In Vitro Maturation of Oocytes Retrieved from Ovarian Tissue: Outcomes from Current Approaches and Future Perspectives
Abstract
1. Introduction
2. Materials and Methods
3. Results
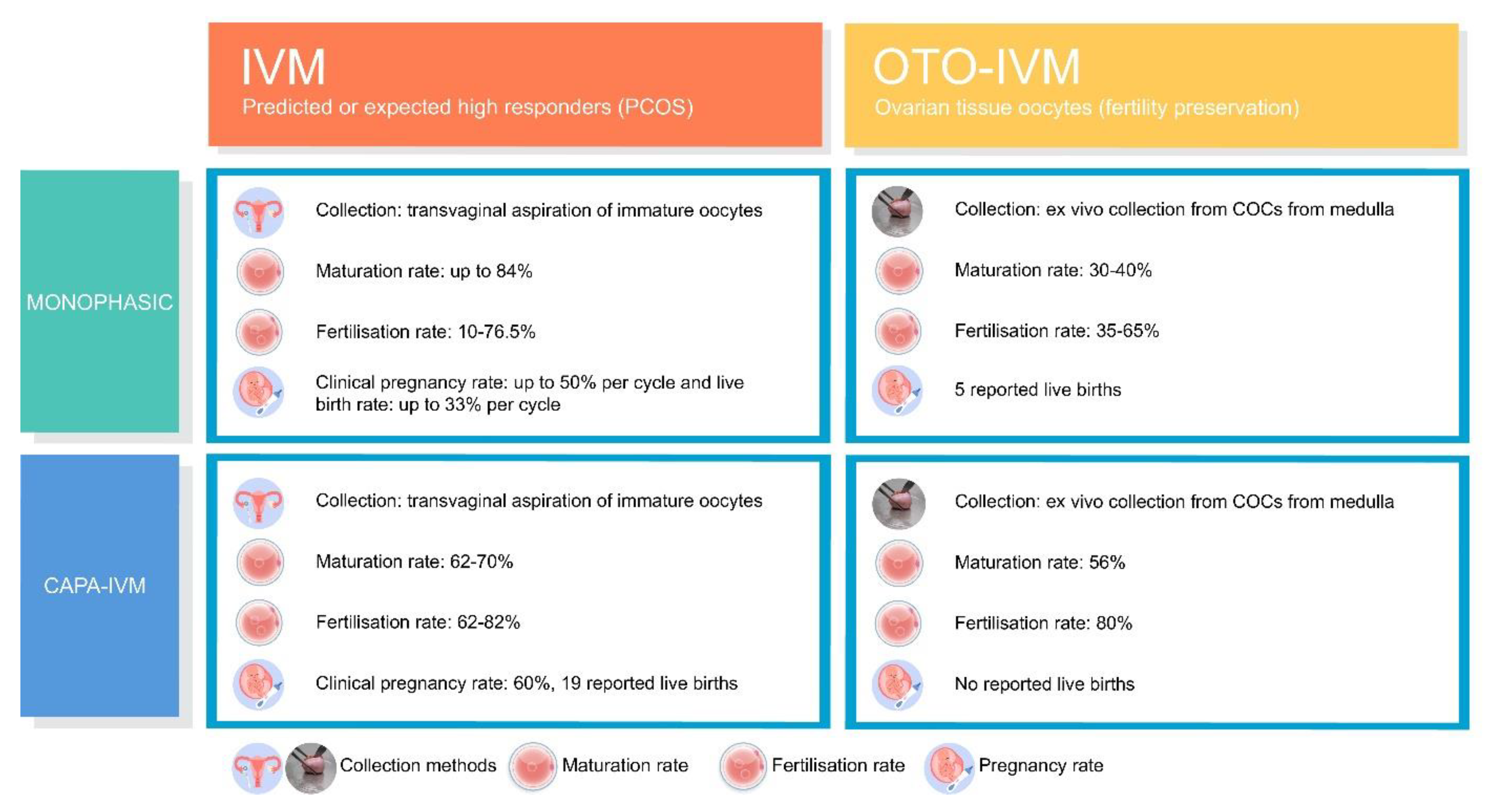
3.1. Monophasic OTO-IVM
3.2. Biphasic IVM
3.3. Biphasic IVM in Human
| Study Type | Methodology | Results | Reference |
|---|---|---|---|
| Prospective pilot study n = 15 PCOS patients age mean ± SD 28.9 ± 3.9 years Single center | Stimulation: 3 days HP-hMG (225 IU, 225 IU, 150 IU) (+2 days if applicable) COC retrieved via aspiration Sibling oocyte study: Monophasic IVM (n = 264 COCs) vs. biphasic IVM (n = 117 COCs) | COCs collected via ultrasound-guided in situ ovarian aspiration Monophasic vs. biphasic IVM: Maturation rate: 48% vs. 70% (p < 0.001) Fertilization rate: 62% vs. 82% (p < 0.001) Yield good quality embryos day 3: 23% vs. 43% (p < 0.001) Blastocyst rate: 14% vs. 23% (p < 0.05) | Sanchez F. et al., 2017 [11] |
| Prospective pilot study n = 40 PCOS patients Single center | Stimulation: 3 doses of 150 IU rFSH (no HCG trigger) COC retrieved via aspiration Sibling oocyte study: Monophasic IVM: (n = 20 patients; age: mean ± SD 28.8 ± 2.9 years; n = 238 COCs) vs biphasic IVM (n = 20 patients, age: mean ± SD 29.1 ± 3.4 y; n = 305 COCs) | COCs collected via ultrasound-guided in situ ovarian aspiration Monophasic vs. biphasic IVM: Maturation rate: 48% vs. 62% (p < 0.05) Fertilization rate was similar Yield good quality embryos day 3: 24% vs. 38% (p < 0.05) Blastocyst rate: 14% vs. 23% (p < 0.05) 1.8x higher amounts of usable embryos per patient: 2.2% vs. 4.2% (p < 0.001) | Sanchez F. et al., 2019 [58] |
| Prospective pilot study n = 80 PCOS patients Single center | Stimulation: 3 doses of gonadotropins (2.5days; ~377 IU used) (no HCG trigger) COC retrieved via aspiration Sibling oocyte study: Monophasic IVM: (n = 40 patients; age: mean ± SD 28.1 ± 3.1 years; n = 16.5 COCs/patient) vs biphasic IVM: (n = 40 patients; age: mean ± SD 28.5 ± 3.4 years; n = 17.5 COCs/patient) | COCs collected via ultrasound-guided in situ ovarian aspiration Monophasic vs. biphasic IVM: Maturation rate: 49% vs. 63.6% (p < 0.001) Fertilization rate similar Yield good quality embryos day 3: 26.8% vs. 30% Vitrified embryos after fresh ET: 1.3 ± 1.9 vs. 2.5 ± 2.5 (p < 0.05) Clinical pregnancy rate: 37.5% vs. 60% (NS; p = 0.06) 19 babies born after biphasic IVM no serious adverse events or reactions found Normal physical appearances were found in the newborn babies Detailed follow-up of the babies is being conducted. | Vuong L. et al., 2020 [59] |
| Prospective pilot study n = 10 patients (mean ± SE 29.40 ± 1.76 years; Range: 16–36 years) with gynecological malignancies Single center | Stimulation: 3 doses of 150 IU rFSH (no HCG trigger) COC retrieved via aspiration Sibling oocyte study: Monophasic OTO-IVM (n = 96 COCs) vs. biphasic OTO-IVM (n = 105 COCs) | COC identification via microscopic evaluation after tissue manipulation Monophasic OTO-IVM vs. biphasic OTO-IVM: Maturation rate: 35% vs. 56% (p < 0.05) Fertilization rate: 68.4% vs. 80% (p < 0.001) Yield good quality embryos day 3: 24% vs. 38%Blastocyst rate: 0% vs. 16% 1.8x higher amounts of usable embryos per patient: 2.2% vs. 4.2% (p < 0.001) | Kirillova A. et al., 2021 [38] |
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Edwards, R. Maturation in vitro of human ovarian oocytes. Lancet 1965, 286, 926–929. [Google Scholar] [CrossRef]
- Cross, P.C.; Brinster, R.L. In vitro development of mouse oocytes. Biol. Reprod. 1970, 3, 298–307. [Google Scholar] [CrossRef]
