163 ± 67 |
| CPB duration, min (mean ± SD) | 139.7 ± 61.0 | 172.5 ± 68.9 | 0.000 | 158 ± 67 |
| Procedure duration, min (mean ± SD) | 315.3 ± 125.4 | 345.7 ± 116.2 | 0.050 | 332 ± 121 |
| Desensitization in HTx, n (%) | 9 (7.8) | 18 (13.2) | 0.120 | 27 (10.6) |
| Post-HTx MCS, n (%) | 21 (18.3) | 50 (36.8) | 0.001 | 71 (28.3) |
| Requiring H/D after HTx, n (%) | 24 (21.2) | 65 (48.5) | 0.000 | 89 (36.0) |
| Extubation after HTx, day (mean ± SD) | 4.6 ± 6.4 | 7.8 ± 9.9 | 0.023 | 6.2 ± 8.5 |
| ICU stay after HTx, day (mean ± SD) | 13.2 ± 8.3 | 18.9 ± 15.5 | 0.002 | 16.1 ± 12.9 |
| Hospital survival, n (%) | 106 (92.2) | 106 (77.9) | 0.001 | 212 (84.5) |
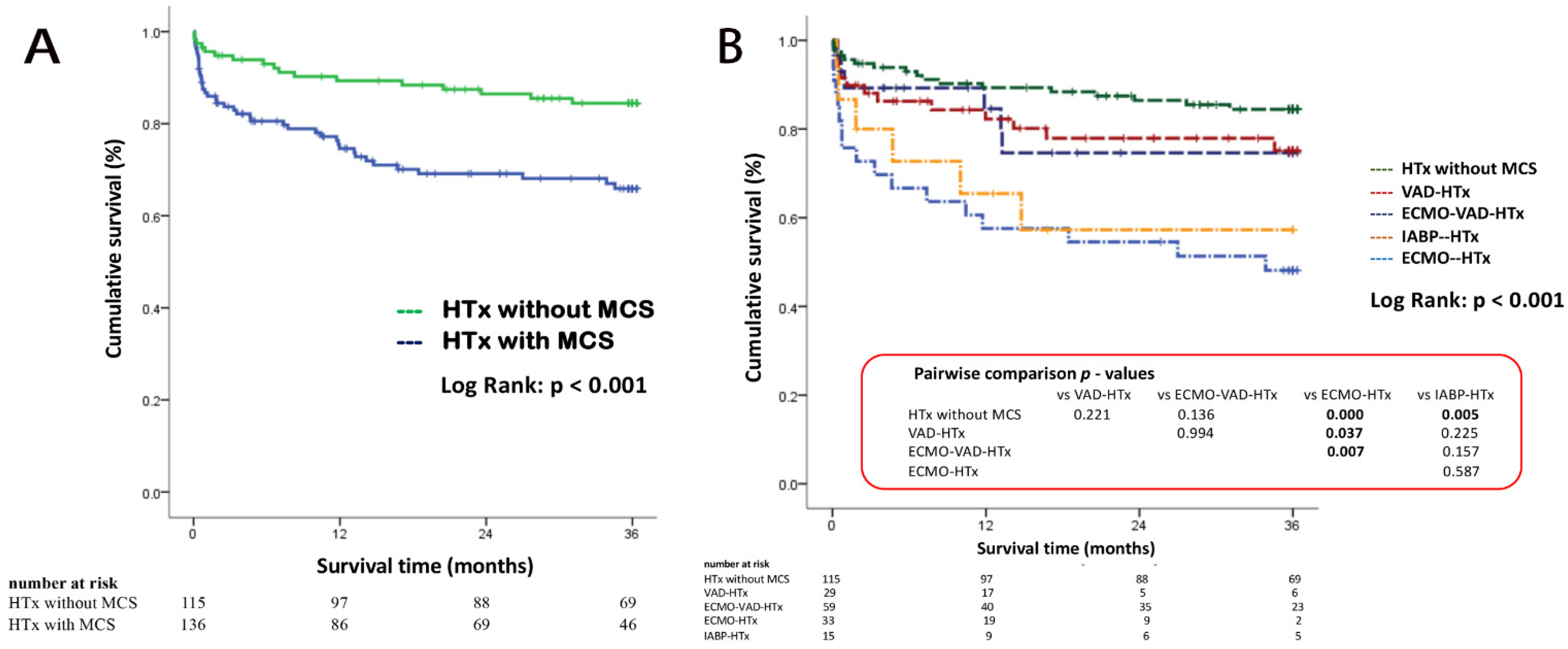
| 90-day survival (%) | 94.8 | 83.7 | 0.004 | 88.8 |
| 1-year survival (%) | 89.3 | 74.6 | 0.002 | 81.4 |
| 3-year survival (%) | 84.4 | 65.9 | 0.002 | 74.1 |
| ACR 1B in 3 years, n (%) | 8 (7.5) | 12 (9.8) | 0.356 | 20 (8.8) |
| AMR in 3 years, n (%) | 7 (6.5) | 5 (4.1) | 0.297 | 12 (5.2) |
| IABP Group 2 | ECMO Group 3 | ECMO-VAD Group 4 | VAD Group 5 | p | Total | |
|---|---|---|---|---|---|---|
| Patient, n (%) | 15 (11) | 33 (24.3) | 59 (43.4) | 29 (21.3) | 136 | |
| Male, n (%) | 15 (100.0) | 28 (84.8) | 47 (79.7) | 26 (89.7) | 0.210 | 116 (85.3) |
| Age, (years ± SD) | 54.0 ± 9.9 | 47.5 ± 16.5 | 44.2 ± 14.5 | 50.4 ± 11.8 | 0.058 | 47.4 ± 14.3 |
| Blood type | 0.571 | |||||
| Type O, n (%) | 4 (26.7) | 14 (42.4) | 24 (40.7) | 10 (34.5) | 52 (38.2) | |
