Female Oncofertility: Current Understandings, Therapeutic Approaches, Controversies, and Future Perspectives
Abstract
1. Introduction
2. Impact of Chemo- and Radio-Therapy on Follicle Quantity
2.1. Clinical Data Describing the Impact of Chemo- and Radio-Therapy on Ovarian Function
2.2. Mechanism of Chemo- and Radio-Therapy Induction of Follicular Loss
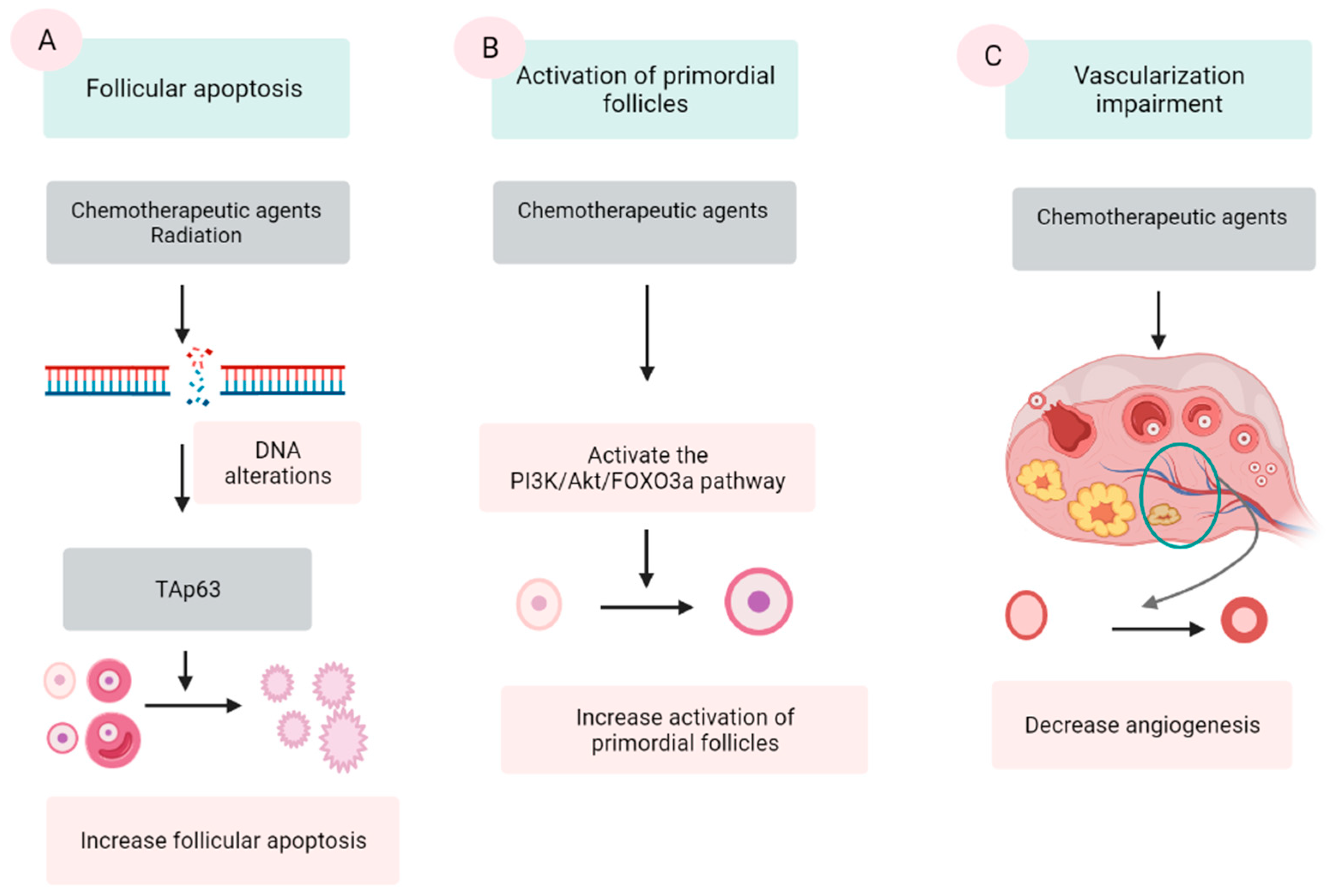
2.2.1. Follicular Apoptosis after Chemotherapy
2.2.2. Activation of PFs Induced by Chemotherapy
2.2.3. Vascularization Impairment
2.2.4. Radiation
3. Impact of Chemo- and Radio-Therapy on Follicle Quality
3.1. Studies Describing Impact of Chemo- and Radio-Therapy on Follicle Quality
3.2. The Mechanism of Chemo- and Radio-Therapy on Follicle Quality
4. The Therapeutic Options for FP
4.1. Oocyte and Embryo Cryopreservation
4.2. OTC
4.3. In Vitro Activation (IVA) of PFs
4.4. Other Experimental Options
5. Protective Approaches to Ovarian Reserve during Chemo- and Radio-Therapy
5.1. The Fertoprotective Agents Preventing Follicular Apoptosis
5.2. The Fertoprotective Agent Preventing Accelerated PFs’ Activation
5.3. Fertoprotective Agents Improving Vascularization
5.4. Ovarian Transposition during Radiotherapy
6. Future Perspectives of FP
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Trama, A.; Bernasconi, A.; McCabe, M.G.; Guevara, M.; Gatta, G.; Botta, L.; Ries, L.; Bleyer, A. Is the cancer survival improvement in European and American adolescent and young adults still lagging behind that in children? Pediatr. Blood Cancer 2019, 66, e27407. [Google Scholar] [CrossRef]
- Trama, A.; Botta, L.; Foschi, R.; Ferrari, A.; Stiller, C.; Desandes, E.; Maule, M.M.; Merletti, F.; Gatta, G. Survival of European adolescents and young adults diagnosed with cancer in 2000–07: Population-based data from EUROCARE-5. Lancet Oncol. 2016, 17, 896–906. [Google Scholar] [CrossRef]
- Anderson, R.A.; Brewster, D.H.; Wood, R.; Nowell, S.; Fischbacher, C.; Kelsey, T.W.; Wallace, W.H.B. The impact of cancer on subsequent chance of pregnancy: A population-based analysis. Hum. Reprod. 2018, 33, 1281–1290. [Google Scholar] [CrossRef]
- Chow, E.J.; Stratton, K.L.; Leisenring, W.M.; Oeffinger, K.C.; Sklar, C.A.; Donaldson, S.S.; Ginsberg, J.P.; Kenney, L.B.; Levine, J.M.; Robison, L.L.; et al. Pregnancy after chemotherapy in male and female survivors of childhood cancer treated between 1970 and 1999: A report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2016, 17, 567–576. [Google Scholar] [CrossRef]
- Overbeek, A.; van den Berg, M.H.; van Leeuwen, F.E.; Kaspers, G.J.; Lambalk, C.B.; van Dulmen-den Broeder, E. Chemotherapy-related late adverse effects on ovarian function in female survivors of childhood and young adult cancer: A systematic review. Cancer Treat. Rev. 2017, 53, 10–24. [Google Scholar] [CrossRef]
- Anderson, R.A.; Mitchell, R.T.; Kelsey, T.W.; Spears, N.; Telfer, E.E.; Wallace, W.H. Cancer treatment and gonadal function: Experimental and established strategies for fertility preservation in children and young adults. Lancet Diabetes Endocrinol. 2015, 3, 556–567. [Google Scholar] [CrossRef]
- Pampanini, V.; Hassan, J.; Oliver, E.; Stukenborg, J.B.; Damdimopoulou, P.; Jahnukainen, K. Fertility Preservation for Prepubertal Patients at Risk of Infertility: Present Status and Future Perspectives. Horm. Res. Paediatr. 2020, 93, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.; Anderson, R.A.; Gourley, C.; Wallace, W.H.; Spears, N. How do chemotherapeutic agents damage the ovary? Hum. Reprod. Update 2012, 18, 525–535. [Google Scholar] [CrossRef]
- Sonigo, C.; Beau, I.; Binart, N.; Grynberg, M. The Impact of Chemotherapy on the Ovaries: Molecular Aspects and the Prevention of Ovarian Damage. Int. J. Mol. Sci. 2019, 20, 5342. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, K.J.; Tan, X.; Oktay, K. Unraveling the mechanisms of chemotherapy-induced damage to human primordial follicle reserve: Road to developing therapeutics for fertility preservation and reversing ovarian aging. Mol. Hum. Reprod. 2020, 26, 553–566. [Google Scholar] [CrossRef]
- Yuksel, A.; Bildik, G.; Senbabaoglu, F.; Akin, N.; Arvas, M.; Unal, F.; Kilic, Y.; Karanfil, I.; Eryılmaz, B.; Yilmaz, P.; et al. The magnitude of gonadotoxicity of chemotherapy drugs on ovarian follicles and granulosa cells varies depending upon the category of the drugs and the type of granulosa cells. Hum. Reprod. 2015, 30, 2926–2935. [Google Scholar] [CrossRef]
- Pascuali, N.; Scotti, L.; Di Pietro, M.; Oubiña, G.; Bas, D.; May, M.; Muñoz, A.G.; Cuasnicú, P.S.; Cohen, D.J.; Tesone, M.; et al. Ceramide-1-phosphate has protective properties against cyclophosphamide-induced ovarian damage in a mice model of premature ovarian failure. Hum. Reprod. 2018, 33, 844–859. [Google Scholar] [CrossRef] [PubMed]
- Wallace, W.H.; Thomson, A.B.; Saran, F.; Kelsey, T.W. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int. J. Radiat Oncol. Biol. Phys. 2005, 62, 738–744. [Google Scholar] [CrossRef]
- Duffy, C.; Allen, S. Medical and psychosocial aspects of fertility after cancer. Cancer J. 2009, 15, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Niedzwiedz, C.L.; Knifton, L.; Robb, K.A.; Katikireddi, S.V.; Smith, D.J. Depression and anxiety among people living with and beyond cancer: A growing clinical and research priority. BMC Cancer 2019, 19, 943. [Google Scholar] [CrossRef] [PubMed]
- Howard-Anderson, J.; Ganz, P.A.; Bower, J.E.; Stanton, A.L. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: A systematic review. J. Natl. Cancer Inst. 2012, 104, 386–405. [Google Scholar] [CrossRef] [PubMed]
- Logan, S.; Perz, J.; Ussher, J.M.; Peate, M.; Anazodo, A. Systematic review of fertility-related psychological distress in cancer patients: Informing on an improved model of care. Psychooncology 2019, 28, 22–30. [Google Scholar] [CrossRef]
- Sun, B.; Yeh, J. Onco-fertility and personalized testing for potential for loss of ovarian reserve in patients undergoing chemotherapy: Proposed next steps for development of genetic testing to predict changes in ovarian reserve. Fertil. Res. Pract. 2021, 7, 13. [Google Scholar] [CrossRef]
- Berjeb, K.K.; Debbabi, L.; Braham, M.; Zemni, Z.; Chtourou, S.; Hannachi, H.; Hamdoun, M.; Ayadi, M.; Kacem, K.; Zhioua, F.; et al. Evaluation of ovarian reserve before and after chemotherapy. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102035. [Google Scholar] [CrossRef]
- Filippi, F.; Meazza, C.; Somigliana, E.; Podda, M.; Dallagiovanna, C.; Massimino, M.; Raspagliesi, F.; Terenziani, M. Fertility preservation in childhood and adolescent female tumor survivors. Fertil. Steril. 2021, 116, 1087–1095. [Google Scholar] [CrossRef]
- Gini, G.; Annibali, O.; Lupasco, D.; Bocci, C.; Tomarchio, V.; Sampaolo, M.; Trappolini, S.; Tafuri, M.A.; Cacciagiù, S.; Ciccarone, M.; et al. Gonadal Function Recovery and Fertility in Women Treated with Chemo- and/or Radiotherapy for Hodgkin’s and Non-Hodgkin Lymphoma. Chemotherapy 2019, 64, 36–41. [Google Scholar] [CrossRef]
- Lehmann, V.; Chemaitilly, W.; Lu, L.; Green, D.M.; Kutteh, W.H.; Brinkman, T.M.; Srivastava, D.K.; Robison, L.L.; Hudson, M.M.; Klosky, J.L. Gonadal Functioning and Perceptions of Infertility Risk Among Adult Survivors of Childhood Cancer: A Report From the St Jude Lifetime Cohort Study. J. Clin. Oncol. 2019, 37, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Shandley, L.M.; Fothergill, A.; Spencer, J.B.; Mertens, A.C.; Cottrell, H.N.; Howards, P.P. Impact of cancer treatment on risk of infertility and diminished ovarian reserve in women with polycystic ovary syndrome. Fertil. Steril. 2018, 109, 516–525.e511. [Google Scholar] [CrossRef]
- Sinha, N.; Letourneau, J.M.; Wald, K.; Xiong, P.; Imbar, T.; Li, B.; Harris, E.; Mok-Lin, E.; Cedars, M.I.; Rosen, M.P. Antral follicle count recovery in women with menses after treatment with and without gonadotropin-releasing hormone agonist use during chemotherapy for breast cancer. J. Assist. Reprod. Genet. 2018, 35, 1861–1868. [Google Scholar] [CrossRef]
- Al-Rawi, S.A.; Saleh, B.O.; Al-Naqqash, M.A. Serum anti-müllerian hormone levels in evaluation of chemotherapy effect on ovarian reserve in women with breast cancer. A follow-up study. Saudi Med. J. 2018, 39, 733–735. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; Remedios, R.; Kirkwood, A.A.; Patrick, P.; Stevens, L.; Clifton-Hadley, L.; Roberts, T.; Hatton, C.; Kalakonda, N.; Milligan, D.W.; et al. Determinants of ovarian function after response-adapted therapy in patients with advanced Hodgkin’s lymphoma (RATHL): A secondary analysis of a randomised phase 3 trial. Lancet Oncol. 2018, 19, 1328–1337. [Google Scholar] [CrossRef]
- Levine, J.M.; Whitton, J.A.; Ginsberg, J.P.; Green, D.M.; Leisenring, W.M.; Stovall, M.; Robison, L.L.; Armstrong, G.T.; Sklar, C.A. Nonsurgical premature menopause and reproductive implications in survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer 2018, 124, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Armuand, G.; Skoog-Svanberg, A.; Bladh, M.; Sydsjö, G. Reproductive Patterns Among Childhood and Adolescent Cancer Survivors in Sweden: A Population-Based Matched-Cohort Study. J. Clin. Oncol. 2017, 35, 1577–1583. [Google Scholar] [CrossRef]
- Chemaitilly, W.; Li, Z.; Krasin, M.J.; Brooke, R.J.; Wilson, C.L.; Green, D.M.; Klosky, J.L.; Barnes, N.; Clark, K.L.; Farr, J.B.; et al. Premature Ovarian Insufficiency in Childhood Cancer Survivors: A Report From the St. Jude Lifetime Cohort. J. Clin. Endocrinol. Metab. 2017, 102, 2242–2250. [Google Scholar] [CrossRef]
- D’Avila, Â.M.; Capp, E.; Corleta, H.V.E. Antral Follicles Count and Anti-Müllerian Hormone Levels after Gonadotoxic Chemotherapy in Patients with Breast Cancer: Cohort Study. Rev. Bras. Ginecol. Obstet. 2017, 39, 162–168. [Google Scholar] [CrossRef]
- Abir, R.; Ben-Aharon, I.; Garor, R.; Yaniv, I.; Ash, S.; Stemmer, S.M.; Ben-Haroush, A.; Freud, E.; Kravarusic, D.; Sapir, O.; et al. Cryopreservation of in vitro matured oocytes in addition to ovarian tissue freezing for fertility preservation in paediatric female cancer patients before and after cancer therapy. Hum. Reprod. 2016, 31, 750–762. [Google Scholar] [CrossRef] [PubMed]
- Hamy, A.S.; Porcher, R.; Eskenazi, S.; Cuvier, C.; Giacchetti, S.; Coussy, F.; Hocini, H.; Tournant, B.; Perret, F.; Bonfils, S.; et al. Anti-Müllerian hormone in breast cancer patients treated with chemotherapy: A retrospective evaluation of subsequent pregnancies. Reprod. Biomed. Online 2016, 32, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Even-Or, E.; Ben-Haroush, A.; Yahel, A.; Yaniv, I.; Stein, J. Fertility After Treatment With High Dose Melphalan in Women With Acute Myelogenous Leukemia. Pediatr. Blood Cancer 2016, 63, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.A.; Chong, A.L.; Deveault, C.; Traubici, J.; Maloney, A.M.; Knight, S.; Lorenzo, A.; Allen, L. Anti-Müllerian Hormone in Female Adolescent Cancer Patients Before, During, and After Completion of Therapy: A Pilot Feasibility Study. J. Pediatr. Adolesc. Gynecol. 2016, 29, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Thomas-Teinturier, C.; Allodji, R.S.; Svetlova, E.; Frey, M.A.; Oberlin, O.; Millischer, A.E.; Epelboin, S.; Decanter, C.; Pacquement, H.; Tabone, M.D.; et al. Ovarian reserve after treatment with alkylating agents during childhood. Hum. Reprod. 2015, 30, 1437–1446. [Google Scholar] [CrossRef]
- Behringer, K.; Thielen, I.; Mueller, H.; Goergen, H.; Eibl, A.D.; Rosenbrock, J.; Halbsguth, T.; Eichenauer, D.A.; Fuchs, M.; Reiners, K.S.; et al. Fertility and gonadal function in female survivors after treatment of early unfavorable Hodgkin lymphoma (HL) within the German Hodgkin Study Group HD14 trial. Ann. Oncol. 2012, 23, 1818–1825. [Google Scholar] [CrossRef]
- Green, D.M.; Kawashima, T.; Stovall, M.; Leisenring, W.; Sklar, C.A.; Mertens, A.C.; Donaldson, S.S.; Byrne, J.; Robison, L.L. Fertility of female survivors of childhood cancer: A report from the childhood cancer survivor study. J. Clin. Oncol. 2009, 27, 2677–2685. [Google Scholar] [CrossRef]
- Soleimani, R.; Heytens, E.; Darzynkiewicz, Z.; Oktay, K. Mechanisms of chemotherapy-induced human ovarian aging: Double strand DNA breaks and microvascular compromise. Aging 2011, 3, 782–793. [Google Scholar] [CrossRef]
- Li, F.; Turan, V.; Lierman, S.; Cuvelier, C.; De Sutter, P.; Oktay, K. Sphingosine-1-phosphate prevents chemotherapy-induced human primordial follicle death. Hum. Reprod. 2014, 29, 107–113. [Google Scholar] [CrossRef]
- Gonfloni, S.; Di Tella, L.; Caldarola, S.; Cannata, S.M.; Klinger, F.G.; Di Bartolomeo, C.; Mattei, M.; Candi, E.; De Felici, M.; Melino, G.; et al. Inhibition of the c-Abl-TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nat. Med. 2009, 15, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Bedoschi, G.; Navarro, P.A.; Oktay, K. Chemotherapy-induced damage to ovary: Mechanisms and clinical impact. Future Oncol. 2016, 12, 2333–2344. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.M. Fertility Preservation in Women. N. Engl. J. Med. 2017, 377, 1657–1665. [Google Scholar] [CrossRef]
- Lambertini, M.; Olympios, N.; Lequesne, J.; Calbrix, C.; Fontanilles, M.; Loeb, A.; Leheurteur, M.; Demeestere, I.; Di Fiore, F.; Perdrix, A.; et al. Impact of Taxanes, Endocrine Therapy, and Deleterious Germline BRCA Mutations on Anti-müllerian Hormone Levels in Early Breast Cancer Patients Treated With Anthracycline- and Cyclophosphamide-Based Chemotherapy. Front. Oncol. 2019, 9, 575. [Google Scholar] [CrossRef] [PubMed]
- Dumontet, C.; Jordan, M.A. Microtubule-binding agents: A dynamic field of cancer therapeutics. Nat. Rev. Drug Discov. 2010, 9, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Edmonds, M.E.; Woodruff, T.K.; Kim, S.Y. Inhibitors of apoptosis protect the ovarian reserve from cyclophosphamide. J. Endocrinol. 2019, 240, 243–256. [Google Scholar] [CrossRef]
- Jeelani, R.; Khan, S.N.; Shaeib, F.; Kohan-Ghadr, H.R.; Aldhaheri, S.R.; Najafi, T.; Thakur, M.; Morris, R.; Abu-Soud, H.M. Cyclophosphamide and acrolein induced oxidative stress leading to deterioration of metaphase II mouse oocyte quality. Free Radic. Biol. Med. 2017, 110, 11–18. [Google Scholar] [CrossRef]
- Kerr, J.B.; Hutt, K.J.; Michalak, E.M.; Cook, M.; Vandenberg, C.J.; Liew, S.H.; Bouillet, P.; Mills, A.; Scott, C.L.; Findlay, J.K.; et al. DNA damage-induced primordial follicle oocyte apoptosis and loss of fertility require TAp63-mediated induction of Puma and Noxa. Mol. Cell 2012, 48, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Winship, A.L.; Stringer, J.M.; Liew, S.H.; Hutt, K.J. The importance of DNA repair for maintaining oocyte quality in response to anti-cancer treatments, environmental toxins and maternal ageing. Hum. Reprod. Update 2018, 24, 119–134. [Google Scholar] [CrossRef]
- Nguyen, Q.N.; Zerafa, N.; Liew, S.H.; Findlay, J.K.; Hickey, M.; Hutt, K.J. Cisplatin- and cyclophosphamide-induced primordial follicle depletion is caused by direct damage to oocytes. Mol. Hum. Reprod. 2019, 25, 433–444. [Google Scholar] [CrossRef]
- Nguyen, Q.N.; Zerafa, N.; Liew, S.H.; Morgan, F.H.; Strasser, A.; Scott, C.L.; Findlay, J.K.; Hickey, M.; Hutt, K.J. Loss of PUMA protects the ovarian reserve during DNA-damaging chemotherapy and preserves fertility. Cell Death Dis. 2018, 9, 618. [Google Scholar] [CrossRef]
- Oktem, O.; Oktay, K. A novel ovarian xenografting model to characterize the impact of chemotherapy agents on human primordial follicle reserve. Cancer Res. 2007, 67, 10159–10162. [Google Scholar] [CrossRef]
- Bildik, G.; Acılan, C.; Sahin, G.N.; Karahuseyinoglu, S.; Oktem, O. C-Abl is not actıvated in DNA damage-induced and Tap63-mediated oocyte apoptosıs in human ovary. Cell Death Dis. 2018, 9, 943. [Google Scholar] [CrossRef] [PubMed]
- Kalich-Philosoph, L.; Roness, H.; Carmely, A.; Fishel-Bartal, M.; Ligumsky, H.; Paglin, S.; Wolf, I.; Kanety, H.; Sredni, B.; Meirow, D. Cyclophosphamide triggers follicle activation and “burnout”; AS101 prevents follicle loss and preserves fertility. Sci. Transl. Med. 2013, 5, 185ra162. [Google Scholar] [CrossRef]
- Xie, Y.; Li, S.; Zhou, L.; Lin, H.; Jiao, X.; Qiu, Q.; Liang, Y.; Zhang, Q. Rapamycin preserves the primordial follicle pool during cisplatin treatment in vitro and in vivo. Mol. Reprod. Dev. 2020, 87, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Rossi, V.; Lispi, M.; Longobardi, S.; Mattei, M.; Di Rella, F.; Salustri, A.; De Felici, M.; Klinger, F.G. LH prevents cisplatin-induced apoptosis in oocytes and preserves female fertility in mouse. Cell Death Differ. 2017, 24, 72–82. [Google Scholar] [CrossRef]
- Allen, C.M.; Lopes, F.; Mitchell, R.T.; Spears, N. Comparative gonadotoxicity of the chemotherapy drugs cisplatin and carboplatin on prepubertal mouse gonads. Mol. Hum. Reprod. 2020, 26, 129–140. [Google Scholar] [CrossRef]
- Morgan, S.; Lopes, F.; Gourley, C.; Anderson, R.A.; Spears, N. Cisplatin and doxorubicin induce distinct mechanisms of ovarian follicle loss; imatinib provides selective protection only against cisplatin. PLoS ONE 2013, 8, e70117. [Google Scholar] [CrossRef]
- Chang, E.M.; Lim, E.; Yoon, S.; Jeong, K.; Bae, S.; Lee, D.R.; Yoon, T.K.; Choi, Y.; Lee, W.S. Cisplatin Induces Overactivation of the Dormant Primordial Follicle through PTEN/AKT/FOXO3a Pathway which Leads to Loss of Ovarian Reserve in Mice. PLoS ONE 2015, 10, e0144245. [Google Scholar] [CrossRef]
- Goldman, K.N.; Chenette, D.; Arju, R.; Duncan, F.E.; Keefe, D.L.; Grifo, J.A.; Schneider, R.J. mTORC1/2 inhibition preserves ovarian function and fertility during genotoxic chemotherapy. Proc. Natl. Acad. Sci. USA 2017, 114, 3186–3191. [Google Scholar] [CrossRef]
- Lande, Y.; Fisch, B.; Tsur, A.; Farhi, J.; Prag-Rosenberg, R.; Ben-Haroush, A.; Kessler-Icekson, G.; Zahalka, M.A.; Ludeman, S.M.; Abir, R. Short-term exposure of human ovarian follicles to cyclophosphamide metabolites seems to promote follicular activation in vitro. Reprod. Biomed. Online 2017, 34, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Xia, H.X.; Guan, H.Y.; Li, B.; Zhang, W. Follicle Loss and Apoptosis in Cyclophosphamide-Treated Mice: What’s the Matter? Int. J. Mol. Sci. 2016, 17, 836. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, M.; Johnson, S.B.; Yuan, G.; Arriba, A.K.; Zubizarreta, M.E.; Chatterjee, S.; Nagarkatti, M.; Nagarkatti, P.; Xiao, S. Doxorubicin obliterates mouse ovarian reserve through both primordial follicle atresia and overactivation. Toxicol. Appl. Pharmacol. 2019, 381, 114714. [Google Scholar] [CrossRef] [PubMed]
- Shai, D.; Aviel-Ronen, S.; Spector, I.; Raanani, H.; Shapira, M.; Gat, I.; Roness, H.; Meirow, D. Ovaries of patients recently treated with alkylating agent chemotherapy indicate the presence of acute follicle activation, elucidating its role among other proposed mechanisms of follicle loss. Fertil. Steril. 2021, 115, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xie, Y.; Li, S.; Liang, Y.; Qiu, Q.; Lin, H.; Zhang, Q. Rapamycin Prevents cyclophosphamide-induced Over-activation of Primordial Follicle pool through PI3K/Akt/mTOR Signaling Pathway in vivo. J. Ovarian Res. 2017, 10, 56. [Google Scholar] [CrossRef]
- Roness, H.; Kashi, O.; Meirow, D. Prevention of chemotherapy-induced ovarian damage. Fertil. Steril. 2016, 105, 20–29. [Google Scholar] [CrossRef]
- Jang, H.; Hong, K.; Choi, Y. Melatonin and Fertoprotective Adjuvants: Prevention against Premature Ovarian Failure during Chemotherapy. Int. J. Mol. Sci. 2017, 18, 1221. [Google Scholar] [CrossRef]
- Roness, H.; Spector, I.; Leichtmann-Bardoogo, Y.; Savino, A.M.; Dereh-Haim, S.; Meirow, D. Pharmacological administration of recombinant human AMH rescues ovarian reserve and preserves fertility in a mouse model of chemotherapy, without interfering with anti-tumoural effects. J. Assist. Reprod. Genet. 2019, 36, 1793–1803. [Google Scholar] [CrossRef]
- Titus, S.; Szymanska, K.J.; Musul, B.; Turan, V.; Taylan, E.; Garcia-Milian, R.; Mehta, S.; Oktay, K. Individual-oocyte transcriptomic analysis shows that genotoxic chemotherapy depletes human primordial follicle reserve in vivo by triggering proapoptotic pathways without growth activation. Sci. Rep. 2021, 11, 407. [Google Scholar] [CrossRef]
- Meirow, D.; Epstein, M.; Lewis, H.; Nugent, D.; Gosden, R.G. Administration of cyclophosphamide at different stages of follicular maturation in mice: Effects on reproductive performance and fetal malformations. Hum. Reprod. 2001, 16, 632–637. [Google Scholar] [CrossRef]
- Meirow, D.; Nugent, D. The effects of radiotherapy and chemotherapy on female reproduction. Hum. Reprod. Update 2001, 7, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Yin, N.; Zhang, L.; Yuan, W.; Zhao, W.; Luan, X.; Zhang, H. Role of SDF-1/CXCR4 and cytokines in the development of ovary injury in chemotherapy drug induced premature ovarian failure mice. Life Sci. 2017, 179, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Meirow, D.; Dor, J.; Kaufman, B.; Shrim, A.; Rabinovici, J.; Schiff, E.; Raanani, H.; Levron, J.; Fridman, E. Cortical fibrosis and blood-vessels damage in human ovaries exposed to chemotherapy. Potential mechanisms of ovarian injury. Hum. Reprod. 2007, 22, 1626–1633. [Google Scholar] [CrossRef] [PubMed]
- Bar-Joseph, H.; Ben-Aharon, I.; Tzabari, M.; Tsarfaty, G.; Stemmer, S.M.; Shalgi, R. In vivo bioimaging as a novel strategy to detect doxorubicin-induced damage to gonadal blood vessels. PLoS ONE 2011, 6, e23492. [Google Scholar] [CrossRef]
- Oktem, O.; Oktay, K. Quantitative assessment of the impact of chemotherapy on ovarian follicle reserve and stromal function. Cancer 2007, 110, 2222–2229. [Google Scholar] [CrossRef]
- Wallace, W.H.; Thomson, A.B.; Kelsey, T.W. The radiosensitivity of the human oocyte. Hum. Reprod. 2003, 18, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Meirow, D.; Biederman, H.; Anderson, R.A.; Wallace, W.H. Toxicity of chemotherapy and radiation on female reproduction. Clin. Obstet. Gynecol. 2010, 53, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Wo, J.Y.; Viswanathan, A.N. Impact of radiotherapy on fertility, pregnancy, and neonatal outcomes in female cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, J.P. New advances in fertility preservation for pediatric cancer patients. Curr. Opin. Pediatr. 2011, 23, 9–13. [Google Scholar] [CrossRef]
- Dolmans, M.M.; von Wolff, M.; Poirot, C.; Diaz-Garcia, C.; Cacciottola, L.; Boissel, N.; Liebenthron, J.; Pellicer, A.; Donnez, J.; Andersen, C.Y. Transplantation of cryopreserved ovarian tissue in a series of 285 women: A review of five leading European centers. Fertil. Steril. 2021, 115, 1102–1115. [Google Scholar] [CrossRef]
- Yasmin, E.; Mitchell, R.; Lane, S. Preservation of fertility in teenagers and young adults treated for haematological malignancies. Lancet Haematol. 2021, 8, e149–e160. [Google Scholar] [CrossRef]
- Abir, R.; Ben-Haroush, A.; Felz, C.; Okon, E.; Raanani, H.; Orvieto, R.; Nitke, S.; Fisch, B. Selection of patients before and after anticancer treatment for ovarian cryopreservation. Hum. Reprod. 2008, 23, 869–877. [Google Scholar] [CrossRef]
- Pligina, K.L.; Zhanataev, A.K.; Kulakova, A.V.; Daugel-Dauge, N.O.; Durnev, A.D. Induced Aneugenic Effects in Mouse Oocytes In Vivo. Bull. Exp. Biol. Med. 2017, 163, 425–429. [Google Scholar] [CrossRef]
- Fabbri, R.; Vicenti, R.; Macciocca, M.; Pasquinelli, G.; Lima, M.; Parazza, I.; Magnani, V.; Venturoli, S. Cryopreservation of ovarian tissue in pediatric patients. Obstet. Gynecol. Int. 2012, 2012, 910698. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, C.; Ferrante, M.G.; Meneghini, C.; Licata, E.; Paciotti, G.; Gallo, M.; Schiavi, M.; Spina, V.; Guarino, A.; Caserta, D.; et al. Female fertility preservation: Impact of cancer on ovarian function and oocyte quality. Int. J. Gynaecol. Obstet. 2021. [Google Scholar] [CrossRef]
- Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. Mature oocyte cryopreservation: A guideline. Fertil. Steril. 2013, 99, 37–43. [Google Scholar] [CrossRef]
- Anderson, R.A.; Amant, F.; Braat, D.; D’Angelo, A.; de Sousa Lopes, S.M.C.; Demeestere, I.; Dwek, S.; Frith, L.; Lambertini, M.; Maslin, C.; et al. ESHRE guideline: Female fertility preservation. Hum. Reprod. Open 2020, 2020, hoaa052. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.R.; Choi, Y.S.; Jee, B.C.; Ku, S.Y.; Suh, C.S.; Kim, K.C.; Lee, W.D.; Kim, S.H. Cryopreserved blastocyst transfer: Impact of gonadotropin-releasing hormone agonist versus antagonist in the previous oocyte retrieval cycles. Fertil. Steril. 2007, 88, 1344–1349. [Google Scholar] [CrossRef]
- Eftekhar, M.; Firouzabadi, R.D.; Karimi, H.; Rahmani, E. Outcome of cryopreserved-thawed embryo transfer in the GnRH agonist versus antagonist protocol. Iran. J. Reprod. Med. 2012, 10, 297–302. [Google Scholar]
- Checa, M.A.; Brassesco, M.; Sastre, M.; Gómez, M.; Herrero, J.; Marque, L.; Brassesco, A.; Espinós, J.J. Random-start GnRH antagonist for emergency fertility preservation: A self-controlled trial. Int. J. Womens Health 2015, 7, 219–225. [Google Scholar] [CrossRef][Green Version]
- Marklund, A.; Eloranta, S.; Wikander, I.; Kitlinski, M.L.; Lood, M.; Nedstrand, E.; Thurin-Kjellberg, A.; Zhang, P.; Bergh, J.; Rodriguez-Wallberg, K.A. Efficacy and safety of controlled ovarian stimulation using GnRH antagonist protocols for emergency fertility preservation in young women with breast cancer-a prospective nationwide Swedish multicenter study. Hum. Reprod. 2020, 35, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, R.J.; Reid, G.D.; Koch, J.; Deans, R.; Ledger, W.L.; Friedlander, M.; Gilchrist, R.B.; Walters, K.A.; Abbott, J.A. The safety and efficacy of controlled ovarian hyperstimulation for fertility preservation in women with early breast cancer: A systematic review. Hum. Reprod. 2017, 32, 1033–1045. [Google Scholar] [CrossRef]
- Rienzi, L.; Gracia, C.; Maggiulli, R.; LaBarbera, A.R.; Kaser, D.J.; Ubaldi, F.M.; Vanderpoel, S.; Racowsky, C. Oocyte, embryo and blastocyst cryopreservation in ART: Systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum. Reprod. Update 2017, 23, 139–155. [Google Scholar] [CrossRef]
- AbdelHafez, F.F.; Desai, N.; Abou-Setta, A.M.; Falcone, T.; Goldfarb, J. Slow freezing, vitrification and ultra-rapid freezing of human embryos: A systematic review and meta-analysis. Reprod. Biomed. Online 2010, 20, 209–222. [Google Scholar] [CrossRef]
- Debrock, S.; Peeraer, K.; Gallardo, E.F.; De Neubourg, D.; Spiessens, C.; D’Hooghe, T.M. Vitrification of cleavage stage day 3 embryos results in higher live birth rates than conventional slow freezing: A RCT. Hum. Reprod. 2015, 30, 1820–1830. [Google Scholar] [CrossRef] [PubMed]
- Alexander, V.M.; Martin, C.E.; Schelble, A.P.; Laufer, A.B.; Hardi, A.; McKenzie, L.J.; Hipp, H.S.; Kawwass, J.F.; Spencer, J.B.; Jungheim, E.S. Ovarian stimulation for fertility preservation in women with cancer: A systematic review and meta-analysis comparing random and conventional starts. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102080. [Google Scholar] [CrossRef]
- Bonardi, B.; Massarotti, C.; Bruzzone, M.; Goldrat, O.; Mangili, G.; Anserini, P.; Spinaci, S.; Arecco, L.; Del Mastro, L.; Ceppi, M.; et al. Efficacy and Safety of Controlled Ovarian Stimulation With or Without Letrozole Co-administration for Fertility Preservation: A Systematic Review and Meta-Analysis. Front. Oncol. 2020, 10, 574669. [Google Scholar] [CrossRef]
- Kim, J.; Turan, V.; Oktay, K. Long-Term Safety of Letrozole and Gonadotropin Stimulation for Fertility Preservation in Women With Breast Cancer. J. Clin. Endocrinol. Metab. 2016, 101, 1364–1371. [Google Scholar] [CrossRef]
- Maman, E.; Meirow, D.; Brengauz, M.; Raanani, H.; Dor, J.; Hourvitz, A. Luteal phase oocyte retrieval and in vitro maturation is an optional procedure for urgent fertility preservation. Fertil. Steril. 2011, 95, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Chian, R.C.; Uzelac, P.S.; Nargund, G. In vitro maturation of human immature oocytes for fertility preservation. Fertil. Steril. 2013, 99, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, J.K.; Trawick, E.C.; Grifo, J.A.; Goldman, K.N. Prognostic role of preimplantation genetic testing for aneuploidy in medically indicated fertility preservation. Fertil. Steril. 2020, 113, 408–416. [Google Scholar] [CrossRef]
- Sciorio, R.; Anderson, R.A. Fertility preservation and preimplantation genetic assessment for women with breast cancer. Cryobiology 2020, 92, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ghunaim, S.; Ghazeeri, G.; Khalife, D.; Azim, H.A., Jr. Fertility preservation in patients with BRCA mutation. Ecancermedicalscience 2020, 14, 1033. [Google Scholar] [CrossRef] [PubMed]
- Melnick, A.P.; Rosenwaks, Z. Oocyte donation: Insights gleaned and future challenges. Fertil. Steril. 2018, 110, 988–993. [Google Scholar] [CrossRef]
- Diaz-Garcia, C.; Domingo, J.; Garcia-Velasco, J.A.; Herraiz, S.; Mirabet, V.; Iniesta, I.; Cobo, A.; Remohí, J.; Pellicer, A. Oocyte vitrification versus ovarian cortex transplantation in fertility preservation for adult women undergoing gonadotoxic treatments: A prospective cohort study. Fertil. Steril. 2018, 109, 478–485.e472. [Google Scholar] [CrossRef]
- Cobo, A.; García-Velasco, J.; Domingo, J.; Pellicer, A.; Remohí, J. Elective and Onco-fertility preservation: Factors related to IVF outcomes. Hum. Reprod. 2018, 33, 2222–2231. [Google Scholar] [CrossRef]
- Perachino, M.; Massarotti, C.; Razeti, M.G.; Parisi, F.; Arecco, L.; Damassi, A.; Fregatti, P.; Solinas, C.; Lambertini, M. Gender-specific aspects related to type of fertility preservation strategies and access to fertility care. ESMO Open 2020, 5, e000771. [Google Scholar] [CrossRef]
- Cobo, A.; García-Velasco, J.A.; Remohí, J.; Pellicer, A. Oocyte vitrification for fertility preservation for both medical and nonmedical reasons. Fertil. Steril. 2021, 115, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Moravek, M.B.; Confino, R.; Lawson, A.K.; Smith, K.N.; Kazer, R.R.; Klock, S.C.; Gradishar, W.J.; Jeruss, J.S.; Pavone, M.E. Predictors and outcomes in breast cancer patients who did or did not pursue fertility preservation. Breast Cancer Res. Treat. 2021, 186, 429–437. [Google Scholar] [CrossRef]
- Oktay, K.; Turan, V.; Bedoschi, G.; Pacheco, F.S.; Moy, F. Fertility Preservation Success Subsequent to Concurrent Aromatase Inhibitor Treatment and Ovarian Stimulation in Women With Breast Cancer. J. Clin. Oncol. 2015, 33, 2424–2429. [Google Scholar] [CrossRef]
- Lawrenz, B.; Jauckus, J.; Kupka, M.; Strowitzki, T.; von Wolff, M. Efficacy and safety of ovarian stimulation before chemotherapy in 205 cases. Fertil. Steril. 2010, 94, 2871–2873. [Google Scholar] [CrossRef]
- Villarreal-Garza, C.; Mesa-Chavez, F.; de la Mora, A.P.; Miaja-Avila, M.; Garcia-Garcia, M.; Fonseca, A.; de la Rosa-Pacheco, S.; Cruz-Ramos, M.; Garza, M.R.G.; Mohar, A.; et al. Prospective Study of Fertility Preservation in Young Women with Breast Cancer in Mexico. J. Natl. Compr. Cancer Netw. 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, S.; Cervelló, I. New insights for fertility preservation by ovarian tissue cryopreservation and transplantation in pediatric cancer patients. Fertil. Steril. 2020, 114, 1191. [Google Scholar] [CrossRef]
- Corkum, K.S.; Rhee, D.S.; Wafford, Q.E.; Demeestere, I.; Dasgupta, R.; Baertschiger, R.; Malek, M.M.; Aldrink, J.H.; Heaton, T.E.; Weil, B.R.; et al. Fertility and hormone preservation and restoration for female children and adolescents receiving gonadotoxic cancer treatments: A systematic review. J. Pediatr. Surg. 2019, 54, 2200–2209. [Google Scholar] [CrossRef]
- Wallace, W.H.; Kelsey, T.W.; Anderson, R.A. Fertility preservation in pre-pubertal girls with cancer: The role of ovarian tissue cryopreservation. Fertil. Steril. 2016, 105, 6–12. [Google Scholar] [CrossRef]
- Ovarian tissue cryopreservation: A committee opinion. Fertil. Steril. 2014, 101, 1237–1243. [CrossRef] [PubMed]
- Oktay, K.; Harvey, B.E.; Loren, A.W. Fertility Preservation in Patients With Cancer: ASCO Clinical Practice Guideline Update Summary. J. Oncol. Pract. 2018, 14, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Revel, A.; Revel-Vilk, S.; Aizenman, E.; Porat-Katz, A.; Safran, A.; Ben-Meir, A.; Weintraub, M.; Shapira, M.; Achache, H.; Laufer, N. At what age can human oocytes be obtained? Fertil. Steril. 2009, 92, 458–463. [Google Scholar] [CrossRef]
- Feigin, E.; Abir, R.; Fisch, B.; Kravarusic, D.; Steinberg, R.; Nitke, S.; Avrahami, G.; Ben-Haroush, A.; Freud, E. Laparoscopic ovarian tissue preservation in young patients at risk for ovarian failure as a result of chemotherapy/irradiation for primary malignancy. J. Pediatr. Surg. 2007, 42, 862–864. [Google Scholar] [CrossRef]
- Michalczyk, K.; Cymbaluk-Płoska, A. Fertility Preservation and Long-Term Monitoring of Gonadotoxicity in Girls, Adolescents and Young Adults Undergoing Cancer Treatment. Cancers 2021, 13, 202. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.M.; Demylle, D.; Jadoul, P.; Pirard, C.; Squifflet, J.; Martinez-Madrid, B.; van Langendonckt, A. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet 2004, 364, 1405–1410. [Google Scholar] [CrossRef]
- Gellert, S.E.; Pors, S.E.; Kristensen, S.G.; Bay-Bjørn, A.M.; Ernst, E.; Andersen, C.Y. Transplantation of frozen-thawed ovarian tissue: An update on worldwide activity published in peer-reviewed papers and on the Danish cohort. J. Assist. Reprod. Genet. 2018, 35, 561–570. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.M.; Pellicer, A.; Diaz-Garcia, C.; Serrano, M.S.; Schmidt, K.T.; Ernst, E.; Luyckx, V.; Andersen, C.Y. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: A review of 60 cases of reimplantation. Fertil. Steril. 2013, 99, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Dolmans, M.M.; Diaz, C.; Pellicer, A. Ovarian cortex transplantation: Time to move on from experimental studies to open clinical application. Fertil. Steril. 2015, 104, 1097–1098. [Google Scholar] [CrossRef] [PubMed]
- Poirot, C.; Brugieres, L.; Yakouben, K.; Prades-Borio, M.; Marzouk, F.; de Lambert, G.; Pacquement, H.; Bernaudin, F.; Neven, B.; Paye-Jaouen, A.; et al. Ovarian tissue cryopreservation for fertility preservation in 418 girls and adolescents up to 15 years of age facing highly gonadotoxic treatment. Twenty years of experience at a single center. Acta Obstet. Gynecol. Scand. 2019, 98, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Demeestere, I.; Simon, P.; Dedeken, L.; Moffa, F.; Tsépélidis, S.; Brachet, C.; Delbaere, A.; Devreker, F.; Ferster, A. Live birth after autograft of ovarian tissue cryopreserved during childhood. Hum. Reprod. 2015, 30, 2107–2109. [Google Scholar] [CrossRef] [PubMed]
- Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: A committee opinion. Fertil. Steril. 2019, 112, 1022–1033. [CrossRef] [PubMed]
- Martinez, F. Update on fertility preservation from the Barcelona International Society for Fertility Preservation-ESHRE-ASRM 2015 expert meeting: Indications, results and future perspectives. Hum. Reprod. 2017, 32, 1802–1811. [Google Scholar] [CrossRef]
- Sheshpari, S.; Shahnazi, M.; Mobarak, H.; Ahmadian, S.; Bedate, A.M.; Nariman-Saleh-Fam, Z.; Nouri, M.; Rahbarghazi, R.; Mahdipour, M. Ovarian function and reproductive outcome after ovarian tissue transplantation: A systematic review. J. Transl. Med. 2019, 17, 396. [Google Scholar] [CrossRef]
- Silber, S. Ovarian tissue cryopreservation and transplantation: Scientific implications. J. Assist. Reprod. Genet. 2016, 33, 1595–1603. [Google Scholar] [CrossRef]
- Herraiz, S.; Novella-Maestre, E.; Rodríguez, B.; Díaz, C.; Sánchez-Serrano, M.; Mirabet, V.; Pellicer, A. Improving ovarian tissue cryopreservation for oncologic patients: Slow freezing versus vitrification, effect of different procedures and devices. Fertil. Steril. 2014, 101, 775–784. [Google Scholar] [CrossRef]
- Shi, Q.; Xie, Y.; Wang, Y.; Li, S. Vitrification versus slow freezing for human ovarian tissue cryopreservation: A systematic review and meta-anlaysis. Sci. Rep. 2017, 7, 8538. [Google Scholar] [CrossRef]
- Lee, S.; Ryu, K.J.; Kim, B.; Kang, D.; Kim, Y.Y.; Kim, T. Comparison between Slow Freezing and Vitrification for Human Ovarian Tissue Cryopreservation and Xenotransplantation. Int. J. Mol. Sci 2019, 20, 3346. [Google Scholar] [CrossRef]
- Fabbri, R.; Vicenti, R.; Macciocca, M.; Martino, N.A.; Dell’Aquila, M.E.; Pasquinelli, G.; Morselli-Labate, A.M.; Seracchioli, R.; Paradisi, R. Morphological, ultrastructural and functional imaging of frozen/thawed and vitrified/warmed human ovarian tissue retrieved from oncological patients. Hum. Reprod. 2016, 31, 1838–1849. [Google Scholar] [CrossRef]
- Wang, T.R.; Yan, J.; Lu, C.L.; Xia, X.; Yin, T.L.; Zhi, X.; Zhu, X.H.; Ding, T.; Hu, W.H.; Guo, H.Y.; et al. Human single follicle growth in vitro from cryopreserved ovarian tissue after slow freezing or vitrification. Hum. Reprod. 2016, 31, 763–773. [Google Scholar] [CrossRef]
- Dalman, A.; Farahani, N.S.D.G.; Totonchi, M.; Pirjani, R.; Ebrahimi, B.; Rezazadeh Valojerdi, M. Slow freezing versus vitrification technique for human ovarian tissue cryopreservation: An evaluation of histological changes, WNT signaling pathway and apoptotic genes expression. Cryobiology 2017, 79, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Hourvitz, A.; Yerushalmi, G.M.; Maman, E.; Raanani, H.; Elizur, S.; Brengauz, M.; Orvieto, R.; Dor, J.; Meirow, D. Combination of ovarian tissue harvesting and immature oocyte collection for fertility preservation increases preservation yield. Reprod. Biomed. Online 2015, 31, 497–505. [Google Scholar] [CrossRef]
- Delattre, S.; Segers, I.; Van Moer, E.; Drakopoulos, P.; Mateizel, I.; Enghels, L.; Tournaye, H.; De Vos, M. Combining fertility preservation procedures to spread the eggs across different baskets: A feasibility study. Hum. Reprod. 2020, 35, 2524–2536. [Google Scholar] [CrossRef] [PubMed]
- Vuong, L.N.; Ho, V.N.A.; Ho, T.M.; Dang, V.Q.; Phung, T.H.; Giang, N.H.; Le, A.H.; Pham, T.D.; Wang, R.; Smitz, J.; et al. In-vitro maturation of oocytes versus conventional IVF in women with infertility and a high antral follicle count: A randomized non-inferiority controlled trial. Hum. Reprod. 2020, 35, 2537–2547. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Cheng, Y.; Kawamura, K.; Takae, S.; Hsueh, A.J. C-type natriuretic peptide stimulates ovarian follicle development. Mol. Endocrinol. 2012, 26, 1158–1166. [Google Scholar] [CrossRef]
- Kawamura, K.; Cheng, Y.; Kawamura, N.; Takae, S.; Okada, A.; Kawagoe, Y.; Mulders, S.; Terada, Y.; Hsueh, A.J. Pre-ovulatory LH/hCG surge decreases C-type natriuretic peptide secretion by ovarian granulosa cells to promote meiotic resumption of pre-ovulatory oocytes. Hum. Reprod. 2011, 26, 3094–3101. [Google Scholar] [CrossRef] [PubMed]
- Soto-Heras, S.; Menéndez-Blanco, I.; Catalá, M.G.; Izquierdo, D.; Thompson, J.G.; Paramio, M.T. Biphasic in vitro maturation with C-type natriuretic peptide enhances the developmental competence of juvenile-goat oocytes. PLoS ONE 2019, 14, e0221663. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, Q.; Cai, J.; Zhao, X.; Ma, B. Effect of C-Type Natriuretic Peptide on Maturation and Developmental Competence of Goat Oocytes Matured In Vitro. PLoS ONE 2015, 10, e0132318. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Yang, X.; Liu, K. Treatment of cattle oocytes with C-type natriuretic peptide before in vitro maturation enhances oocyte mitochondrial function. Anim. Reprod. Sci. 2021, 225, 106685. [Google Scholar] [CrossRef]
- Zhenwei, J.; Xianhua, Z. Pre-IVM treatment with C-type natriuretic peptide in the presence of cysteamine enhances bovine oocytes antioxidant defense ability and developmental competence in vitro. Iran. J. Vet. Res. 2019, 20, 173–179. [Google Scholar]
- Zhao, Y.; Liao, X.; Krysta, A.E.; Bertoldo, M.J.; Richani, D.; Gilchrist, R.B. Capacitation IVM improves cumulus function and oocyte quality in minimally stimulated mice. J. Assist. Reprod. Genet. 2020, 37, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Vuong, L.N.; Le, A.H.; Ho, V.N.A.; Pham, T.D.; Sanchez, F.; Romero, S.; De Vos, M.; Ho, T.M.; Gilchrist, R.B.; Smitz, J. Live births after oocyte in vitro maturation with a prematuration step in women with polycystic ovary syndrome. J. Assist. Reprod. Genet. 2020, 37, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Plancha, C.E.; Rodrigues, P.; Marques, M.; Almeida, J.M.; Navarro-Costa, P. The time is ripe for oocyte in vitro maturation. J. Assist. Reprod. Genet. 2021, 38, 1281–1283. [Google Scholar] [CrossRef]
- Richani, D.; Wang, X.; Zeng, H.T.; Smitz, J.; Thompson, J.G.; Gilchrist, R.B. Pre-maturation with cAMP modulators in conjunction with EGF-like peptides during in vitro maturation enhances mouse oocyte developmental competence. Mol. Reprod. Dev. 2014, 81, 422–435. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Nagano, M.; Kang, S.S.; Yanagawa, Y.; Takahashi, Y. Prematurational culture with 3-isobutyl-1-methylxanthine synchronizes meiotic progression of the germinal vesicle stage and improves nuclear maturation and embryonic development in in vitro-grown bovine oocytes. J. Reprod. Dev. 2014, 60, 9–13. [Google Scholar] [CrossRef]
- Van, N.T.T.; My, L.B.A.; Van Thuan, N.; Bui, H.T. Improve the developmental competence of porcine oocytes from small antral follicles by pre-maturation culture method. Theriogenology 2020, 149, 139–148. [Google Scholar] [CrossRef]
- Fisch, B.; Abir, R. Female fertility preservation: Past, present and future. Reproduction 2018, 156, F11–F27. [Google Scholar] [CrossRef]
- Vilela, J.M.V.; Dolmans, M.M.; Amorim, C.A. Ovarian tissue transportation: A systematic review. Reprod. Biomed. Online 2021, 42, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Roness, H.; Meirow, D. FERTILITY PRESERVATION: Follicle reserve loss in ovarian tissue transplantation. Reproduction 2019, 158, F35–F44. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Huang, Y.; Li, M.; Zhao, H.; Zhao, Y.; Li, R.; Yan, J.; Yu, Y.; Qiao, J. Effect of Local Basic Fibroblast Growth Factor and Vascular Endothelial Growth Factor on Subcutaneously Allotransplanted Ovarian Tissue in Ovariectomized Mice. PLoS ONE 2015, 10, e0134035. [Google Scholar] [CrossRef]
- Kang, B.J.; Wang, Y.; Zhang, L.; Xiao, Z.; Li, S.W. bFGF and VEGF improve the quality of vitrified-thawed human ovarian tissues after xenotransplantation to SCID mice. J. Assist. Reprod. Genet. 2016, 33, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, M.; Mehranjani, M.S.; Shariatzadeh, S.M.; Eimani, H.; Shahverdi, A. N-acetylcysteine improves function and follicular survival in mice ovarian grafts through inhibition of oxidative stress. Reprod. Biomed. Online 2015, 30, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Manavella, D.D.; Cacciottola, L.; Pommé, S.; Desmet, C.M.; Jordan, B.F.; Donnez, J.; Amorim, C.A.; Dolmans, M.M. