In Search of Clinical Impact: Advanced Monitoring Technologies in Daily Heart Failure Care
Abstract
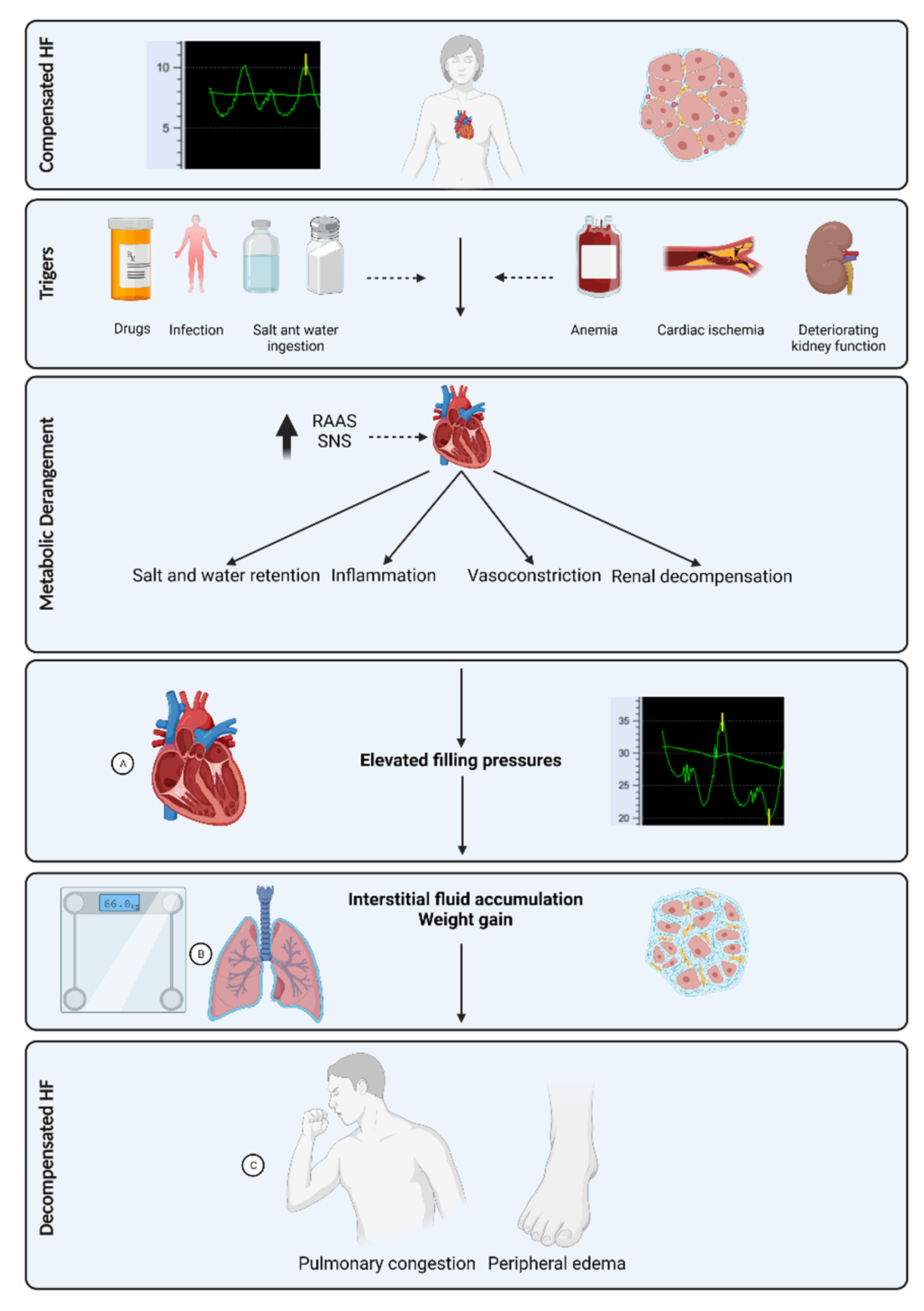
:1. Introduction
2. Non-Invasive HF Monitoring
2.1. Lung Fluid Volume Assessment
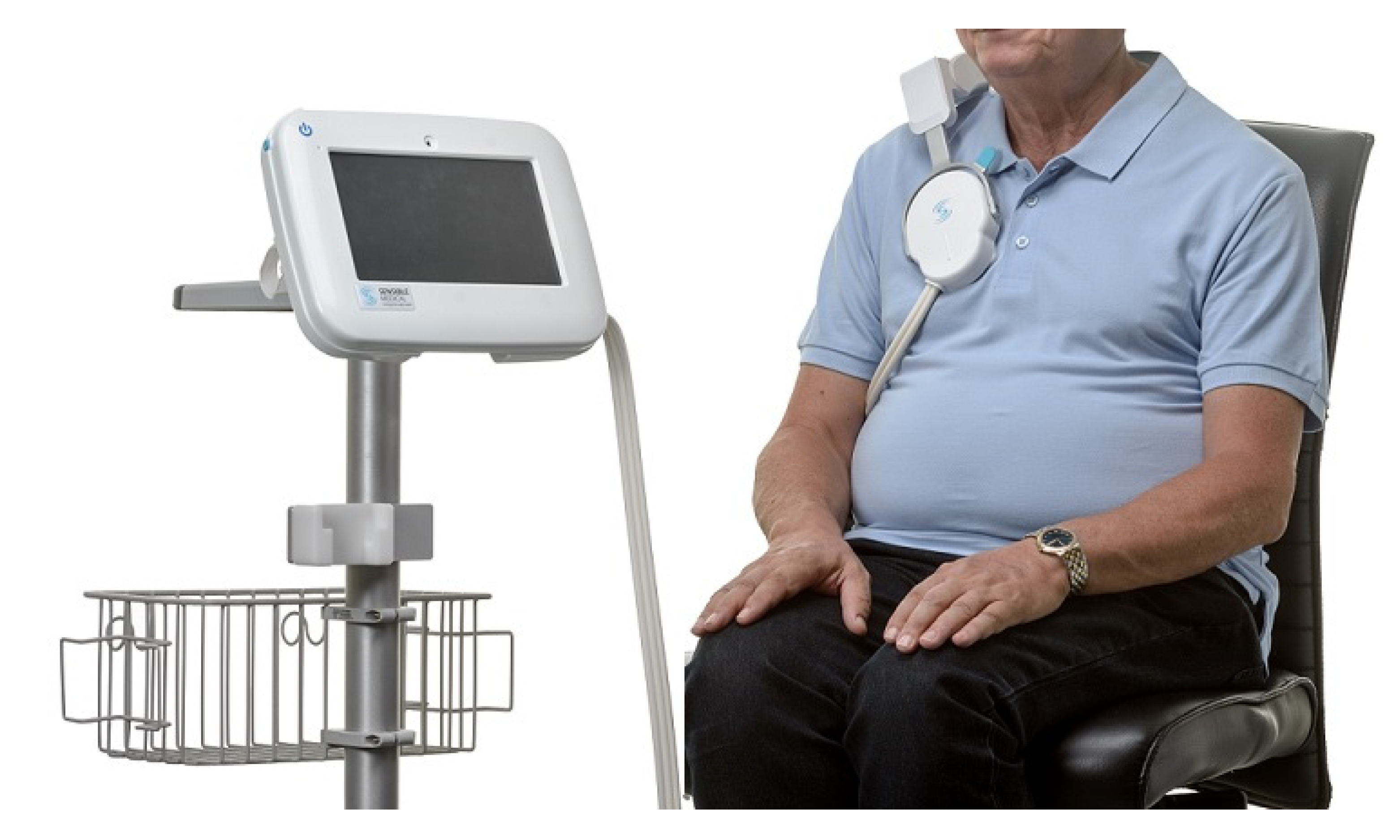
2.2. Whole-Body Electrical Bioimpedance
2.3. Piezoelectric Sensor for Physiologic Vibration Monitoring
2.4. Wearable Devices
3. Invasive HF Monitoring
3.1. Cardiac Implanted Electronic Devices
3.2. Pulmonary Artery Pressure Monitoring
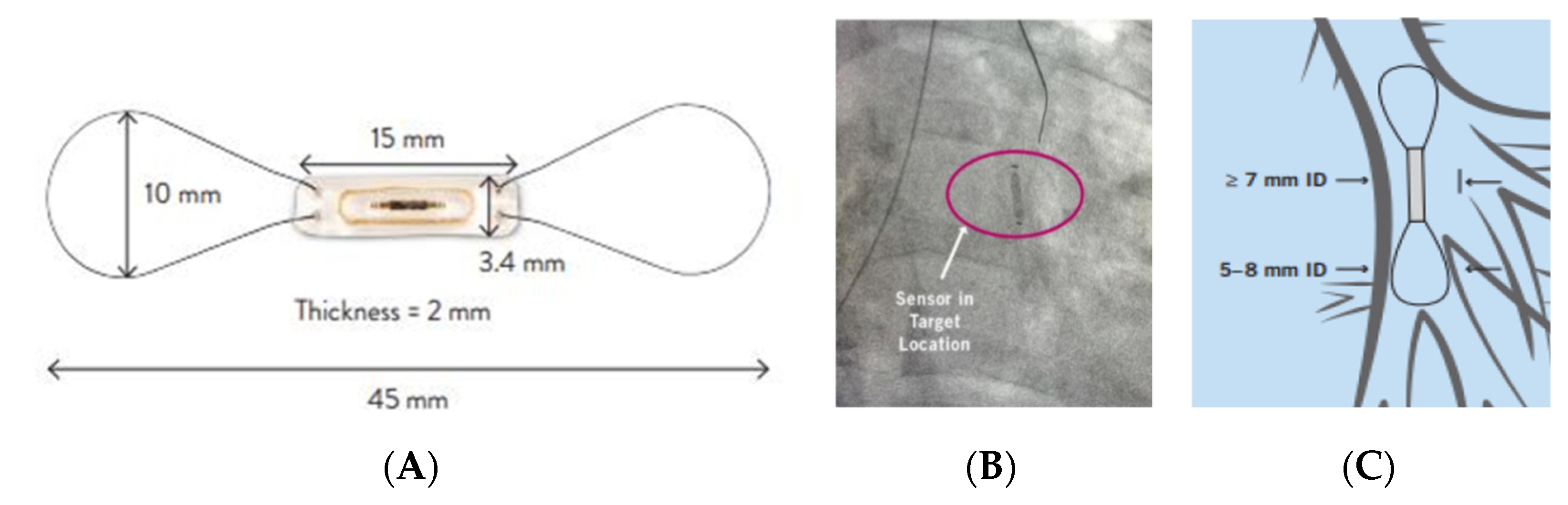
3.3. Left Atrial Pressure Monitoring
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rossignol, P.; Hernandez, A.F.; Solomon, S.D.; Zannad, F. Heart failure drug treatment. Lancet 2019, 393, 1034–1044. [Google Scholar] [CrossRef]
- Metra, M.; Teerlink, J.R. Heart failure. Lancet 2017, 390, 1981–1995. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Albert, N.M.; Allen, L.A.; Bluemke, D.A.; Butler, J.; Fonarow, G.C.; Ikonomidis, J.S.; Khavjou, O.; Konstam, M.A.; Maddox, T.M.; et al. Forecasting the impact of heart failure in the United States: A policy statement from the American Heart Association. Circ. Heart Fail. 2013, 6, 606–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roger, V.L.; Go, A.S.; Lloyd-Jones, D.M.; Benjamin, E.J.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; Fox, C.S.; et al. Heart disease and stroke statistics—2012 update: A report from the American Heart Association. Circulation 2012, 125, e2–e220. [Google Scholar] [PubMed]
- Barker, W.H.; Mullooly, J.P.; Getchell, W. Changing incidence and survival for heart failure in a well-defined older population, 1970-1974 and 1990-1994. Circulation 2006, 113, 799–805. [Google Scholar] [CrossRef] [Green Version]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics—2017 Update: A Report From the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics—2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar]
- Cook, C.; Cole, G.; Asaria, P.; Jabbour, R.; Francis, D.P. The annual global economic burden of heart failure. Int. J. Cardiol. 2014, 171, 368–376. [Google Scholar] [CrossRef]
- Shah, K.S.; Xu, H.; Matsouaka, R.A.; Bhatt, D.L.; Heidenreich, P.A.; Hernandez, A.F.; Devore, A.D.; Yancy, C.W.; Fonarow, G.C. Heart Failure With Preserved, Borderline, and Reduced Ejection Fraction: 5-Year Outcomes. J. Am. Coll. Cardiol. 2017, 70, 2476–2486. [Google Scholar] [CrossRef]
- Hunt, S.A.; Abraham, W.T.; Chin, M.H.; Feldman, A.M.; Francis, G.S.; Ganiats, T.G.; Jessup, M.; Konstam, M.A.; Mancini, D.M.; Michl, K.; et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: Developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 2009, 119, e391–e479. [Google Scholar]
- Abid, L.; Charfeddine, S.; Kammoun, I.; Halima, M.B.; Slima, H.B.; Drissa, M.; Mzoughi, K.; Mbarek, D.; Riahi, L.; Antit, S.; et al. Epidemiology of heart failure and long-term follow-up outcomes in a north-African population: Results from the NAtional TUnisian REgistry of Heart Failure (NATURE-HF). PLoS ONE 2021, 16, e0251658. [Google Scholar] [CrossRef]
- Dunlay, S.M.; Shah, N.D.; Shi, Q.; Morlan, B.; VanHouten, H.; Long, K.H.; Roger, V.L. Lifetime costs of medical care after heart failure diagnosis. Circ. Cardiovasc. Qual. Outcomes 2011, 4, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Bello, N.A.; Claggett, B.; Desai, A.S.; McMurray, J.J.V.; Granger, C.B.; Yusuf, S.; Swedberg, K.; Pfeffer, M.A.; Solomon, S.D. Influence of previous heart failure hospitalization on cardiovascular events in patients with reduced and preserved ejection fraction. Circ. Heart Fail. 2014, 7, 590–595. [Google Scholar] [CrossRef] [Green Version]
- Solomon, S.D.; Dobson, J.; Pocock, S.; Skali, H.; McMurray, J.J.V.; Granger, C.B.; Yusuf, S.; Swedberg, K.; Young, J.B.; Michelson, E.L.; et al. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation 2007, 116, 1482–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatia, R.S.; Tu, J.V.; Lee, D.S.; Austin, P.C.; Fang, J.; Haouzi, A.; Gong, Y.; Liu, P.P. Outcome of heart failure with preserved ejection fraction in a population-based study. N. Engl. J. Med. 2006, 355, 260–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jencks, S.F.; Williams, M.V.; Coleman, E.A. Rehospitalizations among patients in the Medicare fee-for-service program. N. Engl. J. Med. 2009, 360, 1418–1428. [Google Scholar] [CrossRef]
