Association between Severity of Diffuse Idiopathic Skeletal Hyperostosis and Ossification of Other Spinal Ligaments in Patients with Ossification of the Posterior Longitudinal Ligament
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Methods
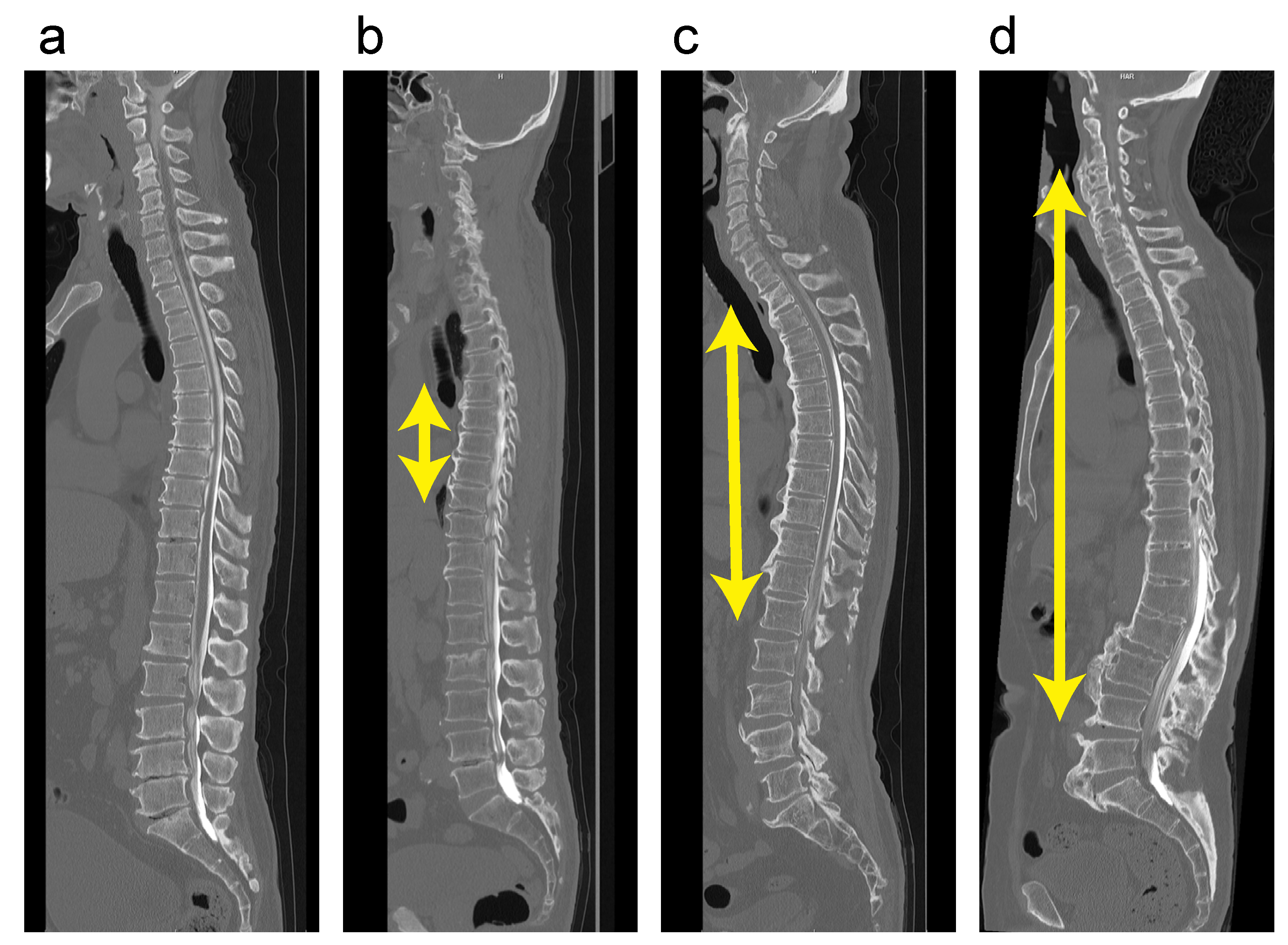
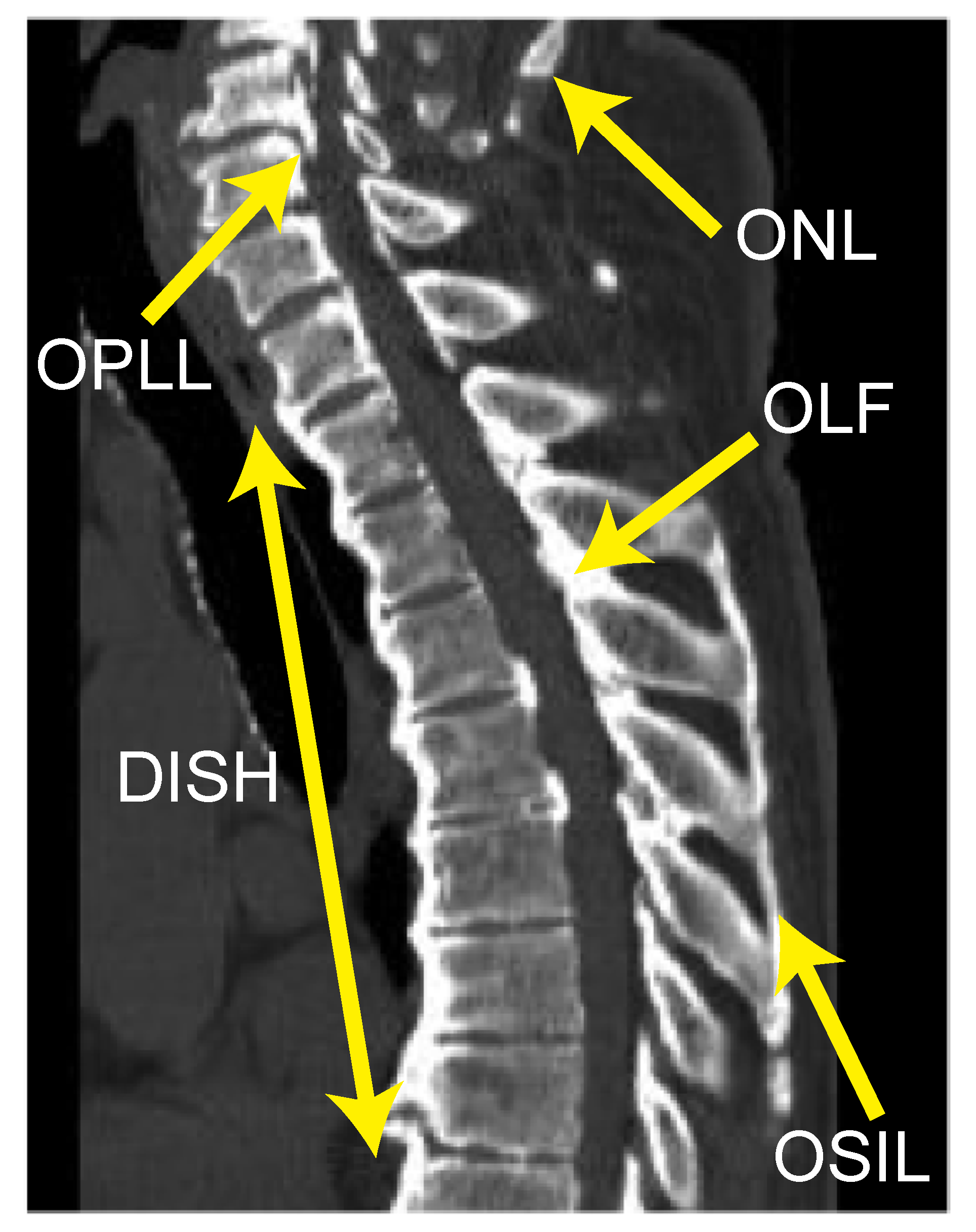
2.2. Radiographic Examinations
2.3. Statistical Analysis
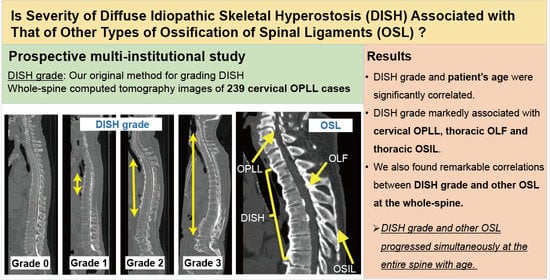
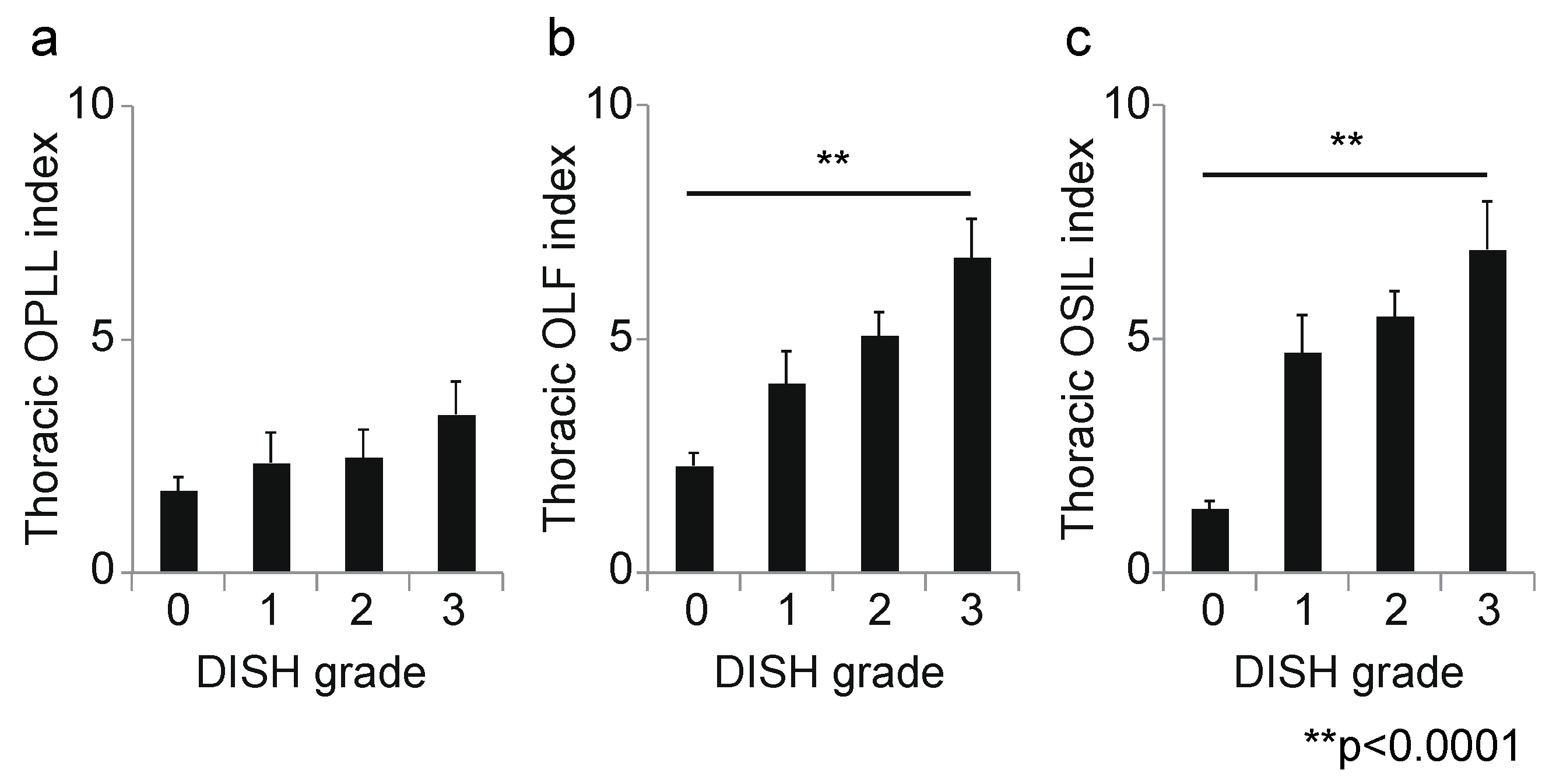
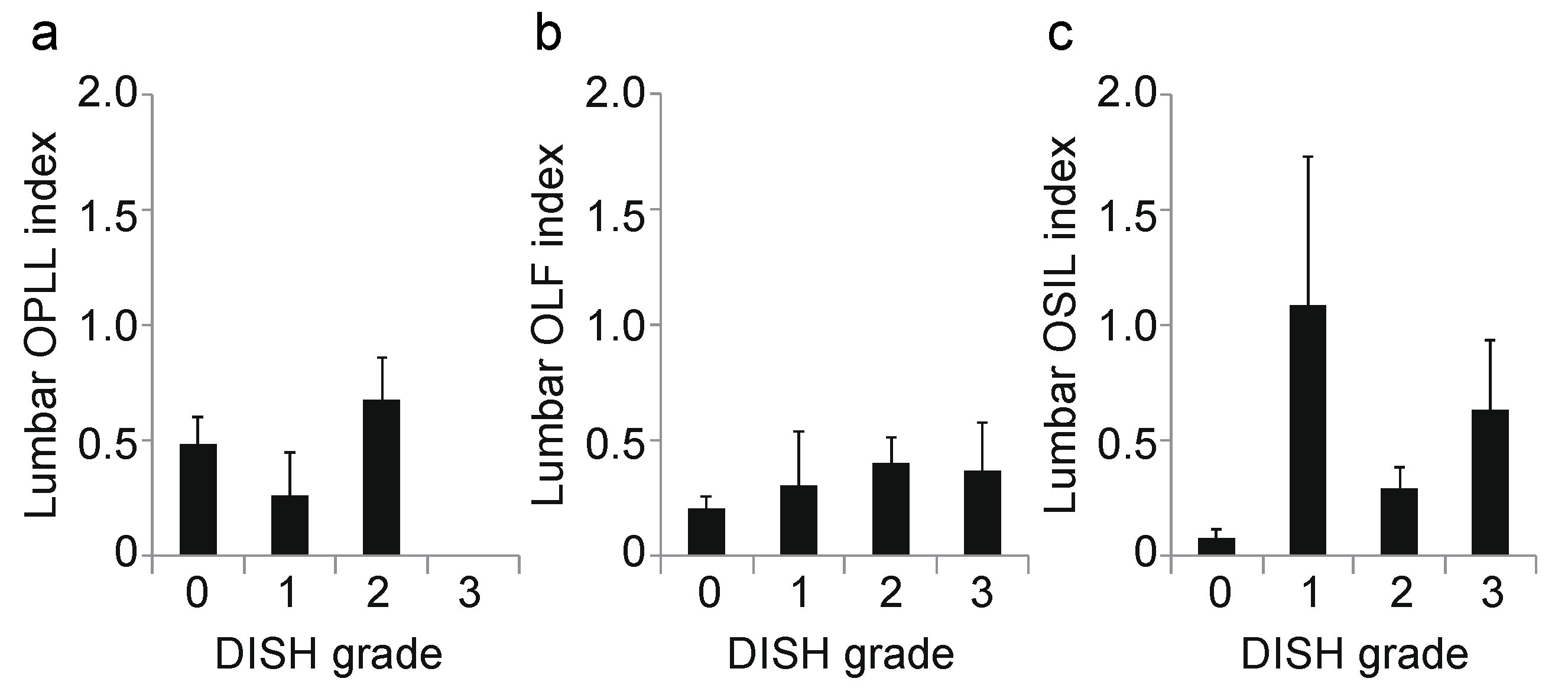
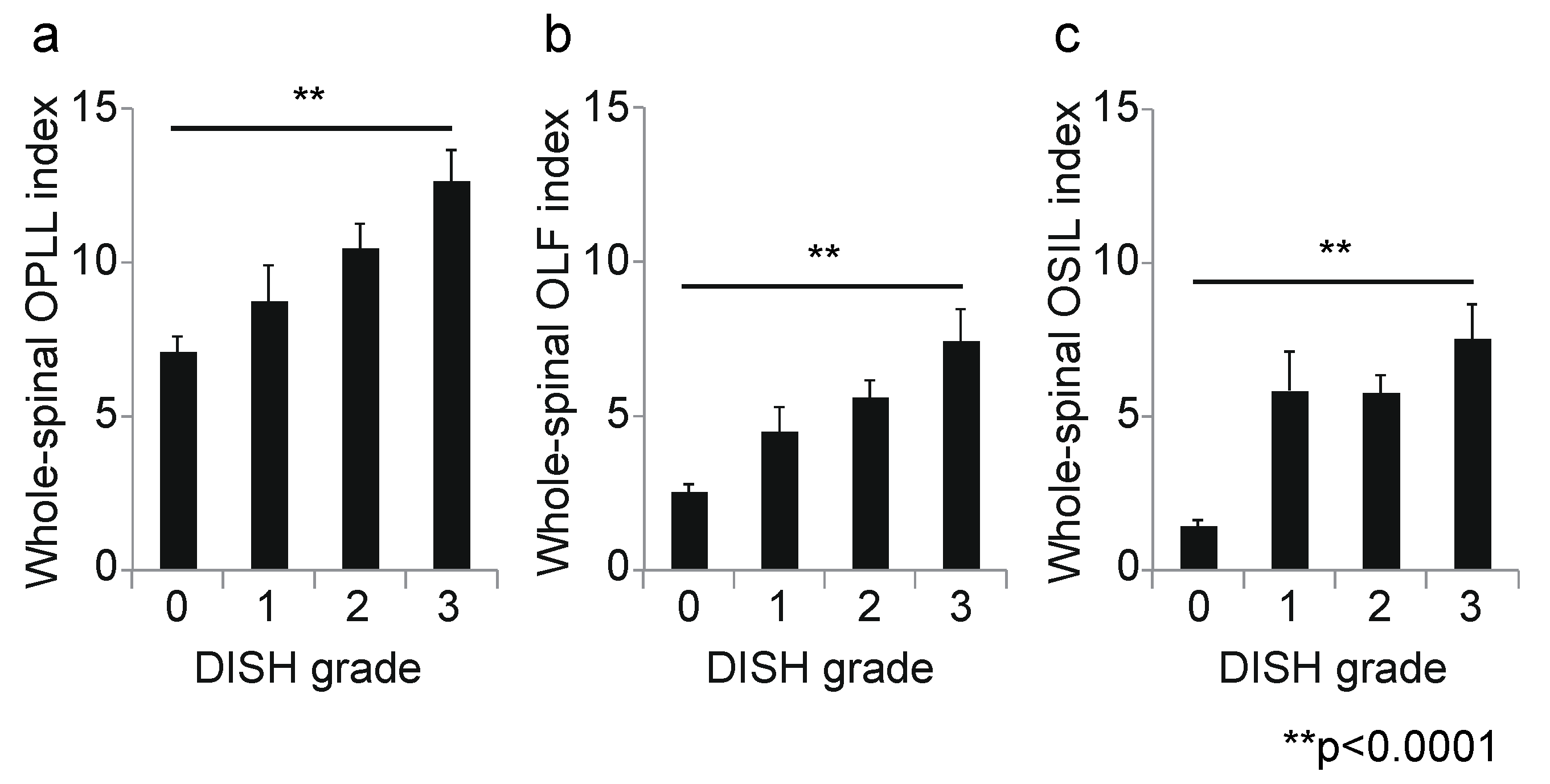
3. Results
3.1. Demographic Data and Surgery-Related Data According to DISH Grade
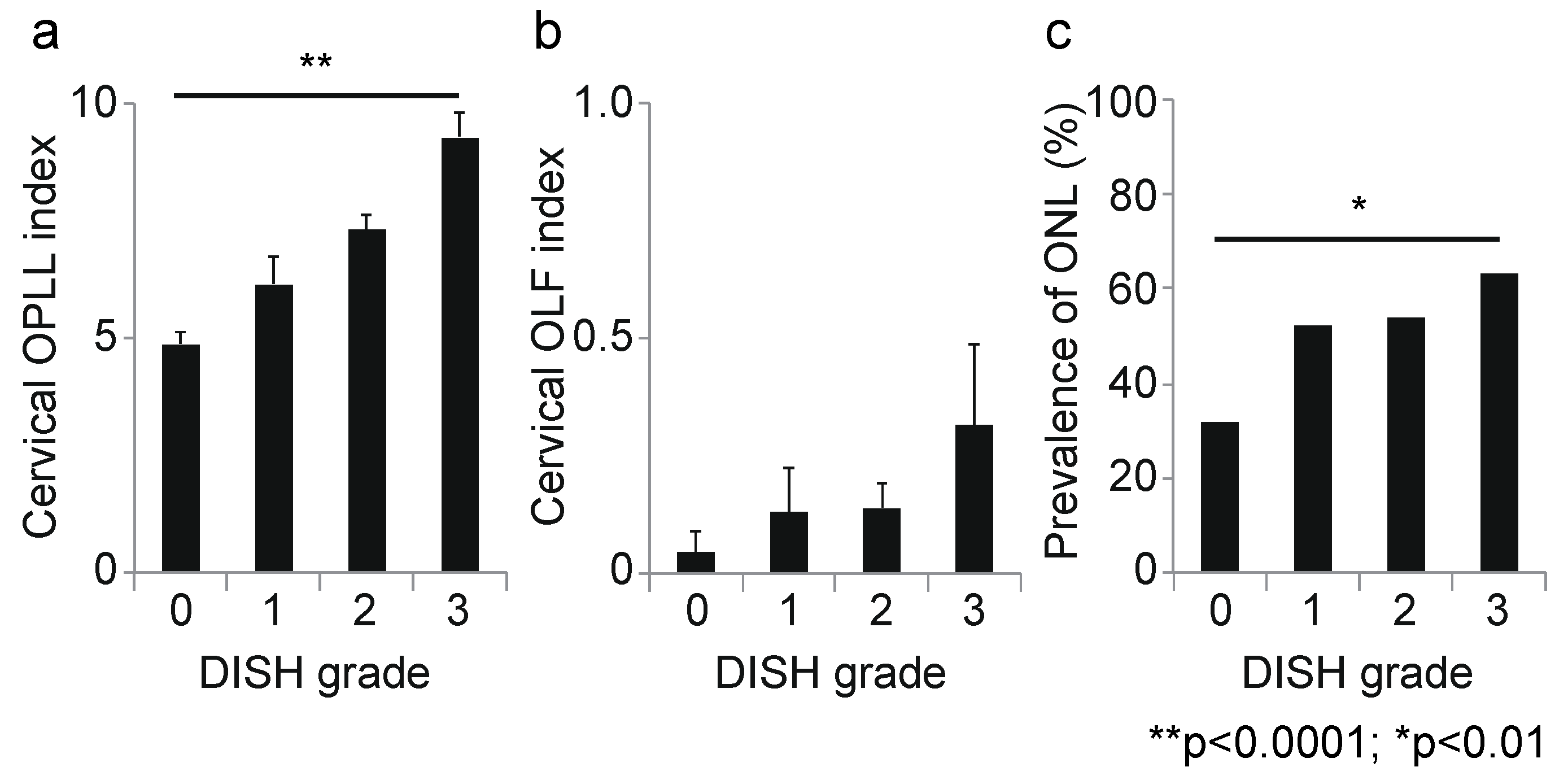
3.2. Association between DISH Grade and OSL
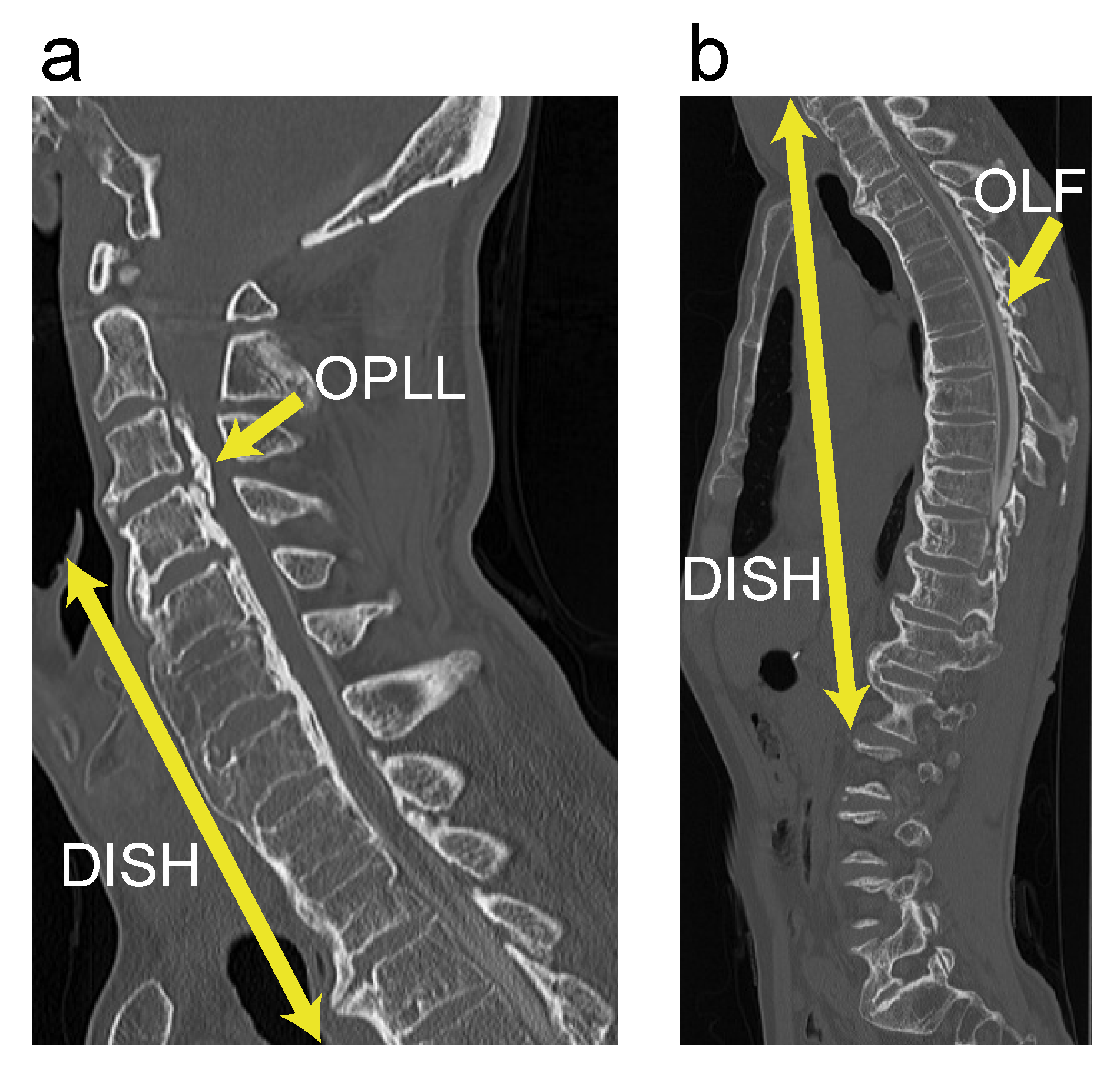
3.3. Case Presentation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Matsunaga, S.; Sakou, T. Ossification of the posterior longitudinal ligament of the cervical spine: Etiology and natural history. Spine 2012, 37, E309–E314. [Google Scholar] [CrossRef]
- Stetler, W.R.; La Marca, F.; Park, P. The genetics of ossification of the posterior longitudinal ligament. Neurosurg. Focus 2011, 30, E7. [Google Scholar] [CrossRef]
- Ehara, S.; Shimamura, T.; Nakamura, R.; Yamazaki, K. Paravertebral ligamentous ossification: DISH, OPLL and OLF. Eur. J. Radiol. 1998, 27, 196–205. [Google Scholar] [CrossRef]
- Epstein, N.E. Simultaneous cervical diffuse idiopathic skeletal hyperostosis and ossification of the posterior longitudinal ligament resulting in dysphagia or myelopathy in two geriatric North Americans. Surg. Neurol. 2000, 53, 427–431, discussion 431. [Google Scholar] [CrossRef]
- Fujimori, T.; Watabe, T.; Iwamoto, Y.; Hamada, S.; Iwasaki, M.; Oda, T. Prevalence, concomitance, and distribution of ossification of the spinal ligaments: Results of whole spine CT scans in 1500 Japanese patients. Spine 2016, 41, 1668–1676. [Google Scholar] [CrossRef]
