Adequacy of Anesthesia and Pupillometry for Endoscopic Sinus Surgery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Anesthesia Technique
2.2.1. Stage 1
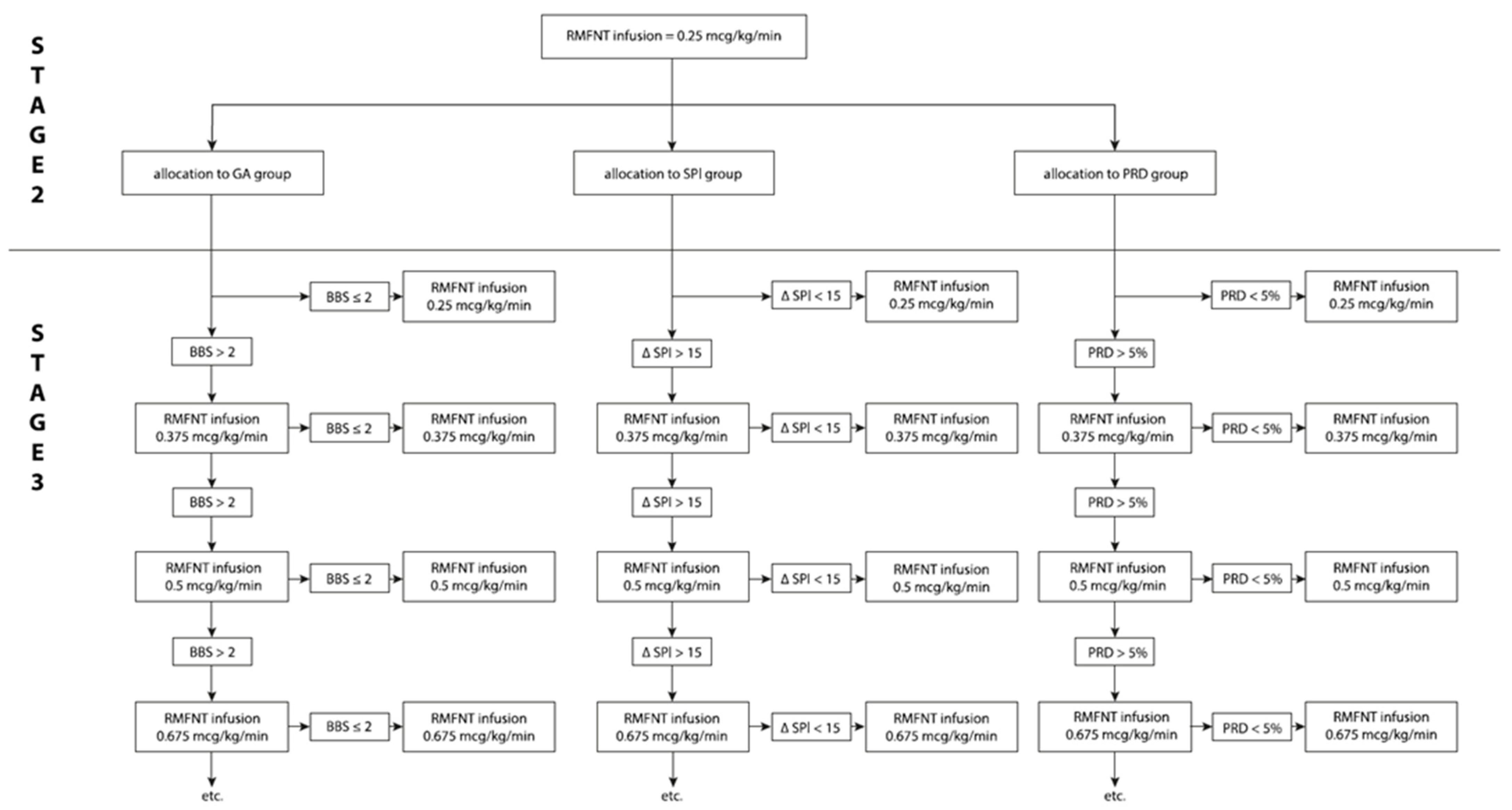
2.2.2. Stage 2
2.2.3. Stage 3 Intraoperatively
2.2.4. SPI Group
2.2.5. PRD Group
2.2.6. GA Group
2.3. Technique of FESS and Surgical Considerations
2.4. Statistical Analysis
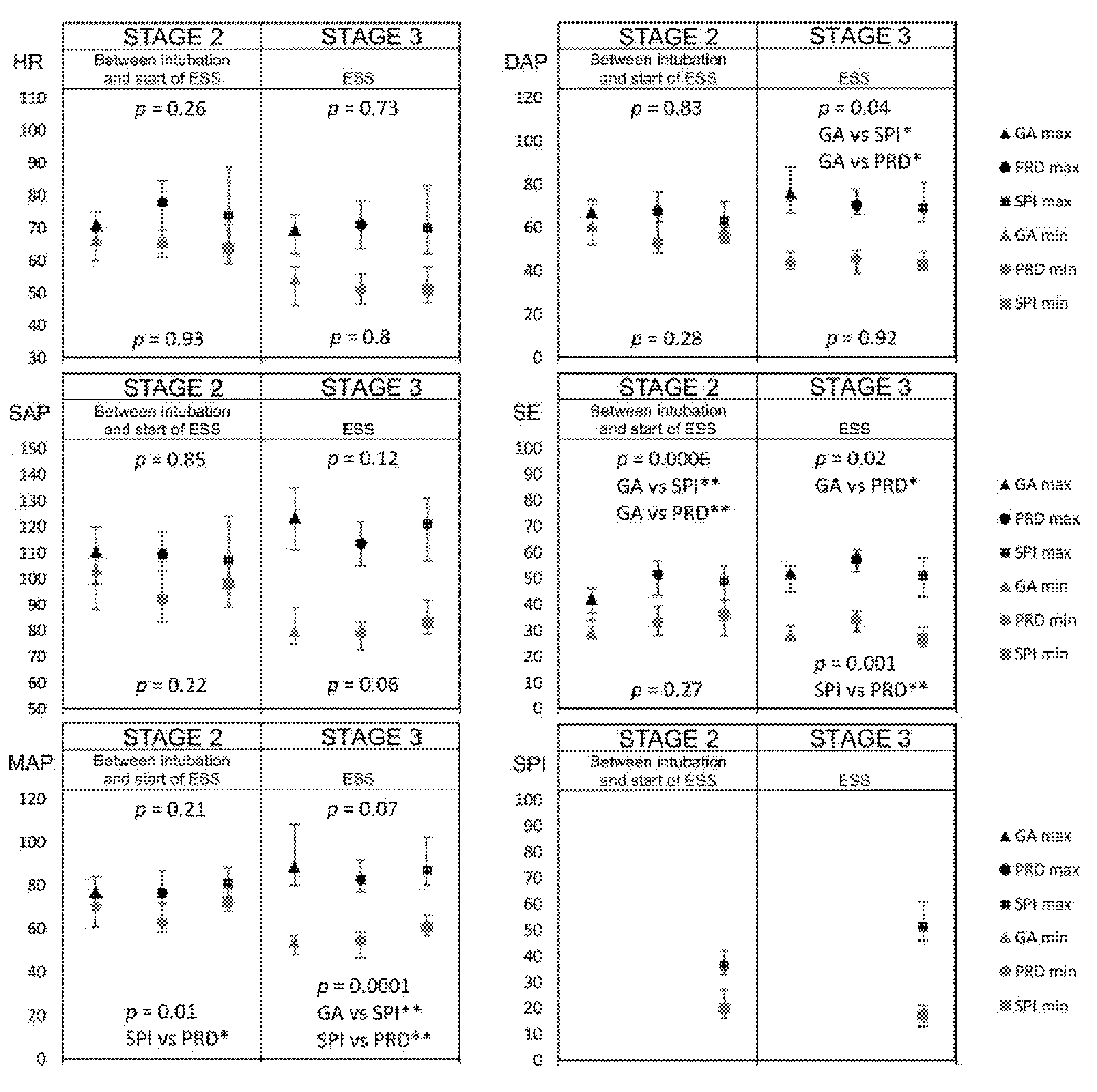
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeican, I.I.; Trombitas, V.; Crivii, C.; Dumitru, M.; Aluaș, M.; Dogaru, G.; Albu, S. Rehabilitation of patients with chronic rhinosinusitis after functional endoscopic sinus surgery. Balneo PRM Res. J. 2021, 12, 65–72. [Google Scholar] [CrossRef]
- Wawrzyniak, K.; Kusza, K.; Cywinski, J.B.; Burduk, P.K.; Kazmierczak, W. Premedication with clonidine before TIVA optimizes surgical field visualization and shortens duration of endoscopic sinus surgery—Results of a clinical trial. Rhinology 2013, 51, 259–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeMaria, S.; Govindaraj, S.; Huang, A.; Hyman, J.; McCormick, P.M.; Hung, M.; Levine, A. The influence of positive end-expiratory pressure on surgical field conditions during functional endoscopic sinus surgery. Anesth. Analg. 2015, 120, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Bafna, U.; Sharma, P.; Singhal, R.K.; Gurjar, S.S.; Bhargava, S.K. Comparison of hypotensive properties of dexmedetomidine versus clonidine for induced hypotension during functional endoscopic sinus surgery: A randomised, double-blind interventional study. Indian J Anaesth. 2021, 65, 579. [Google Scholar]
- Eberhart, L.H.; Folz, B.J.; Wulf, H.; Geldner, G. Intravenous anesthesia provides optimal surgical conditions during microscopic and endoscopic sinus surgery. Laryngoscope 2003, 113, 1369–1373. [Google Scholar] [CrossRef]
- Lund, V.J.; Mackay, I.S. Staging in rhinosinusitis. Rhinology 1993, 31, 183–184. [Google Scholar] [CrossRef]
- Misiołek, H.; Cettler, M.; Woroń, J.; Wordliczek, J.; Dobrogowski, J.; Mayzner-Zawadzka, E. The 2014 guidelines for post-operative pain management. Anaesthesiol. Intensive Ther. 2014, 46, 221–244. [Google Scholar] [CrossRef]