| Non type O, n (%) | 11 (73.3) | 19 (57.6) | 35 (59.3) | 19 (65.5) | 74 (61.8) | |
| Hypertension, n (%) | 13 (39.4) | 20 (33.9) | 8 (27.6) | 0.540 | 44 (32.4) | |
| DM, n (%) | 5 (33.3) | 12 (36.4) | 11 (18.6) | 9 (31.0) | 0.253 | 37 (27.2) |
| Hyperlipidemia, n (%) | 3 (20.0) | 8 (24.2) | 10 (16.9) | 9 (31.0) | 0.497 | 30 (22.1) |
| Smoking, n (%) | 4 (26.7) | 4 (12.1) | 20 (33.9) | 13 (44.8) | 0.036 | 41 (30.1) |
| Primary etiology | 0.638 | |||||
| ICMP, n (%) | 8 (53.3) | 15 (45.5) | 27(45.8) | 10 (34.5) | 60 (44.1) | |
| Non ICMP, n (%) | 7 (46.7) | 18 (54.5) | 32 (54.2) | 19 (65.) | 76 (55.9) | |
| Previous cardiac surgery, n (%) | 3 (20.0) | 14 (42.4) | 16 (27.1) | 16 (55.2) | 0.031 | 49 (36) |
| CPR *, n (%) | 6 (42.9) | 21 (67.7) | 36 (63.2) | 10 (35.7) | 0.035 | 73 (56.2) |
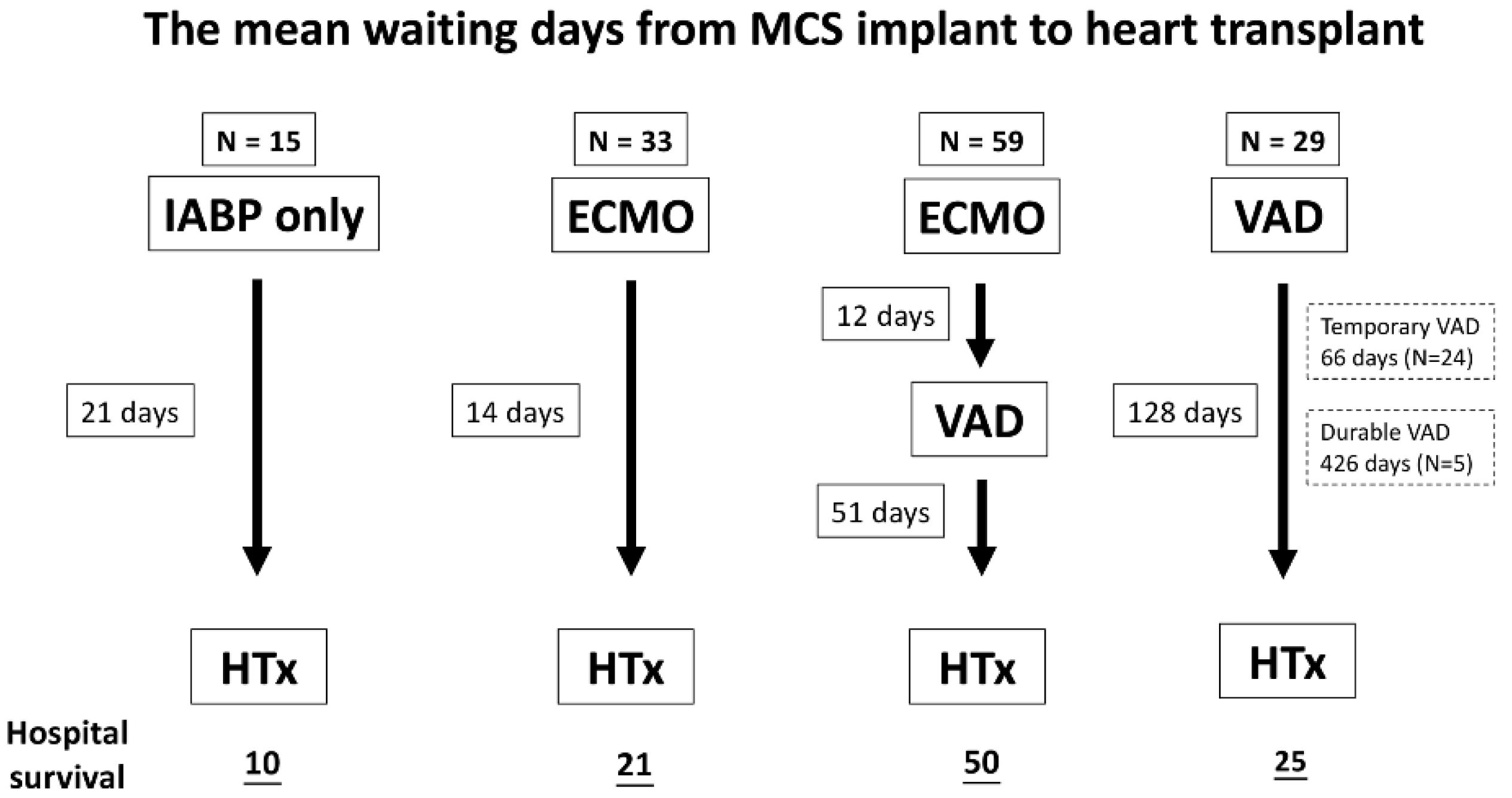
| Waiting days from index admission to HTx, days (days ± SD) | 42.1 ± 47.9 | 19.7 ± 18.0 | 52.8 ± 47.2 | 127.9 ± 311.6 | 0.000 | 47.2 ± 47.7 |
| Listing duration #, days, median (IQR) | 59 (14–156) | 16 (11–295) | 53 (18–81) | 112 (45–173) | 0.017 | 54 (16–134) |
| Waiting days from MCS to HTx, day (mean ± SD) | 21 ± 15 | 14 ± 9 | 63 ± 56 | 128 ± 312 | 0.019 | 60 ± 152 |
| ECMO-VAD 12 ± 12 VAD-HTx 51 ± 53 | Temporary VAD 66 ± 78 Durable VAD 426 ± 714 | |||||
| H/D before HTx, n (%) | 2 (14.3) | 16 (48.5) | 39 (67.2) | 9 (31.0) | 0.000 | 66 (49.3) |
| IABP Group 2 | ECMO Group 3 | ECMO-VAD Group 4 | VAD Group 5 | p | Total | |
|---|---|---|---|---|---|---|
| Patients, n (%) | 15 (11) | 33 (24.3) | 59 (43.4) | 29 (21.3) | 136 | |
| Requiring H/D after HTx, n (%) | 6 (40.0) | 22 (66.7) | 26 (45.6) | 11 (37.9) | 0.099 | 65 (48.5) |
| Desensitization in HTx, n (%) | 1 (6.7) | 0 (0.0) | 13 (22) | 4 (13.8) | 0.022 | 18 (13.2) |
| Ischemic time, min (±SD) | 162.5 ± 67.1 | 165.6 ± 58.5 | 175.5 ± 71.7 | 191.2 ± 73.6 | 0.439 | 175.0 ± 68.6 |
| Bypass duration, min (±SD) | 144.0 ± 57.4 | 157.1 ± 75.7 | 183.5 ± 60.0 | 183.1 ± 78.6 | 0.093 | 172.6 ± 68.9 |
| Procedure duration, min (±SD) | 336.5 ± 138.8 | 357.1 ± 120.0 | 357.7 ± 116.1 | 313.9 ± 98.0 | 0.366 | 345.7 ±116.2 |
| MCS after HTx, n(%) | 8 (53.3) | 15 (45.5) | 22 (37.3) | 5 (17.2) | 0.055 | 50 (36.8) |
| Extubation after HTx, day (±SD) | 3.3 ± 5.3 | 9.1 ± 8.5 | 9.3 ± 12.2 | 4.6 ± 5.3 | 0.266 | 7.8 ± 9.9 |
| ICU stay after HTx, day (±SD) | 8.8 ± 3.6 | 18.4 ± 12.9 | 22.2 ± 18.8 | 18.1 ± 12.1 | 0.081 | 18.9 ± 15.5 |
| Hospital survival, n (%) | 10 (66.7) | 21 (63.6) | 50 (84.7) | 25 (86.2) | 0.051 | 106 (77.9) |
| 90 days survival rate | 80.0% | 72.7% | 88.1% | 89.3% | 0.093 | 83.7% |
| 1-year survival rate | 65.5% | 57.6% | 82.3% | 84.6% | 0.012 | 74.6% |
| 3-year survival rate | 57.3% | 48.1% | 75.1% | 74.6% | 0.031 | 65.9% |
| Rejection 1B in 3 years, n (%) | 0 (0.0) | 3 (12.5) | 8 (14.0) | 1(3.6) | 0.263 | 12 (9.8) |
| AMR in 3 years, n (%) | 0 (0.0) | 1 (4.2) | 3 (5.3) | 1(3.6) | 0.856 | 5 (4.1) |
| Rejection 1B in 5 years, n (%) | 0 (0.0) | 4 (16.7) | 8 (14.0) | 1 (3.6) | 0.202 | 13 (10.7) |
| AMR in 5 years, n (%) | 0 (0.0) | 1 (4.2) | 4 (7.0) | 1 (3.6) | 0.718 | 6 (4.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, N.-K.; Chou, H.-W.; Tsao, C.-I.; Wang, C.-H.; Chen, K.P.-H.; Chi, N.-H.; Huang, S.-C.; Yu, H.-Y.; Chen, Y.-S. Impact of the Pre-Transplant Circulatory Supportive Strategy on Post-Transplant Outcome: Double Bridge May Work. J. Clin. Med. 2021, 10, 4697. https://doi.org/10.3390/jcm10204697
Chou N-K, Chou H-W, Tsao C-I, Wang C-H, Chen KP-H, Chi N-H, Huang S-C, Yu H-Y, Chen Y-S. Impact of the Pre-Transplant Circulatory Supportive Strategy on Post-Transplant Outcome: Double Bridge May Work. Journal of Clinical Medicine. 2021; 10(20):4697. https://doi.org/10.3390/jcm10204697
Chicago/Turabian StyleChou, Nai-Kuan, Heng-Wen Chou, Chuan-I Tsao, Chih-Hsien Wang, Kevin Po-Hsun Chen, Nai-Hsin Chi, Shu-Chien Huang, Hsi-Yu Yu, and Yih-Sharng Chen. 2021. "Impact of the Pre-Transplant Circulatory Supportive Strategy on Post-Transplant Outcome: Double Bridge May Work" Journal of Clinical Medicine 10, no. 20: 4697. https://doi.org/10.3390/jcm10204697
APA StyleChou, N.-K., Chou, H.-W., Tsao, C.-I., Wang, C.-H., Chen, K. P.-H., Chi, N.-H., Huang, S.-C., Yu, H.-Y., & Chen, Y.-S. (2021). Impact of the Pre-Transplant Circulatory Supportive Strategy on Post-Transplant Outcome: Double Bridge May Work. Journal of Clinical Medicine, 10(20), 4697. https://doi.org/10.3390/jcm10204697