- Nishimura, S.; Nagoshi, N.; Iwanami, A.; Takeuchi, A.; Hirai, T.; Yoshii, T.; Takeuchi, K.; Mori, K.; Yamada, T.; Seki, S.; et al. Prevalence and distribution of diffuse idiopathic skeletal hyperostosis on whole-spine computed tomography in patients with cervical ossification of the posterior longitudinal ligament: A multicenter study. Clin. Spine Surg. 2018, 31, E460–E465. [Google Scholar] [CrossRef] [PubMed]
- Kagotani, R.; Yoshida, M.; Muraki, S.; Oka, H.; Hashizume, H.; Yamada, H.; Enyo, Y.; Nagata, K.; Ishimoto, Y.; Teraguchi, M.; et al. Prevalence of diffuse idiopathic skeletal hyperostosis (DISH) of the whole spine and its association with lumbar spondylosis and knee osteoarthritis: The ROAD study. J. Bone Miner. Metab. 2015, 33, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Kasahara, T.; Mimura, T.; Nishizawa, K.; Nakamura, A.; Imai, S. Prevalence of thoracic diffuse idiopathic skeletal hyperostosis (DISH) in Japanese: Results of chest CT-based cross-sectional study. J. Orthop. Sci. 2017, 22, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, H.; Terai, H.; Yamada, K.; Suzuki, A.; Dohzono, S.; Matsumoto, T.; Nakamura, H. Prevalence of diffuse idiopathic skeletal hyperostosis in patients with spinal disorders. Asian Spine J. 2017, 11, 63–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, H.; Liu, G.; Lu, S.; Chen, S.; Jiang, D.; Shi, H.; Fei, Q. Epidemiology of ossification of the spinal ligaments and associated factors in the Chinese population: A cross-sectional study of 2000 consecutive individuals. BMC Musculoskelet. Disord. 2019, 20, 253. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, B.R.; Fehlings, M.G. Ankylosing spinal disorders--falls, flawed flexibility, and fixations. World Neurosurg. 2015, 83, 724–726. [Google Scholar] [CrossRef]