- Strom, J.B.; Kramer, D.B.; Wang, Y.; Shen, C.; Wasfy, J.H.; Landon, B.E.; Wilker, E.H.; Yeh, R.W. Short-term rehospitalization across the spectrum of age and insurance types in the United States. PLoS ONE 2017, 12, e0180767. [Google Scholar] [CrossRef] [PubMed]
- Joynt, K.E.; Jha, A.K. Who has higher readmission rates for heart failure, and why? Implications for efforts to improve care using financial incentives. Circ. Cardiovasc. Qual. Outcomes 2011, 4, 53–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dharmarajan, K.; Hsieh, A.F.; Lin, Z.; Bueno, H.; Ross, J.; Horwitz, L.; Barreto-Filho, J.A.; Kim, N.; Bernheim, S.M.; Suter, L.G.; et al. Diagnoses and Timing of 30-Day Readmissions After Hospitalization for Heart Failure, Acute Myocardial Infarc-tion, or Pneumonia. JAMA 2013, 309, 355–363. [Google Scholar] [CrossRef]
- Loehr, L.R.; Rosamond, W.D.; Chang, P.P.; Folsom, A.R.; Chambless, L.E. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am. J. Cardiol. 2008, 101, 1016–1022. [Google Scholar] [CrossRef]
- Rich, M.W.; Beckham, V.; Wittenberg, C.; Leven, C.L.; Freedland, K.E.; Carney, R.M. A Multidisciplinary Intervention to Prevent the Readmission of Elderly Patients with Congestive Heart Failure. N. Engl. J. Med. 1995, 333, 1190–1195. [Google Scholar] [CrossRef]
- Kitzman, D.W.; Whellan, D.J.; Duncan, P.; Pastva, A.M.; Mentz, R.J.; Reeves, G.R.; Nelson, M.B.; Chen, H.; Upadhya, B.; Reed, S.D.; et al. Physical Rehabilitation for Older Patients Hospitalized for Heart Failure. N. Engl. J. Med. 2021, 385, 203–216. [Google Scholar] [CrossRef]
- Bekfani, T.; Fudim, M.; Cleland, J.G.F.; Jorbenadze, A.; von Haehling, S.; Lorber, A.; Rothman, A.M.K.; Stein, K.; Abraham, W.T.; Sievert, H.; et al. A current and future outlook on upcoming technologies in remote monitoring of patients with heart failure. Eur. J. Heart Fail. 2021, 23, 175–185. [Google Scholar] [CrossRef]
- Cheng, R.; Cox, M.; Neely, M.L.; Heidenreich, P.A.; Bhatt, D.L.; Eapen, Z.J.; Hernandez, A.F.; Butler, J.; Yancy, C.W.; Fonarow, G.C. Outcomes in patients with heart failure with preserved, borderline, and reduced ejection fraction in the Medicare popultion. Am. Hear. J. 2014, 168, 721–730. [Google Scholar] [CrossRef]
- Zile, M.R.; Bennett, T.D.; Sutton, M.S.J.; Cho, Y.K.; Adamson, P.B.; Aaron, M.F.; Aranda, J.M., Jr.; Abraham, W.T.; Smart, F.W.; Stevenson, L.W.; et al. Transition from chronic compensated to acute decompensated heart failure: Pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation 2008, 118, 1433–1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abraham, W.T.; Perl, L. Implantable Hemodynamic Monitoring for Heart Failure Patients. J. Am. Coll. Cardiol. 2017, 70, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Adamson, P.B. Pathophysiology of the transition from chronic compensated and acute decompensated heart failure: New insights from continuous monitoring devices. Curr. Heart Fail. Rep. 2009, 6, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Schrier, R.W.; Abraham, W.T. Hormones and hemodynamics in heart failure. N. Engl. J. Med. 1999, 341, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Kaye, D.M.; Lefkovits, J.; Jennings, G.; Bergin, P.; Broughton, A.; Esler, M.D. Adverse consequences of high sympathetic nervous activity in the failing human heart. J. Am. Coll. Cardiol. 1995, 26, 1257–1263. [Google Scholar] [CrossRef] [Green Version]
- Mentz, R.J.; O’Connor, C.M. Pathophysiology and clinical evaluation of acute heart failure. Nat. Rev. Cardiol. 2016, 13, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Radhoe, S.P.; Veenis, J.F.; Brugts, J.J. Invasive Devices and Sensors for Remote Care of Heart Failure Patients. Sensors 2021, 21, 2014. [Google Scholar] [CrossRef]