- Wynn, P.; Picton, H.M.; Krapez, J.A.; Rutherford, A.J.; Balen, A.H.; Gosden, R.G. Pretreatment with follicle stimulating hormone promotes the numbers of human oocytes reaching metaphase II by in-vitro maturation. Hum. Reprod. 1998, 13, 3132–3138. [Google Scholar] [CrossRef]
- De Vos, M.; Smitz, J.; Thompson, J.G.; Gilchrist, R.B. The definition of IVM is clear—Variations need defining. Hum. Reprod. 2016, 31, 2411–2415. [Google Scholar] [CrossRef]
- Pincus, G.; Enzmann, E.V. Can mammalian eggs undergo normal development in vitro? Proc. Natl. Acad. Sci. USA 1934, 20, 121–122. [Google Scholar] [CrossRef]
- Cha, K.Y.; Koo, J.J.; Ko, J.J.; Choi, D.H.; Han, S.Y.; Yoon, T.K. Pregnancy after in vitro fertilization of human follicular oocytes collected from nonstimulated cycles, their culture in vitro and their transfer in a donor oocyte program. Fertil. Steril. 1991, 55, 109–113. [Google Scholar] [CrossRef]
- Trounson, A.; Wood, C.; Kausche, A. In vitro maturation and the fertilization and developmental competence of oocytes recovered from untreated polycystic ovarian patients. Fertil. Steril. 1994, 62, 353–362. [Google Scholar] [CrossRef]
- Shirasawa, H.; Terada, Y. In vitro maturation of human immature oocytes for fertility preservation and research material. Reprod. Med. Biol. 2017, 16, 258–267. [Google Scholar] [CrossRef] [PubMed]
- De Vos, M.; Grynberg, M.; Ho, T.M.; Yuan, Y.; Albertini, D.F.; Gilchrist, R.B. Perspectives on the development and future of oocyte IVM in clinical practice. J. Assist. Reprod. Genet. 2021, 38, 1265–1280. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, R.B.; Luciano, A.M.; Richani, D.; Zeng, H.T.; Wang, X.; de Vos, M.; Sugimura, S.; Smitz, J.; Richard, F.J.; Thompson, J.G. Oocyte maturation and quality: Role of cyclic nucleotides. Reproduction 2016, 152, R143–R157. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, F.; Lolicato, F.; Romero, S.; de Vos, M.; van Ranst, H.; Verheyen, G.; Anckaert, E.; Smitz, J. An improved IVM method for cumulus-oocyte complexes from small follicles in polycystic ovary syndrome patients enhances oocyte competence and embryo yield. Hum. Reprod. 2017, 32, 2056–2068. [Google Scholar] [CrossRef]
- Vesztergom, D.; Segers, I.; Mostinckx, L.; Blockeel, C.; de Vos, M. Live births after in vitro maturation of oocytes in women who had suffered adnexal torsion and unilateral oophorectomy following conventional ovarian stimulation. J. Assist. Reprod. Genet. 2021, 38, 1–7. [Google Scholar] [CrossRef]
- Siristatidis, C.S.; Maheshwari, A.; Vaidakis, D.; Bhattacharya, S. In vitro maturation in subfertile women with polycystic ovarian syndrome undergoing assisted reproduction. Cochrane Database Syst. Rev. 2018, 2018, CD006606. [Google Scholar] [CrossRef]
- Child, T.J.; Abdul-Jalil, A.K.; Gulekli, B.; Tan, S.L. In vitro maturation and fertilization of oocytes from unstimulated normal ovaries, polycystic ovaries, and women with polycystic ovary syndrome. Fertil. Steril. 2001, 76, 936–942. [Google Scholar] [CrossRef]
- Söderström-Anttila, V.; Mäkinen, S.; Tuuri, T.; Suikkari, A.-M. Favourable pregnancy results with insemination of in vitro matured oocytes from unstimulated patients. Hum. Reprod. 2005, 20, 1534–1540. [Google Scholar] [CrossRef] [PubMed]
- Galvão, A.; Segers, I.; Smitz, J.; Tournaye, H.; de Vos, M. In vitro maturation (IVM) of oocytes in patients with resistant ovary syndrome and in patients with repeated deficient oocyte maturation. J. Assist. Reprod. Genet. 2018, 35, 2161–2171. [Google Scholar] [CrossRef] [PubMed]
- Chian, R.; Buckett, W.; Tulandi, T.; Tan, S. Prospective randomized study of human chorionic gonadotrophin priming before immature oocyte retrieval from unstimulated women with polycystic ovarian syndrome. Hum. Reprod. 2000, 15, 165–170. [Google Scholar] [CrossRef]