- Okada, E.; Yoshii, T.; Yamada, T.; Watanabe, K.; Katsumi, K.; Hiyama, A.; Watanabe, M.; Nakagawa, Y.; Okada, M.; Endo, T.; et al. Spinal fractures in patients with Diffuse idiopathic skeletal hyperostosis: A nationwide multi-institution survey. J. Orthop. Sci. 2019, 24, 601–606. [Google Scholar] [CrossRef]
- Westerveld, L.A.; Verlaan, J.J.; Oner, F.C. Spinal fractures in patients with ankylosing spinal disorders: A systematic review of the literature on treatment, neurological status and complications. Eur. Spine J. 2009, 18, 145–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Q.; Ni, B.; Yang, J.; Zhu, Z.; Yang, J. Simultaneous ossification of the posterior longitudinal ligament and ossification of the ligamentum flavum causing upper thoracic myelopathy in DISH: Case report and literature review. Eur. Spine J. 2011, 20, S195–S201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloom, R.A. The prevalence of ankylosing hyperostosis in a Jerusalem population--with description of a method of grading the extent of the disease. Scand. J. Rheumatol. 1984, 13, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Fornasier, V.L.; Littlejohn, G.; Urowitz, M.B.; Keystone, E.C.; Smythe, H.A. Spinal entheseal new bone formation: The early changes of spinal diffuse idiopathic skeletal hyperostosis. J. Rheumatol. 1983, 10, 939–947. [Google Scholar]
- Haller, J.; Resnick, D.; Miller, C.W.; Schils, J.P.; Kerr, R.; Bielecki, D.; Sartoris, D.J.; Gundry, C.R. Diffuse idiopathic skeletal hyperostosis: Diagnostic significance of radiographic abnormalities of the pelvis. Radiology 1989, 172, 835–839. [Google Scholar] [CrossRef]
- Hirai, T.; Yoshii, T.; Iwanami, A.; Takeuchi, K.; Mori, K.; Yamada, T.; Wada, K.; Koda, M.; Matsuyama, Y.; Takeshita, K.; et al. Prevalence and distribution of ossified lesions in the whole spine of patients with cervical ossification of the posterior longitudinal ligament a multicenter study (JOSL CT study). PLoS ONE 2016, 11, e0160117. [Google Scholar] [CrossRef] [Green Version]