- Kapelios, C.J.; Malliaras, K.; Kaldara, E.; Vakrou, S.; Nanas, J.N. Loop diuretics for chronic heart failure: A foe in disguise of a friend? Eur. Heart J. Cardiovasc. Pharmacother. 2018, 4, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Lyngå, P.; Persson, H.; Martinell, A.H.; Hägglund, E.; Hagerman, I.; Langius-Eklöf, A.; Rosenqvist, M. Weight monitoring in patients with severe heart failure (WISH). A randomized controlled trial. Eur. J. Hear. Fail. 2012, 14, 438–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Goode, K.M.; Cuddihy, P.E.; Cleland, J.G.F.; TEN-HMS Investigators. Predicting hospitalization due to worsening heart failure using daily weight measurement: Analysis of the Trans-European Network-Home-Care Management System (TEN-HMS) study. Eur. J. Heart Fail. 2009, 11, 420–427. [Google Scholar] [CrossRef]
- Cleland, J.G.F.; Louis, A.A.; Rigby, A.S.; Janssens, U.; Balk, A.H.M.M.; TEN-HMS Investigators. Noninvasive home telemonitoring for patients with heart failure at high risk of recurrent admission and death: The Trans-European Network-Home-Care Management System (TEN-HMS) study. J. Am. Coll. Cardiol. 2005, 45, 1654–1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koehler, F.; Koehler, K.; Deckwart, O.; Prescher, S.; Wegscheider, K.; Kirwan, B.-A.; Winkler, S.; Vettorazzi, E.; Bruch, L.; Oeff, M.; et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): A randomised, controlled, parallel-group, unmasked trial. Lancet 2018, 392, 1047–1057. [Google Scholar] [CrossRef]
- Poelzl, G.; Egelseer-Bruendl, T.; Pfeifer, B.; Modre-Osprian, R.; Welte, S.; Fetz, B.; Krestan, S.; Haselwanter, B.; Zaruba, M.M.; Doerler, J.; et al. Feasibility and effectiveness of a multidimensional post-discharge disease management programme for heart failure patients in clinical practice: The HerzMobil Tirol programme. Clin. Res. Cardiol. 2021. [CrossRef]
- Ong, M.K.; Romano, P.S.; Edgington, S.; Aronow, H.U.; Auerbach, A.D.; Black, J.T.; de Marco, T.; Escarce, J.J.; Evangelista, L.S.; Hanna, B.; et al. Effectiveness of Remote Patient Monitoring After Discharge of Hospitalized Patients With Heart Failure: The Better Effectiveness After Transition—Heart Failure (BEAT-HF) Randomized Clinical Trial. JAMA Intern. Med. 2016, 176, 310–318. [Google Scholar] [CrossRef]
- Coiro, S.; Rossignol, P.; Ambrosio, G.; Carluccio, E.; Alunni, G.; Murrone, A.; Tritto, I.; Zannad, F.; Girerd, N. Prognostic value of residual pulmonary congestion at discharge assessed by lung ultrasound imaging in heart failure. Eur. J. Heart Fail. 2015, 17, 1172–1181. [Google Scholar] [CrossRef]
- Peacock, W.I.; Albert, N.M.; Kies, P.; White, R.D.; Emerman, C.L. Bioimpedance monitoring: Better than chest x-ray for predicting abnormal pulmonary fluid? Congest. Heart Fail. 2000, 6, 86–89. [Google Scholar] [CrossRef]
- Ramos, M.U.; LaBree, J.W.; Remole, W.; Kubicek, W.G. Transthoracic electric impedance. A clinical guide of pulmonary fluid accumulation in congestive heart failure. Minn. Med. 1975, 58, 671–676. [Google Scholar]
- Ito, H.; Yamakoshi, K.I.; Togawa, T. Transthoracic admittance plethysmograph for measuring cardiac output. J. Appl. Physiol. 1976, 40, 451–454. [Google Scholar] [CrossRef]
- Patterson, R.P.; Kubicek, W.G.; Witsoe, D.A.; From, A.H. Studies on the effect of controlled volume change on the thoracic electrical impedance. Med. Biol. Eng. Comput. 1978, 16, 531–536. [Google Scholar] [CrossRef]
- Shochat, M.K.; Shotan, A.; Blondheim, D.S.; Kazatsker, M.; Dahan, I.; Asif, A.; Rozenman, Y.; Kleiner, I.; Weinstein, J.M.; Frimerman, A.; et al. Non-Invasive Lung IMPEDANCE-Guided Preemptive Treatment in Chronic Heart Failure Patients: A Randomized Controlled Trial (IMPEDANCE-HF Trial). J. Card. Fail. 2016, 22, 713–722. [Google Scholar] [CrossRef]
- Shochat, M.K.; Fudim, M.; Shotan, A.; Blondheim, D.S.; Kazatsker, M.; Dahan, I.; Asif, A.; Rozenman, Y.; Kleiner, I.; Weinstein, J.M.; et al. Prediction of readmissions and mortality in patients with heart failure: Lessons from the IMPEDANCE-HF extended trial. ESC Heart Fail. 2018, 5, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Shochat, M.; Charach, G.; Meyler, S.; Kazatzker, M.; Mosseri, M.; Frimerman, A.; Rabinovich, P.; Shotan, A.; Meisel, S. In-ternal thoracic impedance monitoring: A novel method for the preclinical detection of acute heart failure. Cardiovasc. Revasc. Med. 2006, 7, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Shochat, M.; Shotan, A.; Blondheim, D.S.; Kazatsker, M.; Dahan, I.; Asif, A.; Shochat, I.; Frimerman, A.; Rozenman, Y.; Meisel, S.R. Derivation of baseline lung impedance in chronic heart failure patients: Use for monitoring pulmonary congestion and predicting admissions for decompensation. J. Clin. Monit. Comput. 2015, 29, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Khandwalla, R.M.; Birkeland, K.; Zimmer, R.; Banet, M.; Pede, S.; Kedan, I. Predicting heart failure events with home monitoring: Use of a novel, wearable necklace to measure stroke volume, cardiac output and thoracic impedance. J. Am. Coll. Cardiol. 2016, 67, 1296. [Google Scholar] [CrossRef]