- Ge, H.-S.; Huang, X.-F.; Zhang, W.; Zhao, J.-Z.; Lin, J.-J.; Zhou, W. Exposure to human chorionic gonadotropin during in vitro maturation does not improve the maturation rate and developmental potential of immature oocytes from patients with polycystic ovary syndrome. Fertil. Steril. 2008, 89, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Holzer, H.E.G.; Scharf, E.; Chian, R.-C.; Demirtas, E.; Buckett, W.; Tan, S.L. In vitro maturation of oocytes collected from unstimulated ovaries for oocyte donation. Fertil. Steril. 2007, 88, 62–67. [Google Scholar] [CrossRef]
- Huang, J.Y.; Chian, R.-C.; Tan, S.L. Ovarian hyperstimulation syndrome prevention strategies: In vitro maturation. Semin. Reprod. Med. 2010, 28, 519–531. [Google Scholar] [CrossRef]
- Kedem, A.; Yerushalmi, G.M.; Brengauz, M.; Raanani, H.; Orvieto, R.; Hourvitz, A.; Meirow, D. Outcome of immature oocytes collection of 119 cancer patients during ovarian tissue harvesting for fertility preservation. J. Assist. Reprod. Genet. 2018, 35, 851–856. [Google Scholar] [CrossRef]
- Meirow, D.; Hardan, I.; Dor, J.; Fridman, E.; Elizur, S.; Ra’Anani, H.; Slyusarevsky, E.; Amariglio, N.; Schiff, E.; Rechavi, G.; et al. Searching for evidence of disease and malignant cell contamination in ovarian tissue stored from hematologic cancer patients. Hum. Reprod. 2008, 23, 1007–1013. [Google Scholar] [CrossRef]
- De Roo, C.; Lierman, S.; Tilleman, K.; Peynshaert, K.; Braeckmans, K.; Caanen, M.; Lambalk, C.; Weyers, S.; T’Sjoen, G.; Cornelissen, R.; et al. Ovarian tissue cryopreservation in female-to-male transgender persons: Insights in ovarian histology and physiology after prolonged androgen treatment. Reprod. Biomed. Online 2017, 34, 557–566. [Google Scholar] [CrossRef]
- Revel, A.; Safran, A.; Benshushan, A.; Shushan, A.; Laufer, N.; Simon, A. In vitro maturation and fertilization of oocytes from an intact ovary of a surgically treated patient with endometrial carcinoma: Case report. Hum. Reprod. 2004, 19, 1608–1611. [Google Scholar] [CrossRef] [PubMed]
- Leonel, E.C.R.; Corral, A.; Risco, R.; Camboni, A.; Taboga, S.R.; Kilbride, P.; Vazquez, M.; Morris, J.; Dolmans, M.-M.; Amorim, C.A. Stepped vitrification technique for human ovarian tissue cryopreservation. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Kristensen, S.G.; Rasmussen, A.; Byskov, A.G.; Andersen, C.Y. Isolation of pre-antral follicles from human ovarian medulla tissue. Hum. Reprod. 2011, 26, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Segers, I.; Mateizel, I.; van Moer, E.; Smitz, J.; Tournaye, H.; Verheyen, G.; de Vos, M. In vitro maturation (IVM) of oocytes recovered from ovariectomy specimens in the laboratory: A promising “ex vivo” method of oocyte cryopreservation resulting in the first report of an ongoing pregnancy in Europe. J. Assist. Reprod. Genet. 2015, 32, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Hourvitz, A.; Yerushalmi, G.; Maman, E.; Raanani, H.; Elizur, S.; Brengauz, M.; Orvieto, R.; Dor, J.; Meirow, D. Combination of ovarian tissue harvesting and immature oocyte collection for fertility preservation increases preservation yield. Reprod. Biomed. Online 2015, 31, 497–505. [Google Scholar] [CrossRef]
- Park, C.W.; Lee, S.H.; Yang, K.M.; Lee, I.H.; Lim, K.T.; Lee, K.H.; Kim, T.J. Cryopreservation of in vitro matured oocytes after ex vivo oocyte retrieval from gynecologic cancer patients undergoing radical surgery. Clin. Exp. Reprod. Med. 2016, 43, 119–125. [Google Scholar] [CrossRef]