- Mori, K.; Yoshii, T.; Hirai, T.; Iwanami, A.; Takeuchi, K.; Yamada, T.; Seki, S.; Tsuji, T.; Fujiyoshi, K.; Furukawa, M.; et al. Prevalence and distribution of ossification of the supra/interspinous ligaments in symptomatic patients with cervical ossification of the posterior longitudinal ligament of the spine: A CT-based multicenter cross-sectional study. BMC Musculoskelet. Disord. 2016, 17, 492. [Google Scholar] [CrossRef]
- Yoshii, T.; Hirai, T.; Iwanami, A.; Nagoshi, N.; Takeuchi, K.; Mori, K.; Yamada, T.; Seki, S.; Tsuji, T.; Fujiyoshi, K.; et al. Co-existence of ossification of the nuchal ligament is associated with severity of ossification in the whole spine in patients with cervical ossification of the posterior longitudinal ligament—A multi-center CT study. J. Orthop. Sci. 2019, 24, 35–41. [Google Scholar] [CrossRef]
- Resnick, D.; Niwayama, G. Radiographic and pathologic features of spinal involvement in diffuse idiopathic skeletal hyperostosis (DISH). Radiology 1976, 119, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.; Nakano, M.; Yasuda, T.; Seki, S.; Hori, T.; Suzuki, K.; Makino, H.; Kimura, T. Characteristics of ossification of the spinal ligament; incidence of ossification of the ligamentum flavum in patients with cervical ossification of the posterior longitudinal ligament—Analysis of the whole spine using multidetector CT. J. Orthop. Sci. 2016, 21, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Kasahara, T.; Mimura, T.; Nishizawa, K.; Murakami, Y.; Matsusue, Y.; Imai, S. Prevalence, distribution, and morphology of thoracic ossification of the yellow ligament in Japanese: Results of CT-based cross-sectional study. Spine 2013, 38, E1216–E1222. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, T.; Morishita, S.; Inose, H.; Yuasa, M.; Hirai, T.; Okawa, A.; Fushimi, K.; Fujiwara, T. Comparison of perioperative complications in anterior decompression with fusion and posterior decompression with fusion for cervical ossification of the posterior longitudinal ligament: Propensity score matching analysis using a nation-wide inpatient database. Spine 2020, 45, E1006–E1012. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, N.; Nagata, K.; Muraki, S.; Oka, H.; Yoshida, M.; Enyo, Y.; Kagotani, R.; Hashizume, H.; Yamada, H.; Ishimoto, Y.; et al. Prevalence and progression of radiographic ossification of the posterior longitudinal ligament and associated factors in the Japanese population: A 3-year follow-up of the ROAD study. Osteoporos. Int. 2014, 25, 1089–1098. [Google Scholar] [CrossRef]
- Latourte, A.; Charlon, S.; Etcheto, A.; Feydy, A.; Allanore, Y.; Dougados, M.; Molto, A. Imaging findings suggestive of axial spondyloarthritis in diffuse idiopathic skeletal hyperostosis. Arthritis Care Res. 2018, 70, 145–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mader, R.; Baraliakos, X.; Eshed, I.; Novofastovski, I.; Bieber, A.; Verlaan, J.-J.; Kiefer, D.; Pappone, N.; Atzeni, F. Imaging of diffuse idiopathic skeletal hyperostosis (DISH). RMD Open 2020, 6, e001151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, K.; Yayama, T.; Nishizawa, K.; Nakamura, A.; Mimura, T.; Imai, S. Aortic pulsation prevents the development of ossification of anterior longitudinal ligament toward the aorta in patients with diffuse idiopathic skeletal hyperostosis (DISH) in Japanese: Results of chest CT-based cross-sectional study. J. Orthop. Sci. 2019, 24, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, I.; D’Angelo, S.; Palazzi, C.; Padula, A.; Mader, R.; Khan, M.A. Diffuse idiopathic skeletal hyperostosis: Differentiation from ankylosing spondylitis. Curr. Rheumatol. Rep. 2009, 11, 321–328. [Google Scholar] [CrossRef]