- May, M.; Levine, H.L.; Mester, S.J.; Schaitkin, B. Complications of endoscopic sinus surgery: Analysis of 2108 patients—Incidence and prevention. Laryngoscope 1994, 104, 1080–1083. [Google Scholar] [CrossRef]
- Gilbey, P.; Kukuev, Y.; Samet, A.; Talmon, Y.; Ivry, S. The quality of the surgical field during functional endoscopic sinus surgery—The effect of the mode of ventilation—A randomized, prospective, double-blind study. Laryngoscope 2009, 119, 2449–2453. [Google Scholar] [CrossRef]
- Maniglia, A.J. Fatal and other major complications of endoscopic sinus surgery. Laryngoscope 1991, 101, 349–354. [Google Scholar] [CrossRef]
- Stankiewicz, J.A. Complications in endoscopic intranasal ethmoidectomy: An update. Laryngoscope 1989, 99, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Stankiewicz, J.A.; Lal, D.; Connor, M.; Welch, K. Complications in endoscopic sinus surgery for chronic rhino sinusitis: A 25-year experience. Laryngoscope 2011, 121, 2684–2701. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.; Crosby, G.J.; Bhattacharyya, N. Airway protection and the laryngeal mask airway in sinus and nasal surgery. Laryngoscope 2004, 114, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Kinsella, J.B.; Calhoun, K.H.; Bradfield, J.J.; Hokanson, J.A.; Bailey, B.J. Complications of endoscopic sinus surgery in a residency training program. Laryngoscope 1995, 105, 376–379. [Google Scholar] [CrossRef]
- Yang, J.J.; Wang, Q.P.; Wang, T.Y.; Sun, J.; Wang, Z.Y.; Zuo, D.; Xu, J.G. Marked hypotension induced by adrenaline contained in local anesthetic. Laryngoscope 2005, 115, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Collins, M.; Hung, P.; Rees, G.; Close, D.; Wormald, P.J. The effect of β-Blocker premedication on the surgical field during endoscopic sinus surgery. Laryngoscope 2004, 114, 1042–1046. [Google Scholar] [CrossRef]
- Degoute, C.S. Controlled hypotension: A guide to drug choice. Drugs 2007, 67, 1053–1076. [Google Scholar] [CrossRef]
- Higgins, T.S.; Hwang, P.H.; Kingdom, T.T.; Orlandi, R.R.; Stammberger, H.; Han, J.K. Systematic review of topical vasoconstrictors in endoscopic sinus surgery. Laryngoscope 2011, 121, 422–432. [Google Scholar] [CrossRef]
- Drozdowski, A.; Sieśkiewicz, A.; Siemiatkowski, A. Reduction of intraoperative bleeding during functional endoscopic sinus surgery. Anestezjol. Intens. Ter. 2011, 43, 45–50. [Google Scholar]
- Ko, M.T.; Chuang, K.C.; Su, C.Y. Multiple analyses of factors related to intraoperative blood loss and the role of reverse Trendelenburg position in endoscopic sinus surgery. Laryngoscope 2018, 118, 1687–1691. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.; Park, C.; Jeon, Y.; Kim, D.; Joo, J.; Kang, H. Comparison between dexmedetomidine and remifentanil for controlled hypotension and recovery in endoscopic sinus surgery. Ann. Otol. Rhinol. Laryngol. 2013, 122, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Manola, M.; De Luca, E.; Moscillo, L.; Mastella, A. Using remifentanil and sufentanil in functional endoscopic sinus surgery to improve surgical conditions. ORL J. Otorhinolaryngol. Relat. Spec. 2005, 67, 83–86. [Google Scholar] [CrossRef]
- Gruenewald, M.; Meybohm, P.; Ilies, C.; Hocker, J.; Hanss, R.; Scholz, J.; Bein, B. Influence of different remifentanil concentrations on the performance of the surgical stress index to detect a standardized painful stimulus during sevoflurane anaesthesia. Br. J. Anaesth. 2009, 103, 586–593. [Google Scholar] [CrossRef] [Green Version]
- Boonmak, P.; Boonmak, S.; Laopaiboon, M. Deliberate hypotension with propofol under anaesthesia for functional endoscopic sinus surgery (FESS). Cochrane Database Syst. Rev. 2016, 10, CD006623. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, I.; Göhner, A.; Crozier, T.A.; Hesjedal, B.; Wiese, C.H.; Popov, A.F.; Bauer, M.; Hinz, J.M. Surgical pleth index-guided remifentanil administration reduces remifentanil and propofol consumption and shortens recovery times in outpatient anaesthesia. Br. J. Anaesth. 2013, 110, 622–628. [Google Scholar] [CrossRef] [Green Version]
- Miłoński, J.; Urbaniak, J.; Zielińska-Bliźniewska, H.; Olszewski, J. Analiza średniego ciśnienia tętniczego krwi i częstości akcji serca u chorych operowanych z powodów laryngologicznych w okresie przedoperacyjnym oraz śródoperacyjnym i pooperacyjnym. Otolaryngol. Pol. 2011, 65, 357–362. [Google Scholar] [CrossRef]