- Yin, H.; Jiang, H.; Kristensen, S.G.; Andersen, C.Y. Vitrification of in vitro matured oocytes collected from surplus ovarian medulla tissue resulting from fertility preservation of ovarian cortex tissue. J. Assist. Reprod. Genet. 2016, 33, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Lierman, S.; Tilleman, K.; Braeckmans, K.; Peynshaert, K.; Weyers, S.; T’Sjoen, G.; de Sutter, P. Fertility preservation for trans men: Frozen-thawed in vitro matured oocytes collected at the time of ovarian tissue processing exhibit normal meiotic spindles. J. Assist. Reprod. Genet. 2017, 34, 1449–1456. [Google Scholar] [CrossRef]
- Fasano, G.; Moffa, F.; Dechène, J.; Englert, Y.; Demeestere, I. Vitrification of in vitro matured oocytes collected from antral follicles at the time of ovarian tissue cryopreservation. Reprod. Biol. Endocrinol. 2011, 9, 150. [Google Scholar] [CrossRef]
- Walls, M.L.; Douglas, K.; Ryan, J.P.; Tan, J.; Hart, R. In-vitro maturation and cryopreservation of oocytes at the time of oophorectomy. Gynecol. Oncol. Rep. 2015, 13, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Wilken-Jensen, H.N.; Kristensen, S.G.; Jeppesen, J.V.; Andersen, C.Y. Developmental competence of oocytes isolated from surplus medulla tissue in connection with cryopreservation of ovarian tissue for fertility preservation. Acta Obstet. Gynecol. Scand. 2014, 93, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Fasano, G.; Dechène, J.; Antonacci, R.; Biramane, J.; Vannin, A.-S.; van Langendonckt, A.; Devreker, F.; Demeestere, I. Outcomes of immature oocytes collected from ovarian tissue for cryopreservation in adult and prepubertal patients. Reprod. Biomed. Online 2017, 34, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Nikiforov, D.; Junping, C.; Cadenas, J.; Shukla, V.; Blanshard, R.; Pors, S.E.; Kristensen, S.G.; Macklon, K.T.; Colmorn, L.; Ernst, E.; et al. Improving the maturation rate of human oocytes collected ex vivo during the cryopreservation of ovarian tissue. J. Assist. Reprod. Genet. 2020, 37, 891–904. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Zarchi, M.; Mohsenzadeh, M.; Khalili, M.A.; Tabibnejad, N.; Yari, N.; Agha-Rahimi, A. Embryo cryopreservation following in-vitro maturation for fertility preservation in a woman with Mullerian adenosarcoma: A case report. J. Hum. Reprod. Sci. 2017, 10, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, A.; Bunyaeva, E.; van Ranst, H.; Khabas, G.; Farmakovskaya, M.; Kamaletdinov, N.; Nazarenko, T.; Abubakirov, A.; Sukhikh, G.; Smitz, J.E.J. Improved maturation competence of ovarian tissue oocytes using a biphasic in vitro maturation system for patients with gynecological malignancy: A study on sibling oocytes. J. Assist. Reprod. Genet. 2021, 38, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lierman, S.; Tolpe, A.; de Croo, I.; de Gheselle, S.; Defreyne, J.; Baetens, M.; Dheedene, A.; Colman, R.; Menten, B.; T’Sjoen, G.; et al. Low feasibility of in vitro matured oocytes originating from cumulus complexes found during ovarian tissue preparation at the moment of gender confirmation surgery and during testosterone treatment for fertility preservation in transgender men. Fertil. Steril. 2021, 116, 1068–1076. [Google Scholar] [CrossRef]
- Fadini, R.; Renzini, M.M.; Guarnieri, T.; Canto, M.D.; de Ponti, E.; Sutcliffe, A.; Shevlin, M.; Comi, R.; Coticchio, G. Comparison of the obstetric and perinatal outcomes of children conceived from in vitro or in vivo matured oocytes in in vitro maturation treatments with births from conventional ICSI cycles. Hum. Reprod. 2012, 27, 3601–3608. [Google Scholar] [CrossRef][Green Version]
- Dietrich, J.E.; Jauckus, J.; Hoffmann, S.; Liebenthron, J.; Capp, E.; Strowitzki, T.; Germeyer, A. In vitro maturation of immature oocytes from ovarian tissue prior to shipment to a cryobank. Arch. Gynecol. Obstet. 2020, 302, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Prasath, E.B.; Chan, M.L.H.; Wong, W.H.W.; Lim, C.J.W.; Tharmalingam, M.D.; Hendricks, M.; Loh, S.F.; Chia, Y.N. First pregnancy and live birth resulting from cryopreserved embryos obtained from in vitro matured oocytes after oophorectomy in an ovarian cancer patient. Hum. Reprod. 2014, 29, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Uzelac, P.S.; Delaney, A.A.; Christensen, G.L.; Bohler, H.C.; Nakajima, S.T. Live birth following in vitro maturation of oocytes retrieved from extracorporeal ovarian tissue aspiration and embryo cryopreservation for 5 years. Fertil. Steril. 2015, 104, 1258–1260. [Google Scholar] [CrossRef] [PubMed]
- Segers, I.; Bardhi, E.; Mateizel, I.; van Moer, E.; Schots, R.; Verheyen, G.; Tournaye, H.; de Vos, M. Live births following fertility preservation using in-vitro maturation of ovarian tissue oocytes. Hum. Reprod. 2020, 35, 2026–2036. [Google Scholar] [CrossRef] [PubMed]
- Coticchio, G.; Canto, M.D.; Renzini, M.M.; Guglielmo, M.C.; Brambillasca, F.; Turchi, D.; Novara, P.V.; Fadini, R. Oocyte maturation: Gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization. Hum. Reprod. Updat. 2015, 21, 427–454. [Google Scholar] [CrossRef]
- Ferreira, E.; Vireque, A.; Adona, P.; Meirelles, F.; Ferriani, R.; Navarro, P. Cytoplasmic maturation of bovine oocytes: Structural and biochemical modifications and acquisition of developmental competence. Theriogenology 2009, 71, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, H.; Cao, X.; Motola, S.; Popliker, M.; Conti, M.; Tsafriri, A. Epidermal growth factor family members: Endogenous mediators of the ovulatory response. Endocrinology 2005, 146, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Zamah, A.M.; Hsieh, M.; Chen, J.; Vigne, J.L.; Rosen, M.P.; Cedars, M.I.; Conti, M. Human oocyte maturation is dependent on LH-stimulated accumulation of the epidermal growth factor-like growth factor, amphiregulin†. Hum. Reprod. 2010, 25, 2569–2578. [Google Scholar] [CrossRef]
- Kawamura, K.; Cheng, Y.; Kawamura, N.; Takae, S.; Okada, A.; Kawagoe, Y.; Mulders, S.; Terada, Y.; Hsueh, A.J. Pre-ovulatory LH/hCG surge decreases C-type natriuretic peptide secretion by ovarian granulosa cells to promote meiotic resumption of pre-ovulatory oocytes. Hum. Reprod. 2011, 26, 3094–3101. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, Q.; Cai, J.; Zhao, X.; Ma, B. Effect of C-type natriuretic peptide on maturation and developmental competence of goat oocytes matured in vitro. PLoS ONE 2015, 10, e0132318. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, C.; Fan, X.; Li, R.; Zhang, J. Effect of C-type natriuretic peptide pretreatment on in vitro bovine oocyte maturation. In Vitro Cell. Dev. Biol. Anim. 2017, 53, 199–206. [Google Scholar] [CrossRef]
- Jia, Z.; Yang, X.; Liu, K. Treatment of cattle oocytes with C-type natriuretic peptide before in vitro maturation enhances oocyte mitochondrial function. Anim. Reprod. Sci. 2021, 225, 106685. [Google Scholar] [CrossRef]
- Goud, P.T.; Goud, A.P.; Qian, C.; Laverge, H.; van der Elst, J.; de Sutter, P.; Dhont, M. In-vitro maturation of human germinal vesicle stage oocytes: Role of cumulus cells and epidermal growth factor in the culture medium. Hum. Reprod. 1998, 13, 1638–1644. [Google Scholar] [CrossRef]
- Peluffo, M.C.; Ting, A.Y.; Zamah, A.M.; Conti, M.; Stouffer, R.L.; Zelinski, M.; Hennebold, J.D. Amphiregulin promotes the maturation of oocytes isolated from the small antral follicles of the rhesus macaque. Hum. Reprod. 2012, 27, 2430–2437. [Google Scholar] [CrossRef]
- Ben-Ami, I.; Komsky, A.; Bern, O.; Kasterstein, E.; Komarovsky, D.; Ron-El, R. In vitro maturation of human germinal vesicle-stage oocytes: Role of epidermal growth factor-like growth factors in the culture medium. Hum. Reprod. 2011, 26, 76–81. [Google Scholar] [CrossRef]