- Kuperus, J.S.; Waalwijk, J.F.; Regan, E.A.; van der Horst-Bruinsma, I.E.; Oner, F.C.; de Jong, P.A.; Verlaan, J.-J. Simultaneous occurrence of ankylosing spondylitis and diffuse idiopathic skeletal hyperostosis: A systematic review. Rheumatology 2018, 57, 2120–2128. [Google Scholar] [CrossRef]
- Kobashi, G.; Washio, M.; Okamoto, K.; Sasaki, S.; Yokoyama, T.; Miyake, Y.; Sakamoto, N.; Ohta, K.; Inaba, Y.; Tanaka, H. High body mass index after age 20 and diabetes mellitus are independent risk factors for ossification of the posterior longitudinal ligament of the spine in Japanese subjects: A case-control study in multiple hospitals. Spine 2004, 29, 1006–1010. [Google Scholar] [CrossRef]
- Akune, T.; Ogata, N.; Seichi, A.; Ohnishi, I.; Nakamura, K.; Kawaguchi, H. Insulin secretory response is positively associated with the extent of ossification of the posterior longitudinal ligament of the spine. J. Bone Jt. Surg. Am. 2001, 83, 1537–1544. [Google Scholar] [CrossRef]
- Nagoshi, N.; Watanabe, K.; Nakamura, M.; Matsumoto, M.; Li, N.; Ma, S.; He, D.; Tian, W.; Jeon, H.; Lee, J.J.; et al. Does diabetes affect the surgical outcomes in cases with cervical ossification of the posterior longitudinal ligament? A multicenter study from Asia pacific spine study group. Glob. Spine J. 2021, 10, 2192568221996300. [Google Scholar] [CrossRef]
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | p-Value | |
|---|---|---|---|---|---|
| Patients, n | 132 | 23 | 65 | 19 | |
| Mean age (years) | 60.9 ± 11.6 | 65.4 ± 12.7 | 66.9 ± 12.3 | 72.8 ± 9.9 | <0.0001 |
| Male sex (%) | 61.4 | 78.3 | 75.4 | 78.9 | 0.09 |
| Body mass index | 26.1 ± 4.7 | 25.7 ± 5.0 | 25.9 ± 3.7 | 24.7 ± 4.9 | 0.32 |
| DM (%) | 21.2 | 30.4 | 32.3 | 15.8 | 0.25 |
| FH of OPLL (%) | 3.03 | 4.35 | 3.08 | 5.26 | 0.95 |
| Trauma history (%) | 6.82 | 0.00 | 7.69 | 10.5 | 0.53 |
| Cervical level | |||||
| Patients treated surgically, n (%) | 74 (56.1) | 8 (34.5) | 37 (56.9) | 10 (52.6) | 0.27 |