- Ilies, C.; Gruenewald, M.; Ludwigs, J.; Thee, C.; Hocker, J.; Hanss, R.; Steinfath, M.; Bein, B. Evaluation of the surgical stress index during spinal and general anaesthesia. Br. J. Anaesth. 2010, 105, 533–537. [Google Scholar] [CrossRef] [Green Version]
- Larson, M.D.; Behrends, M. Portable infrared pupillometry: A review. Anesth. Analg. 2015, 120, 1242–1253. [Google Scholar] [CrossRef]
- Shen, P.-H.; Weitzel, E.K.; Lai, J.-T.; Wormald, P.-J.; Ho, C.-S. Intravenous esmolol infusion improves surgical fields during sevoflurane-anesthetized endoscopic sinus surgery: A double-blind, randomized, placebo-controlled trial. Am. J. Rhinol. Allergy 2011, 25, e208–e211. [Google Scholar] [CrossRef]
- Torrent, A.A.; Bustamante, V.R.; Fons, N.C.; Tutusaus, F.J.R.; Vargas, D.B.; Garcia, C.G. The use of pupillometry as monitoring of intraoperative analgesia in the consumption of analgesics during the first 12 h after surgery. Rev. Esp. Anestesiol. Reanim. 2015, 63, 253–260. [Google Scholar]
- Karkanevatos, A.; Lancaster, J.L.; Osman, I.; Swift, A.C. Pupil size and reaction during functional endoscopic sinus surgery (FESS). Clin. Otolaryngol. Allied Sci. 2003, 28, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Sabourdin, N.; Barrois, J.; Louvet, N.; Rigouzzo, A.; Guye, M.-L.; Dadure, C.; Constant, I. Pupillometry-guided intraoperative remifentanil administration vs. standard practice influences opioid use: A randomized study. Anesthesiology 2017, 127, 284–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bharti, N.; Chari, P.; Kumar, P. Effect of sevoflurane versus propofol-based anesthesia on the hemodynamic response and recovery characteristics in patients undergoing microlaryngeal surgery. Saudi J. Anaesth. 2012, 6, 380–384. [Google Scholar] [CrossRef]
- Cardesín, A.; Pontes, C.; Rosell, R.; Escamilla, Y.; Marco, J.; Escobar, M.J.; Bernal-Sprekelsen, M. Hypotensive anaesthesia and bleeding during endoscopic sinus surgery: An observational study. Eur. Arch. Otorhinolaryngol. 2014, 271, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Boezaart, A.P.; van der Merwe, J.; Coetzee, A. Comparison of sodium nitroprusside- and esmolol-induced controlled hypotension for functional endoscopic sinus surgery. Can. J. Anesth. 1995, 42, 373–376. [Google Scholar] [CrossRef] [Green Version]
- Lindop, M.J. Complications and morbidity of controlled hypotension. Br. J. Anaesth. 1975, 47, 799–803. [Google Scholar] [CrossRef]
- Gruenewald, M.; Ilies, C. Monitoring the nociception-anti-nociception balance. Best Pr. Res. Clin. Anaesthesiol. 2013, 27, 235–247. [Google Scholar] [CrossRef]
- Struys, M.M.; Vanpeteghem, C.; Huiku, M.; Uutela, K.; Blyaert, N.B.K.; Mortier, E.P. Changes in a surgical stress index in response to standardized pain stimuli during propofol-remifentanil infusion. Br. J. Anaesth. 2007, 99, 359–367. [Google Scholar] [CrossRef] [Green Version]
- Wennervirta, J.; Hynynen, M.; Koivusalo, A.M.; Uutela, K.; Huiku, M.; Vakkuri, A. Surgical stress index as a measure of nociception/antinociception balance during general anesthesia. Acta Anaesthesiol. Scand. 2008, 52, 1038–1045. [Google Scholar] [CrossRef]
- Ahonen, J.; Jokela, R.; Uutela, K.; Huiku, M. Surgical stress index reflects surgical stress in gynaecological laparoscopic day-case surgery. Br. J. Anaesth. 2007, 98, 456–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledowski, T.; Pascoe, E.; Ang, B.; Schmarbeck, T.; Clarke, M.W.; Fuller, C.; Kapoor, V. Monitoring of intra-operative nociception: Skin conductance and surgical stress index versus stress hormone plasma levels. Anaesthesia 2010, 65, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Schuttler, J.; Albrecht, S.; Breivik, H.; Osnes, S.; Prys-Roberts, C.; Holder, K.; Chauvin, M.; Viby-Mogensen, J.; Mogensen, T.; Gustafson, I.; et al. A comparison of remifentanil and alfentanil in patients undergoing major abdominal surgery. Anaesthesia 1997, 52, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Stasiowski, M.; Missir, A.; Pluta, A.; Szumera, I.; Stasiak, M.; Szopa, W.; Błaszczyk, B.; Możdżyński, B.; Majchrzak, K.; Tymowski, M.; et al. Influence of infiltration anaesthesia on perioperative outcomes following lumbar discectomy under surgical pleth index-guided general anaesthesia: A prelimi-nary report from a randomised controlled prospective trial. Adv. Med. Sci. 2020, 65, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Stasiowski, M.J.; Starzewska, M.; Niewiadomska, E.; Król, S.; Marczak, K.; Żak, J.; Pluta, A.; Eszyk, J.; Grabarek, B.O.; Szumera, I.; et al. Adequacy of anesthesia guidance for colonoscopy procedures. Pharmaceuticals 2021, 14, 464. [Google Scholar] [CrossRef]
- Stasiowski, M.J.; Pluta, A.; Lyssek-Boroń, A.; Kawka, M.; Krawczyk, L.; Niewiadomska, E.; Dobrowolski, D.; Rejdak, R.; Król, S.; Żak, J.; et al. Preventive analgesia, hemodynamic stability, and pain in vitreoretinal surgery. Medicina 2021, 57, 262. [Google Scholar] [CrossRef]
- Pluta, A.; Stasiowski, M.J.; Lyssek-Boroń, A.; Król, S.; Krawczyk, L.; Niewiadomska, E.; Żak, J.; Kawka, M.; Dobrowolski, D.; Grabarek, B.O.; et al. Adverse Events during vitrectomy under adequacy of anesthesia-An additional report. J. Clin. Med. 2021, 10, 4172. [Google Scholar] [CrossRef]
- Hogue, C.W., Jr.; Bowdl, T.A.; O’Leary, C.; Duncalf, D.; Miguel, R.; Pitts, M.; Strisand, J.; Kirvassilis, G.; Jamerson, B.; McNeal, S.; et al. A multicenter evaluation of total intravenous anesthesia with remifentanil and propofol for elective inpatient surgery. Anesth. Analg. 1996, 83, 279–285. [Google Scholar] [CrossRef]



| Metrics | Total | GA Group | SPI Group | PRD Group | p-Value | |
| n=89 (100%) | n=30 (33.7%) | n=31 (34.8%) | n=28 (31.5%) | |||
| age | [years] | 50.2 ± 14.6 51 (27) | 49 ± 15.4 51 (26) | 47.7 ± 13.9 46 (21) | 54.1 ± 14.4 59.5 (23) | 0.22 |
| gender | male | 56 (62.9) | 20 (66.7) | 18 (58.1) | 18 (64.3) | 0.77 |
| female | 33 (37.1) | 10 (33.3) | 13 (41.9) | 10 (35.7) | ||
| Anthropometric Data | Total | GA Group | SPI Group | PRD Group | p-Value | |
| n= 89 (100%) | n= 30 (33.7%) | n= 31 (34.8%) | n= 28 (31.5%) | |||
| height | [cm] | 171.4 ± 9 171 (12) | 173.5 ± 9.3 175 (9) | 170.9 ± 8.7 170 (12) | 169.6 ± 9.1 170 (12) | 0.26 |
| weight | [kg] | 78.2 ± 14.6 79 (20) | 80.3 ± 11.5 81 (18) | 78.3 ± 14.7 79 (25) | 75.9 ± 17.3 74 (23.5) | 0.52 |
| BMI | [kg/m2] | 26.5 ± 3.9 26.6 (5.2) | 26.8 ± 3.9 26.9 (4.4) | 26.6 ± 3.6 27 (4.6) | 26.1 ± 4.2 25.2 (5.2) | 0.82 |
| BMI | norm | 29 (32.6) | 9 (30) | 7 (22.6) | 13 (46.4) | 0.38 |
| overweight | 43 (48.3) | 15 (50) | 18 (58.1) | 10 (35.7) | ||
| obesity | 17 (19.1) | 6 (20) | 6 (19.4) | 5 (17.9) | ||
| ASA | I | 25 (29.8) | 9 (31) | 11 (37.9) | 5 (19.2) | 0.31 |
| II | 45 (53.6) | 18 (62.1) | 14 (48.3) | 13 (50) | 0.52 | |
| III | 14 (16.7) | 2 (6.9) | 4 (13.8) | 8 (30.8) | 0.05 | |
| LM CT scale mean | LM < 12 | 50 (56.2) | 12 (40) | 19 (61.3) | 19 (67.9) | 0.08 |
| LM ≥ 12 | 39 (43.8) | 18 (60) | 12 (38.7) | 9 (32.1) | ||
| primary EES/revision | 74 (84.1) | 25 (86.2) | 26 (83.9) | 23 (82.1) | 0.91 | |
| samter’s triad | 7 (8.3) | 5 (17.2) | 0 (0) | 2 (7.7) | 0.05 | |
| asthma | 17 (20.2) | 10 (34.5) | 4 (13.8) | 3 (11.5) | 0.06 | |
| arterial hypertension | 31 (36.9) | 10 (34.5) | 10 (34.5) | 11 (42.3) | 0.79 | |
| coronary artery disease | 7 (8.3) | 2 (6.9) | 1 (3.4) | 4 (15.4) | 0.27 | |
| Surgery | Total | GA Group | SPI Group | PRD Group | p-Value |
|---|---|---|---|---|---|
| n = 89 (100%) | n = 30 (33.7%) | n = 31 (34.8%) | n = 28 (31.5%) | ||
| unilateral/bilateral | 26 (29.2) | 6 (20.0) | 10 (32.3) | 10 (35.7) | 0.42 |
| 62 (70.8) | 23 (77.0) | 21 (67.7) | 18 (64.3) | ||
| antrosthomy with sfenoetmiodectomy | 41 (46.6) | 17 (58.6) | 12 (38.7) | 12 (42.9) | 0.27 |
| antrosthomy with total etmoidectomy | 19 (21.6) | 6 (20.7) | 6 (19.4) | 7 (25) | 0.86 |
| antrosthomy with anterior etmoidectomy | 28 (31.8) | 6 (20.7) | 13 (41.9) | 9 (32.1) | 0.21 |
| isolated anthrostomy | - | - | - | - | - |
| Intraoperative Parameters | Total | GA Group | SPI Group | PRD Group | p-Value |
|---|---|---|---|---|---|
| n = 89 (100%) | n = 30 (33.7%) | n = 31 (34.8%) | n = 28 (31.5%) | ||
| total intraoperative blood loss tibl (mL) | 207.5 ± 154.3 170 (200) | 283.3 ± 193.5 220 (300) | 165.2 ± 100.2 150 (150) | 173.1 ± 128.6 135 (189) | 0.04 p < 0.05 ga vs. spi * |
| length of operation lop (min) | 74.1 ± 32.3 73 (35) | 82.6 ± 33.1 85 (40) | 75.8 ± 34.2 70 (39) | 63.1 ± 26.7 65.5 (35) | 0.05 ga vs. prd * |
| total propofol consumption (mg) | 666 ± 269.5 620 (340) | 762.3 ± 273.2 750 (350) | 665.8 ± 237.8 650 (400) | 562.9 ± 269.1 520 (285) | 0.008 p < 0.01 ga vs. prd ** |
| total RMFNT consumption (mg) | 1.6 ± 1.2 1.5 (1.1) | 1.7 ± 1.1 1.5 (1) | 1.8 ± 0.9 1.8 (0.9) | 1.3 ± 1.4 1 (0.7) | 0.005 p < 0.01 spi vs. prd ** |
| max speed of RMFNT infusion (mcg/kg/min) | 0.38 ± 0.16 0.38 (0.25) | 0.42 ± 0.21 0.38 (0.25) | 0.4 ± 0.12 0.38 (0.25) | 0.33 ± 0.15 0.25 (0.13) | 0.02 p < 0.05 ga vs. prd * |
| min speed of RMFNT infusion (mcg/kg/min) | 0.22 ± 0.06 0.25 (0.13) | 0.21 ± 0.06 0.25 (0.13) | 0.25 ± 0.05 0.25 (0) | 0.21 ± 0.07 0.25 (0.13) | 0.03 p < 0.05 |
| mean speed of RMFNT infusion (mcg/kg/min) | 0.31 ± 0.12 0.28 (0.12) | 0.32 ± 0.14 0.28 (0.16) | 0.33 ± 0.09 0.34 (0.16) | 0.27 ± 0.12 0.25 (0.1) | 0.007 p < 0.01 spi vs. prd ** |
| max values of BBS | 2.7 ± 0.7 3 (1) | 2.9 ± 0.7 3 (1) | 2.5 ± 0.6 3 (1) | 2.6 ± 0.7 3 (1) | 0.2 |
| min values of BBS | 1.7 ± 0.5 2 (1) | 1.8 ± 0.4 2 (0) | 1.7 ± 0.5 2 (1) | 1.6 ± 0.5 2 (1) | 0.09 |
| mean values of BBS | 2 ± 0.4 2.1 (0.3) | 2.1 ± 0.5 2.2 (0.4) | 1.9 ± 0.5 2 (0.5) | 2 ± 0.3 2 (0.2) | 0.07 |
| mean time duration of BBS > 2 | 12.7 ± 15.3 5 (20) | 16.3 ± 16.8 15 (30) | 11.8 ± 14.9 5 (25) | 9.6 ± 13.7 5 (15) | 0.23 |
| mean number of incidences of increased value of BBS > 2 | 1.07 ± 1.1 1 (2) | 1.4 ± 1.3 1 (2) | 0.9 ± 0.9 1 (1) | 0.89 ± 1 1 (1.5) | 0.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stasiowski, M.J.; Szumera, I.; Wardas, P.; Król, S.; Żak, J.; Missir, A.; Pluta, A.; Niewiadomska, E.; Krawczyk, L.; Jałowiecki, P.; et al. Adequacy of Anesthesia and Pupillometry for Endoscopic Sinus Surgery. J. Clin. Med. 2021, 10, 4683. https://doi.org/10.3390/jcm10204683
Stasiowski MJ, Szumera I, Wardas P, Król S, Żak J, Missir A, Pluta A, Niewiadomska E, Krawczyk L, Jałowiecki P, et al. Adequacy of Anesthesia and Pupillometry for Endoscopic Sinus Surgery. Journal of Clinical Medicine. 2021; 10(20):4683. https://doi.org/10.3390/jcm10204683
Chicago/Turabian StyleStasiowski, Michał Jan, Izabela Szumera, Piotr Wardas, Seweryn Król, Jakub Żak, Anna Missir, Aleksandra Pluta, Ewa Niewiadomska, Lech Krawczyk, Przemysław Jałowiecki, and et al. 2021. "Adequacy of Anesthesia and Pupillometry for Endoscopic Sinus Surgery" Journal of Clinical Medicine 10, no. 20: 4683. https://doi.org/10.3390/jcm10204683
APA StyleStasiowski, M. J., Szumera, I., Wardas, P., Król, S., Żak, J., Missir, A., Pluta, A., Niewiadomska, E., Krawczyk, L., Jałowiecki, P., & Grabarek, B. O. (2021). Adequacy of Anesthesia and Pupillometry for Endoscopic Sinus Surgery. Journal of Clinical Medicine, 10(20), 4683. https://doi.org/10.3390/jcm10204683