- Inoue, Y.; Miyamoto, S.; Fukami, T.; Shirota, K.; Yotsumoto, F.; Kawarabayashi, T. Amphiregulin is much more abundantly expressed than transforming growth factor-alpha and epidermal growth factor in human follicular fluid obtained from patients undergoing in vitro fertilization–embryo transfer. Fertil. Steril. 2009, 91, 1035–1041. [Google Scholar] [CrossRef]
- Heydarnejad, A.; Ostadhosseini, S.; Varnosfaderani, S.R.; Jafarpour, F.; Moghimi, A.; Nasr-Esfahani, M.H. Supplementation of maturation medium with CoQ10 enhances developmental competence of ovine oocytes through improvement of mitochondrial function. Mol. Reprod. Dev. 2019, 86, 812–824. [Google Scholar] [CrossRef]
- Sánchez, F.; Le, A.H.; Ho, V.N.A.; Romero, S.; van Ranst, H.; de Vos, M.; Gilchrist, R.B.; Ho, T.M.; Vuong, L.N.; Smitz, J. Biphasic in vitro maturation (CAPA-IVM) specifically improves the developmental capacity of oocytes from small antral follicles. J. Assist. Reprod. Genet. 2019, 36, 2135–2144. [Google Scholar] [CrossRef] [PubMed]
- Vuong, L.N.; Ho, V.N.A.; Ho, T.M.; Dang, V.Q.; Phung, T.H.; Giang, N.H.; Le, A.H.; Pham, T.D.; Wang, R.; Smitz, J.; et al. In-vitro maturation of oocytes versus conventional IVF in women with infertility and a high antral follicle count: A randomized non-inferiority controlled trial. Hum. Reprod. 2020, 35, 2537–2547. [Google Scholar] [CrossRef] [PubMed]
- Saenz-De-Juano, M.; Ivanova, E.; Romero, S.; Lolicato, F.; Sánchez, F.; van Ranst, H.; Krueger, F.; Segonds-Pichon, A.; de Vos, M.; Andrews, S.; et al. DNA methylation and mRNA expression of imprinted genes in blastocysts derived from an improved in vitro maturation method for oocytes from small antral follicles in polycystic ovary syndrome patients. Hum. Reprod. 2019, 34, 1640–1649. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; McLaughlin, M.; Wallace, W.H.B.; Albertini, D.F.; Telfer, E. The immature human ovary shows loss of abnormal follicles and increasing follicle developmental competence through childhood and adolescence. Hum. Reprod. 2014, 29, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Hambridge, H.L.; Mumford, S.; Mattison, D.; Ye, A.; Pollack, A.; Bloom, M.; Mendola, P.; Lynch, K.L.; Wactawski-Wende, J.; Schisterman, E. The influence of sporadic anovulation on hormone levels in ovulatory cycles. Hum. Reprod. 2013, 28, 1687–1694. [Google Scholar] [CrossRef] [PubMed]
- Revel, A.; Revel-Vilk, S.; Aizenman, E.; Porat-Katz, A.; Safran, A.; Ben-Meir, A.; Weintraub, M.; Shapira, M.; Achache, H.; Laufer, N. At what age can human oocytes be obtained? Fertil. Steril. 2009, 92, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Strowitzki, T.; Bruckner, T.; Roesner, S. Maternal and neonatal outcome and children’s development after medically assisted reproduction with in-vitro matured oocytes—A systematic review and meta-analysis. Hum. Reprod. Updat. 2021, 27, 460–473. [Google Scholar] [CrossRef] [PubMed]
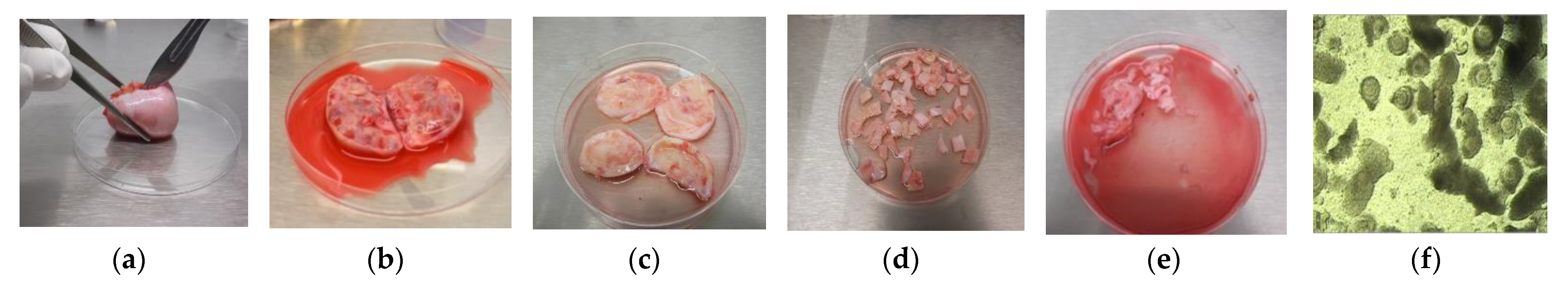
| Study Type | Methodology | Results | Reference |
|---|---|---|---|
| Case report | 43 years old patient Endometrial carcinoma Removal of polycystic ovary 10,000 IU HCG, 36 h prior to surgery, no FSH priming | Follicle aspiration from the ovary ex vivo (17 gauge needle) 17x oocytes retrieved, of which already 2x MII 3x MII after 24 h OTO-IVM; 1x 2PN after ICSI 7x MII after 48 h OTO-IVM; 3x 2PN after ICSI 40% 2PN rate; 4x day 3 embryos cryopreserved (2x ≥ 6 cell) | Revel A. et al., 2004 [24] |
| Case report | 38 years old patient Ovarian adenocarcinoma | Follicle aspiration from the ovary in situ (26 gauge needle) during surgery 3x COCs identified 2x MII after 30 h OTO-IVM; vitrification After warming, 1x 2PN after ICSI 50% 2PN rate; 1x day 2 embryo (2 cell); no pregnancy after ET | Fadini R. et al., 2012 [40] |
| Cohort study | 255 patients (mean ± SE 22.3 ± 1.26 years, range 10–40 years), different types of IVM 56 patients underwent OTO-IVM Hodgkin (n = 15), hematological cancer (n = 8), sarcoma (n = 11), breast cancer (n = 4), cervix cancer (n = 6), other (n = 12) | Follicle aspiration from the ovary ex vivo using needles and medium was analyzed microscopic after manipulation of the tissue 6.95 ± 0.83 COCs identified (mean ± SE) In 2.47 ± 0.43 MII after 48 h OTO-IVM; vitrification or ICSI on fresh oocytes 10/16 2PN after ICSI 62.5% 2PN rate; 1.67 ± 0.56 embryos cryopreserved | Hourvitz A. et al., 2015 [28] |
| Cohort study 1 | 9 patients (mean ± SD 30.6 ± 3.84 years, range 26–36 years) Breast cancer (n = 5), BRCA (n = 1), arteriovenous malformation (n = 1), systemic lupus erythematosus (n = 1), immature teratoma of tuba (n = 1) | COC identification via microscopic evaluation after tissue manipulation 10.8 ± 2.5 COCs identified (mean ± SEM) 4.6 ± 1.2 MII after 24 h or 40 h OTO-IVM 3.5 ± 1.0 2PN after ICSI 65 ± 11% 2PN rate 2.9 ± 0.6 embryos day 3 vitrified | Segers I. et al., 2015 [27] |
| Case series report | 6 patients (mean 29 years, range 19–39 years) Endometrial (n = 2), ovarian (n = 3), double primary endometrial and ovarian cancer (n = 1) | Follicle aspiration from the ovary ex vivo (18 gauge needle) 53x COCs identified (mean 10.6 per patient) 36x MII after 48 h OTO-IVM; Vitrification of 28 MII oocytes for 4 patients, 5x embryos vitrified for 1 patient after ICSI on fresh OTO-IVM oocytes (8x MII injected); 62.5% 2PN rate | Park C.W. et al., 2016 [29] |
| Cross sectional study | 136 patients (mean ± SD 27.6 ± 5.6 years) Different oncological indications (n = 120), hematological benign (n = 8), immunological disorders (n = 8) | Follicle aspiration from the ovary ex vivo, discarded tissue filtered through a cell strainer and microscopic evaluation 559x COCs subjected to OTO-IVM 145x MII after 24 h or 48 h OTO-IVM; Vitrification of 139x MII oocytes for 72 patients, 7x 2PN vitrified for 1 patient after ICSI on fresh OTO-IVM oocytes (12x MII injected in 5 patients); 58.3% 2PN rate 9x MII vitrification-warmed, 2x MII intact after warming, 1x embryo (low quality day 2); biochemical pregnancy after ET | Fasano G. et al., 2017 [35] |
| Case report | 37 years old patient Mullerian adenocarcinoma Ovarian wedge resection (2 × 2 cm) | Follicle aspiration from the ovary ex vivo (20 gauge needle) 3x COCs identified 2x MII after 48 h OTO-IVM; 2x 2PN after ICSI; 100% 2PN rate 2x embryos (grade B and C day 3) vitrified | Mohsenzadeh M. et al., 2017 [37] |
| Cohort study 1 | 8 patients (mean ± SD 27.3 ± 4.8 years range 20–34 years) | Oocyte aspiration from the ovary ex vivo 25x COCs identified 12x MII after 36 h OTO-IVM 5x embryos cryopreserved after ICSI 5x embryos for ET in different patients: no pregnancies after ET | Kedem et al., 2018 [21] |
| Case report | 30 years old patient Breast cancer Ovarian wedge resection (2 × 2 cm) | Follicle aspiration from the ovary ex vivo (21 gauge needle), remaining fluid after manipulation underwent microscopic evaluation 33x COCs identified 11x MII after 48 h OTO-IVM; 6x 2PN after ICSI; 54.5% 2PN rate 6x cleavage embryos (day 3), 3 blastocysts (euploid) vitrified | Kirillova A. et al., 2020 [38] |