| Surgical Method | |||||
| Laminoplasty (%) | 40.5 | 62.5 | 51.4 | 60.0 | 0.40 |
| Laminectomy (%) | 1.35 | 0.00 | 0.00 | 0.00 | 0.86 |
| ADF (%) | 29.7 | 25.0 | 13.5 | 0.00 | 0.08 |
| PDF (%) | 27.0 | 12.5 | 32.4 | 30.0 | 0.71 |
| APF (%) | 1.35 | 0.00 | 2.70 | 10.0 | 0.37 |
| Perioperative complication (%) | 14.9 | 0.00 | 32.4 | 0.20 | 0.07 |
| Neurological deterioration (%) | 1.35 | 0.00 | 2.70 | 0.00 | 0.89 |
| C5 palsy (%) | 8.11 | 0.00 | 13.5 | 0.10 | 0.63 |
| CSF leakage (%) | 1.35 | 0.00 | 2.70 | 0.00 | 0.89 |
| Surgical site infection (%) | 0.00 | 0.00 | 5.41 | 0.00 | 0.17 |
| Screw loosening (%) | 1.35 | 0.00 | 0.00 | 0.00 | 0.86 |
| Screw malposition (%) | 1.35 | 0.00 | 0.00 | 0.00 | 0.86 |
| Dysphasia (%) | 0.00 | 0.00 | 2.70 | 0.10 | 0.10 |
| Deep vein thrombosis (%) | 0.00 | 0.00 | 2.70 | 0.00 | 0.47 |
| Heart failure (%) | 1.35 | 0.00 | 0.00 | 0.00 | 0.86 |
| Delirium (%) | 0.00 | 0.00 | 2.70 | 0.00 | 0.47 |
| Thoracic level | |||||
| Patients treated surgically, n (%) | 11 (8.33) | 4 (17.4) | 9 (13.8) | 2 (10.5) | 0.48 |
| Surgical Method | |||||
| Laminectomy (%) | 36.4 | 0.00 | 22.2 | 50.0 | 0.46 |
| PDF (%) | 54.5 | 75.0 | 66.7 | 50.0 | 0.86 |
| PF (%) | 9.09 | 25.0 | 11.1 | 0.00 | 0.79 |
| Perioperative complication (%) | 36.4 | 0.00 | 22.2 | 0.00 | 0.41 |
| Neurological deterioration (%) | 27.3 | 0.00 | 0.00 | 0.00 | 0.20 |
| Surgical site infection (%) | 9.09 | 0.00 | 11.1 | 0.00 | 0.88 |
| Wound dehiscence (%) | 0.00 | 0.00 | 11.1 | 0.00 | 0.58 |
| Lumbar level | |||||
| Patients treated surgically, n (%) | 6 (4.55) | 2 (8.70) | 3 (4.62) | 0 (0.00) | 0.62 |
| Surgical Method | |||||
| Laminectomy (%) | 33.3 | 50.0 | 66.7 | 0.00 | 0.63 |
| PDF (%) | 66.7 | 0.00 | 33.3 | 0.00 | 0.23 |
| PF (%) | 0.00 | 50.0 | 0.00 | 0.00 | 0.08 |
| Perioperative complication (%) | 0.00 | 0.00 | 0.00 | 0.00 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishimura, S.; Hirai, T.; Nagoshi, N.; Yoshii, T.; Hashimoto, J.; Mori, K.; Maki, S.; Katsumi, K.; Takeuchi, K.; Ushio, S.; et al. Association between Severity of Diffuse Idiopathic Skeletal Hyperostosis and Ossification of Other Spinal Ligaments in Patients with Ossification of the Posterior Longitudinal Ligament. J. Clin. Med. 2021, 10, 4690. https://doi.org/10.3390/jcm10204690
Nishimura S, Hirai T, Nagoshi N, Yoshii T, Hashimoto J, Mori K, Maki S, Katsumi K, Takeuchi K, Ushio S, et al. Association between Severity of Diffuse Idiopathic Skeletal Hyperostosis and Ossification of Other Spinal Ligaments in Patients with Ossification of the Posterior Longitudinal Ligament. Journal of Clinical Medicine. 2021; 10(20):4690. https://doi.org/10.3390/jcm10204690
Chicago/Turabian StyleNishimura, Soraya, Takashi Hirai, Narihito Nagoshi, Toshitaka Yoshii, Jun Hashimoto, Kanji Mori, Satoshi Maki, Keiichi Katsumi, Kazuhiro Takeuchi, Shuta Ushio, and et al. 2021. "Association between Severity of Diffuse Idiopathic Skeletal Hyperostosis and Ossification of Other Spinal Ligaments in Patients with Ossification of the Posterior Longitudinal Ligament" Journal of Clinical Medicine 10, no. 20: 4690. https://doi.org/10.3390/jcm10204690
APA StyleNishimura, S., Hirai, T., Nagoshi, N., Yoshii, T., Hashimoto, J., Mori, K., Maki, S., Katsumi, K., Takeuchi, K., Ushio, S., Furuya, T., Watanabe, K., Nishida, N., Kaito, T., Kato, S., Nagashima, K., Koda, M., Nakashima, H., Imagama, S., ... Kawaguchi, Y., on behalf of the Japanese Organization of the Study for Ossification of Spinal Ligament (JOSL). (2021). Association between Severity of Diffuse Idiopathic Skeletal Hyperostosis and Ossification of Other Spinal Ligaments in Patients with Ossification of the Posterior Longitudinal Ligament. Journal of Clinical Medicine, 10(20), 4690. https://doi.org/10.3390/jcm10204690