- Uriel, N.; Sayer, G.; Imamura, T.; Rodgers, D.; Kim, G.; Raikhelkar, J.; Sarswat, N.; Kalantari, S.; Chung, B.; Nguyen, A.; et al. Relationship Between Noninvasive Assessment of Lung Fluid Volume and Invasively Measured Cardiac Hemodynamics. J. Am. Heart Assoc. 2018, 7, e009175. [Google Scholar] [CrossRef] [Green Version]
- Amir, O.; Rappaport, D.; Zafrir, B.; Abraham, W.T. A novel approach to monitoring pulmonary congestion in heart failure: Initial animal and clinical experiences using remote dielectric sensing technology. Congest. Heart Fail. 2013, 19, 149–155. [Google Scholar] [CrossRef]
- Abraham, W.T.; Amir, O.; Weinstein, J.M.; Abbo, A.; Tuvia, B.G. Remote Dielectric Sensing (ReDS™)—Guided Patient Management of Ambulatory Heart Failure Patients Reduces Rehospitalization Rates. J. Card. Fail. 2015, 21 (Suppl. S77). [Google Scholar] [CrossRef]
- Abraham, W.T.; Anker, S.; Burkhoff, D.; Cleland, J.; Gorodeski, E.; Jaarsma, T.; Small, R.; Lindenfeld, J.; Miller, A.; Ogenstad, S.; et al. Primary Results of the Sensible Medical Innovations Lung Fluid Status Monitor Allows Reducing Readmission Rate of Heart Failure Patients (smile) Trial. J. Card. Fail. 2019, 25, 938. [Google Scholar] [CrossRef]
- Khalil, S.F.; Mohktar, M.S.; Ibrahim, F. The theory and fundamentals of bioimpedance analysis in clinical status monitoring and diagnosis of diseases. Sensors 2014, 14, 10895–10928. [Google Scholar] [CrossRef]
- Bernstein, D.P. Continuous noninvasive real-time monitoring of stroke volume and cardiac output by thoracic electrical bioimpedance. Crit. Care Med. 1986, 14, 898–901. [Google Scholar] [CrossRef]
- Lukaski, H.C. Evolution of bioimpedance: A circuitous journey from estimation of physiological function to assessment of body composition and a return to clinical research. Eur. J. Clin. Nutr. 2013, 67 (Suppl. S1), S2–S9. [Google Scholar] [CrossRef] [Green Version]
- Hoffer, E.C.; Meador, C.K.; Simpson, D.C. Correlation of whole-body impedance with total body water volume. J. Appl. Physiol. 1969, 27, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Nyboer, J. Electrical impedance plethysmography; a physical and physiologic approach to peripheral vascular study. Circulation 1950, 2, 811–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubicek, W.G.; Karnegis, J.N.; Patterson, R.P.; Witsoe, D.A.; Mattson, R.H. Development and evaluation of an impedance cardiac output system. Aerosp. Med. 1966, 37, 1208–1212. [Google Scholar] [PubMed]
- Kubicek, W.G.; From, A.H.; Patterson, R.P.; Witsoe, D.A.; Castaneda, A.; Lillehei, R.C.; Ersek, R. Impedance cardiography as a noninvasive means to monitor cardiac function. JAAMI J. Assoc. Adv. Med. Instrum. 1970, 4, 79–84. [Google Scholar] [PubMed]
- Karnegis, J.N.; Kubicek, W.G. Physiological correlates of the cardiac thoracic impedance waveform. Am. Heart J. 1970, 79, 519–523. [Google Scholar] [CrossRef]
- Kubicek, W.G.; Kottke, J.; Ramos, M.U.; Patterson, R.P.; Witsoe, D.A.; Labree, J.W.; Remole, W.; Layman, T.E.; Schoening, H.; Garamela, J.T. The Minnesota impedance cardiograph—Theory and applications. Biomed. Eng. 1974, 9, 410–416. [Google Scholar] [PubMed]
- Cotter, G.; Moshkovitz, Y.; Kaluski, E.; Cohen, A.J.; Miller, H.; Goor, D.; Vered, Z. Accurate, noninvasive continuous monitoring of cardiac output by whole-body electrical bioimpedance. Chest 2004, 125, 1431–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozenman, Y.; Rotzak, R.; Patterson, R.P. Detection of left ventricular systolic dysfunction using a newly developed, laptop based, impedance cardiographic index. Int. J. Cardiol. 2011, 149, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.J.; Arnaudov, D.; Zabeeda, D.; Schultheis, L.; Lashinger, J.; Schachner, A. Non-invasive measurement of cardiac output during coronary artery bypass grafting. Eur. J. Cardiothorac. Surg. 1998, 14, 64–69. [Google Scholar] [CrossRef] [Green Version]
- Paredes, O.L.; Shite, J.; Shinke, T.; Watanabe, S.; Otake, H.; Matsumoto, D.; Imuro, Y.; Ogasawara, D.; Sawada, T.; Yokoyama, M. Impedance cardiography for cardiac output estimation: Reliability of wrist-to-ankle electrode configuration. Circ. J. 2006, 70, 1164–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tita, C.; Gilbert, E.M.; Van Bakel, A.B.; Grzybowski, J.; Haas, G.J.; Jarrah, M.; Dunlap, S.H.; Gottlieb, S.S.; Klapholz, M.; Patel, P.C.; et al. A Phase 2a dose-escalation study of the safety, tolerability, pharmacokinetics and haemodynamic effects of BMS-986231 in hospitalized patients with heart failure with reduced ejection fraction. Eur. J. Heart Fail. 2017, 19, 1321–1332. [Google Scholar] [CrossRef] [Green Version]
- Narous, M.; Yee, E.; Cowan, K.; Fine, N.M.; Mikami, Y.; White, J.A.; Exner, D.V. Do whole body impedance cardiography estimates of left ventricular structure, volumes and function correlate with the gold standard of cardiac magnetic resonance imaging? J. Cardiovasc. Magn. Reson. 2016, 18 (Suppl. S1), 194. [Google Scholar] [CrossRef] [Green Version]
- Pohanka, M. Overview of Piezoelectric Biosensors, Immunosensors and DNA Sensors and Their Applications. Materials 2018, 11, 448. [Google Scholar] [CrossRef] [Green Version]
- Allataifeh, A.; Ahmad, M.A. Simultaneous piezoelectric noninvasive detection of multiple vital signs. Sci. Rep. 2020, 10, 416. [Google Scholar] [CrossRef]
- Bennett, M.K.; Shao, M.; Gorodeski, E.Z. Home monitoring of heart failure patients at risk for hospital readmission using a novel under-the-mattress piezoelectric sensor: A preliminary single centre experience. J. Telemed. Telecare 2017, 23, 60–67. [Google Scholar] [CrossRef]
- Bayoumy, K.; Gaber, M.; Elshafeey, A.; Mhaimeed, O.; Dineen, E.H.; Marvel, F.A.; Martin, S.S.; Muse, E.D.; Turakhia, M.P.; Tarakji, K.G.; et al. Smart wearable devices in cardiovascular care: Where we are and how to move forward. Nat. Rev. Cardiol. 2021, 18, 581–599. [Google Scholar] [CrossRef]
- Perez, M.V.; Mahaffey, K.W.; Hedlin, H.; Rumsfeld, J.S.; Garcia, A.; Ferris, T.; Balasubramanian, V.; Russo, A.M.; Rajmane, A.; Cheung, L.; et al. Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation. N. Engl. J. Med. 2019, 381, 1909–1917. [Google Scholar] [CrossRef]
- Seshadri, D.R.; Bittel, B.; Browsky, D.; Houghtaling, P.; Drummond, C.K.; Desai, M.Y.; Gillinov, A.M. Accuracy of Apple Watch for Detection of Atrial Fibrillation. Circulation 2020, 141, 702–703. [Google Scholar] [CrossRef]
- Etiwy, M.; Akhrass, Z.; Gillinov, L.; Alashi, A.; Wang, R.; Blackburn, G.; Gillinov, S.; Phelan, D.; Gillinov, A.M.; Houghtaling, P.L.; et al. Accuracy of wearable heart rate monitors in cardiac rehabilitation. Cardiovasc. Diagn. Ther. 2019, 9, 262–271. [Google Scholar] [CrossRef]
- Castelletti, S.; Dagradi, F.; Goulene, K.; Danza, A.I.; Baldi, E.; Stramba-Badiale, M.; Schwartz, P.J. A wearable remote monitoring system for the identification of subjects with a prolonged QT interval or at risk for drug-induced long QT syndrome. Int. J. Cardiol. 2018, 266, 89–94. [Google Scholar] [CrossRef]
- Kario, K.; Shimbo, D.; Tomitani, N.; Kanegae, H.; Schwartz, J.E.; Williams, B. The first study comparing a wearable watch-type blood pressure monitor with a conventional ambulatory blood pressure monitor on in-office and out-of-office settings. J. Clin. Hypertens. 2020, 22, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Nachman, D.; Gepner, Y.; Goldstein, N.; Kabakov, E.; Ishay, A.B.; Littman, R.; Azmon, Y.; Jaffe, E.; Eisenkraft, A. Comparing blood pressure measurements between a photoplethysmography-based and a standard cuff-based manometry device. Sci. Rep. 2020, 10, 16116. [Google Scholar] [CrossRef] [PubMed]
- Stehlik, J.; Schmalfuss, C.; Bozkurt, B.; Nativi-Nicolau, J.; Wohlfahrt, P.; Wegerich, S.; Rose, K.; Ray, R.; Schofield, R.; Deswal, A.; et al. Continuous Wearable Monitoring Analytics Predict Heart Failure Hospitalization: The LINK-HF Multicenter Study. Circ. Heart Fail. 2020, 13, e006513. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Taborsky, M.; Glikson, M.; Heinrich, U.; Schumacher, B.; Katz, A.; Brachmann, J.; Lewalter, T.; Goette, A.; Block, M.; et al. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): A randomised controlled trial. Lancet 2014, 384, 583–590. [Google Scholar] [CrossRef]
- Morgan, J.M.; Kitt, S.; Gill, J.; McComb, J.M.; Ng, G.A.; Raftery, J.; Roderick, P.; Seed, A.; Williams, S.G.; Witte, K.K.; et al. Remote management of heart failure using implantable electronic devices. Eur. Heart J. 2017, 38, 2352–2360. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.-M.; Wang, L.; Chau, E.; Chan, R.H.-W.; Kong, S.-L.; Tang, M.-O.; Christensen, J.; Stadler, R.W.; Lau, C.-P. Intrathoracic impedance monitoring in patients with heart failure: Correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation 2005, 112, 841–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.H.; Tong, W. Measuring impedance in congestive heart failure: Current options and clinical applications. Am. Heart J. 2009, 157, 402–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Veldhuisen, D.J.; Braunschweig, F.; Conraads, V.; Ford, I.; Cowie, M.R.; Jondeau, G.; Kautzner, J.; Aguilera, R.M.; Lunati, M.; Yu, C.M.; et al. Intrathoracic impedance monitoring, audible patient alerts, and outcome in patients with heart failure. Circulation 2011, 124, 1719–1726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udelson, J.E. T.M.I. (Too much information)? Circulation 2011, 124, 1697–1699. [Google Scholar] [CrossRef] [Green Version]
- Vollmann, D.; Nägele, H.; Schauerte, P.; Wiegand, U.; Butter, C.; Zanotto, G.; Quesada, A.; Guthmann, A.; Hill, M.R.; Lamp, B. Clinical utility of intrathoracic impedance monitoring to alert patients with an implanted device of deteriorating chronic heart failure. Eur. Heart J. 2007, 28, 1835–1840. [Google Scholar] [CrossRef]
- Boehmer, J.P.; Hariharan, R.; Devecchi, F.G.; Smith, A.L.; Molon, G.; Capucci, A.; An, Q.; Averina, V.; Stolen, C.M.; Thakur, P.H.; et al. A Multisensor Algorithm Predicts Heart Failure Events in Patients With Implanted Devices: Results From the MultiSENSE Study. JACC Heart Fail. 2017, 5, 216–225. [Google Scholar] [CrossRef]
- Treskes, R.W.; Beles, M.; Caputo, M.; Cordon, A.; Biundo, E.; Maes, E.; Egorova, A.D.; Schalij, M.J.; Van Bockstal, K.; Grazio-li-Gauthier, L.; et al. Clinical and economic impact of HeartLogic™ compared with standard care in heart failure patients. ESC Heart Fail. 2021, 8, 1541–1551. [Google Scholar] [CrossRef] [PubMed]
- Magalski, A.; Adamson, P.; Gadler, F.; Böehm, M.; Steinhaus, D.; Reynolds, D.; Vlach, K.; Linde, C.; Cremers, B.; Sparks, B.; et al. Continuous ambulatory right heart pressure measurements with an implantable hemodynamic monitor: A multicenter, 12-month follow-up study of patients with chronic heart failure. J. Card. Fail. 2002, 8, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Bourge, R.C.; Abraham, W.T.; Adamson, P.B.; Aaron, M.F.; Aranda, J.M., Jr.; Magalski, A.; Zile, M.R.; Smith, A.L.; Smart, F.W.; O’Shaughnessy, M.A.; et al. Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: The COMPASS-HF study. J. Am. Coll. Cardiol. 2008, 51, 1073–1079. [Google Scholar] [CrossRef] [Green Version]
- Pour-Ghaz, I.; Hana, D.; Raja, J.; Ibebuogu, U.N.; Khouzam, R.N. CardioMEMS: Where we are and where can we go? Ann. Transl. Med. 2019, 7, 418. [Google Scholar] [CrossRef]
- Abraham, W.T.; Adamson, P.B.; Bourge, R.C.; Aaron, M.F.; Costanzo, M.R.; Stevenson, L.W.; Strickland, W.; Neelagaru, S.; Raval, N.; Krueger, S.; et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: A randomised controlled trial. Lancet 2011, 377, 658–666. [Google Scholar] [CrossRef]
- Abraham, W.T.; Stevenson, L.W.; Bourge, R.C.; Lindenfeld, J.A.; Bauman, J.G.; Adamson, P.B.; CHAMPION Trial Study Group. Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: Complete follow-up results from the CHAMPION randomised trial. Lancet 2016, 387, 453–461. [Google Scholar] [CrossRef]
- Givertz, M.M.; Stevenson, L.W.; Costanzo, M.R.; Bourge, R.C.; Bauman, J.G.; Ginn, G.; Abraham, W.T. Pulmonary Artery Pressure-Guided Management of Patients With Heart Failure and Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2017, 70, 1875–1886. [Google Scholar] [CrossRef]
- Adamson, P.B.; Abraham, W.T.; Bourge, R.C.; Costanzo, M.R.; Hasan, A.; Yadav, C.; Henderson, J.; Cowart, P.; Stevenson, L.W. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ. Heart Fail. 2014, 7, 935–944. [Google Scholar] [CrossRef] [Green Version]
- Shavelle, D.M.; Desai, A.S.; Abraham, W.T.; Bourge, R.C.; Raval, N.; Rathman, L.D.; Heywood, J.T.; Jermyn, R.A.; Pelzel, J.; Jonsson, O.T.; et al. Lower Rates of Heart Failure and All-Cause Hospitalizations During Pulmonary Artery Pressure-Guided Therapy for Ambulatory Heart Failure: One-Year Outcomes From the CardioMEMS Post-Approval Study. Circ. Heart Fail. 2020, 13, e006863. [Google Scholar] [CrossRef]
- SFDA. PMA Number P100045. 2014. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P100045 (accessed on 10 October 2021).
- Desai, A.S.; Bhimaraj, A.; Bharmi, R.; Jermyn, R.; Bhatt, K.; Shavelle, D.; Redfield, M.M.; Hull, R.; Pelzel, J.; Davis, K.; et al. Ambulatory Hemodynamic Monitoring Reduces Heart Failure Hospitalizations in "Real-World" Clinical Practice. J. Am. Coll. Cardiol. 2017, 69, 2357–2365. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.; Bharmi, R.; Jonsson, O.; Oliveira, G.H.; Artis, A.; Valika, A.; Capodilupo, R.; Adamson, P.B.; Roberts, G.; Dalal, N.; et al. Association of Ambulatory Hemodynamic Monitoring of Heart Failure With Clinical Outcomes in a Concurrent Matched Cohort Analysis. JAMA Cardiol. 2019, 4, 556–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chion-cel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Lindenfeld, J.; Zile, M.R.; Desai, A.S.; Bhatt, K.; Ducharme, A.; Horstmanshof, D.; Krim, S.R.; Maisel, A.; Mehra, M.R.; Paul, S.; et al. Haemodynamic-guided management of heart failure (GUIDE-HF): A randomised controlled trial. Lancet 2021, 398, 991–1001. [Google Scholar] [CrossRef]
- Mullens, W.; Sharif, F.; Dupont, M.; Rothman, A.M.; Wijns, W. Digital health care solution for proactive heart failure management with the Cordella Heart Failure System: Results of the SIRONA first-in-human study. Eur. J. Heart Fail. 2020, 22, 1912–1919. [Google Scholar] [CrossRef] [PubMed]
- Ritzema, J.; Melton, I.C.; Richards, A.M.; Crozier, I.G.; Frampton, C.; Doughty, R.N.; Whiting, J.; Kar, S.; Eigler, N.; Krum, H.; et al. Direct left atrial pressure monitoring in ambulatory heart failure patients: Initial experience with a new permanent implantable device. Circulation 2007, 116, 2952–2959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maurer, M.S.; Adamson, P.B.; Costanzo, M.R.; Eigler, N.; Gilbert, J.; Gold, M.R.; Klapholz, M.; Saxon, L.A.; Singh, J.P.; Troughton, R.; et al. Rationale and Design of the Left Atrial Pressure Monitoring to Optimize Heart Failure Therapy Study (LAPTOP-HF). J. Card. Fail. 2015, 21, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Abraham, W.T.; Adamson, P.B.; Costanzo, M.R.; Eigler, N.; Gold, M.; Klapholz, M.; Maurer, M.; Saxon, L.; Singh, J.; Troughton, R. Hemodynamic Monitoring in Advanced Heart Failure: Results from the LAPTOP-HF Trial. J. Card. Fail. 2016, 22, 940. [Google Scholar] [CrossRef]
- Perl, L.; Soifer, E.; Bartunek, J.; Erdheim, D.; Köhler, F.; Abraham, W.T.; Meerkin, D. A Novel Wireless Left Atrial Pressure Monitoring System for Patients with Heart Failure, First Ex-Vivo and Animal Experience. J. Cardiovasc. Transl. Res. 2019, 12, 290–298. [Google Scholar] [CrossRef]
- D’Amario, D.; Restivo, A.; Canonico, F.; Rodolico, D.; Mattia, G.; Francesco, B.; Vergallo, R.; Trani, C.; Aspromonte, N.; Crea, F. Experience of remote cardiac care during the COVID-19 pandemic: The V-LAP™ device in advanced heart failure. Eur. J. Heart Fail. 2020, 22, 1050–1052. [Google Scholar] [CrossRef] [PubMed]
- Feickert, S.; D’Ancona, G.; Murero, M.; Ince, H. Intra-cardiac microcomputer allows for innovative telemedicine in chronic heartfailure during coronavirus disease-2019 pandemic: A case report. Eur. Heart J. Case Rep. 2020, 4, 1–6. [Google Scholar] [CrossRef]

| Year | Reference | Patient Characteristics | Monitoring Method | Follow Up | Primary Endpoint | Secondary Endpoint |
|---|---|---|---|---|---|---|
| 2012 | WISH [33] | 344 patients hospitalized for ADHF and NFYHA III-IV, LVEF < 50% | Daily weighing using internet connected scale. | 12 months | No difference in cardiac re-hospitalizations (HR 0.90, CI 0.65–1.26, p = 0.54) | No difference in all cause hospitalization, death, or composite of both. |
| 2005 | TEN-HMS [35] | 426 patients with in 6 weeks of ADHF admission and LVEF <40% and on diuretics | Home telemonitoring (automatic BP, electronic scale, ECG), monthly nurse phone call or usual care | 240 days | Days lost for death or hospitalization did not differ (12.7%, 15.9%, 19.5% respectively) | Mortality was higher in usual care group (45 vs. 27% in nurse phone call and 29% in telemonitoring groups) |
| 2016 | BEAT-HF [38] | 1437 patients hospitalized for ADHF | electronic telemonitoring (BP, heart rate, weight, symptoms) + monthly tele-coaching or usual care | 180 days | Similar all cause hospitalization at 180 days- 50.8% vs. 49.2% respectively (HR-1.03; 95% CI, 0.88–1.20; p = 0.74) | no significant differences in 30-day readmission or 180-day mortality. |
| 2016 | IMPEDANCE-HF [44] | 256 patients with ADHF admission in the last year, LVEF < 35%, NYHA II-IV | Monthly lung impedance vs. usual care | 48 ± 32 months | 211 vs. 386 ADHF hospitalizations (p < 0.001) among monitored vs. control | 42 vs. 59 deaths respectively (HR 0.52, 95% CI 0.35–0.78, p = 0.002) |
| 2019 | SMILE [52] (Preliminary results) | 268 patients with current ADHF hospitalization | Remote dielectric sensing vs. usual care | 6.1 ± 3.4 months | 21 vs. 43 readmissions (HR 0.52, 95% CI- 0.31–0.87, p = 0.01) | No mortality benefits. Lower days lost for ADHF (1.37 vs. 2.62, p = 0.006) |
| 2014 | IN-TIME [79] | 664 patients, LVEF < 35%, NYHA II-III, OMT. | CIED based daily monitoring (HR, activity, arrythmia, HR, HR variability, HR at rest, ventricular ectopy) vs. usual care | 12 months | Composite of all-cause death, overnight hospital admission for heart failure, change in NYHA class patient global self-assessment was better in monitored group (18.9% vs. 27.2%, OR 0.63, 95% CI 0.43–0.90, p = 0·013) | Mortality of 10 vs. 27 patients respectively. |
| 2011 | DOT-HF [83] | 335 patients with ADHF admission in the last year, LVEF < 35%, NYHA II-IV | CIED based thoracic impedance monitoring vs. usual care | 14.9 ± 5 months | all-cause mortality and HF hospitalizations was similar (29% vs. 20% (p = 0.063, HR 0.52; 95% CI- 0.97–2.37) | HF hospitalization (HR0 1.79; 95% CI- 1.08–2.95; p = 0.022) and outpatient visits (250 vs. 84, p < 0.0001) were higher in the monitored group |
| 2011 | COMPASS-HF [89] | 274 HF patients, on OMT, NYHA III-IV and ADHF hospitalization in previous 6 months | Implantable RV and ePAD pressure monitor | 6 months | Nonsignificant 21% reduction in HF hospitalizations (p = 0.33) | time to first HF-related hospitalizations was 35% lower (HR-0.64, 95% CI-0.42–0.96, p = 0.03) |
| 2016 | CHAMPION [92] | 550 HF patients with previous ADHF hospitalization and NYHA III | Implantable PA pressure monitor | 18 months (complete follow up) | ADHF admissions were 33% lower (HR- 0.67, 95% CI 0.55–0.80, p < 0.0001) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nachman, D.; Rahamim, E.; Kolben, Y.; Mengesha, B.; Elbaz-Greener, G.; Amir, O.; Asleh, R. In Search of Clinical Impact: Advanced Monitoring Technologies in Daily Heart Failure Care. J. Clin. Med. 2021, 10, 4692. https://doi.org/10.3390/jcm10204692
Nachman D, Rahamim E, Kolben Y, Mengesha B, Elbaz-Greener G, Amir O, Asleh R. In Search of Clinical Impact: Advanced Monitoring Technologies in Daily Heart Failure Care. Journal of Clinical Medicine. 2021; 10(20):4692. https://doi.org/10.3390/jcm10204692
Chicago/Turabian StyleNachman, Dean, Eldad Rahamim, Yotam Kolben, Bethlehem Mengesha, Gabby Elbaz-Greener, Offer Amir, and Rabea Asleh. 2021. "In Search of Clinical Impact: Advanced Monitoring Technologies in Daily Heart Failure Care" Journal of Clinical Medicine 10, no. 20: 4692. https://doi.org/10.3390/jcm10204692
APA StyleNachman, D., Rahamim, E., Kolben, Y., Mengesha, B., Elbaz-Greener, G., Amir, O., & Asleh, R. (2021). In Search of Clinical Impact: Advanced Monitoring Technologies in Daily Heart Failure Care. Journal of Clinical Medicine, 10(20), 4692. https://doi.org/10.3390/jcm10204692