| Prospectiveobservational study | 12 patients (mean ± SD 27.3. ± 4.8 years range 20–34 years) Breast cancer (n = 6), B-cell lymphoma (n = 1), Hodgkin lymphoma (n = 2), cervical embryonal rhabdomyosarcoma (n = 1), pleiomorphic xanthoastrocytoma (n = 1), Ewing’s sarcoma (n = 1) Unilateral ovarian resection | Follicle aspiration from the ovary ex vivo (21 gauge needle), remaining fluid after manipulation underwent microscopic evaluation 37x COCs identified 14x MII after 24 h OTO-IVM; 8x MII were vitrified 4x 2PN vitrified for 3 patients after ICSI on fresh OTO-IVM oocytes (6x MII injected in 3 patients); 66.6% 2PN rate | Dietrich J. et al., 2020 [41] |
| Cross sectional study | 83 patients (median; min-max: 20 years (17.6–38.4 years) Transgender patients Bilateral oophorectomy at the time of gender affirming surgery under testosterone treatment | COC identification via microscopic evaluation after tissue manipulation 1903x COCs identified 453x MII after 48 h OTO-IVM; 410x MII were vitrified, 208x MII warmed 48x 2PN (from 151 warmed intact) after ICSI with 1 sperm donor 34.5% 2PN rate 25x embryos day 3, 1x blastocyst day 5 (4BB) (euploid) 44x embryos arrested (91.7%) | Lierman S. et al., 2021 [39] |
| Study Type | Methodology | Results | Reference |
|---|---|---|---|
| Case report | 21 years old patient Oophorectomy for bilateral large pelvic masses, peritoneal disease and ascites and raised serum CA125 of 1.279 U/mL left salpingo-ophorectomy at day 5 of the menstrual cycle | Follicle aspiration from the ex vivo ovary (18 gauge needle), No data on COCs collection, number of MII oocytes or embryo development. 24 h OTO-IVM performed 3x day 2 embryos cryopreserved by slow rate freezing. 2x embryos thawed, ET in artificial frozen-embryo transfer cycle Outcome: Healthy male singleton, 2.580 g | Prasasth. et al., 2014 [42] |
| Case report | 23 years old patient 8 cm complex mass in the solitary left ovary Left salpingo-oophorectomy | Oocyte aspiration from a healthy piece of ovarian tissue (2 × 3 cm) 10x COCs identified 4x MII after 24 h OTO-IVM 4x 2PN after ICSI, 3x 2PN were cryopreserved by slow freezing 3x 2PN were thawed, DET (cleavage stage embryos) Outcome: Healthy male singleton, 3.883 g | Uzelac et al., 2015 [43] |
| Cohort study 1 | 12 patients (mean ± SD 29.8. ± 5.05 years range 23–36 years) Breast cancer (n = 9), Hodgkin lymphoma (n = 2), arterio-venous malformation of the uterus (n = 1) | COC identification via microscopic evaluation after tissue manipulation 157x COCs identified 51x MII after 28 h or 40 h OTO-IVM 24x MII vitrified 37x fresh OTO-IVM MII ICSI: 21x 2PN (56% 2PN rate) 15x embryos day 3 (good quality) vitrified Outcomes: 7x embryos warmed for 5 patients; 2 healthy female singletons (2.660 g and 3.860 g) for 2 patients 12x vitrification-warmed oocytes used for 2 patients; 1 healthy male singleton, 3.150 g | Segers et al., 2020 [44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Roo, C.; Tilleman, K. In Vitro Maturation of Oocytes Retrieved from Ovarian Tissue: Outcomes from Current Approaches and Future Perspectives. J. Clin. Med. 2021, 10, 4680. https://doi.org/10.3390/jcm10204680
De Roo C, Tilleman K. In Vitro Maturation of Oocytes Retrieved from Ovarian Tissue: Outcomes from Current Approaches and Future Perspectives. Journal of Clinical Medicine. 2021; 10(20):4680. https://doi.org/10.3390/jcm10204680
Chicago/Turabian StyleDe Roo, Chloë, and Kelly Tilleman. 2021. "In Vitro Maturation of Oocytes Retrieved from Ovarian Tissue: Outcomes from Current Approaches and Future Perspectives" Journal of Clinical Medicine 10, no. 20: 4680. https://doi.org/10.3390/jcm10204680
APA StyleDe Roo, C., & Tilleman, K. (2021). In Vitro Maturation of Oocytes Retrieved from Ovarian Tissue: Outcomes from Current Approaches and Future Perspectives. Journal of Clinical Medicine, 10(20), 4680. https://doi.org/10.3390/jcm10204680






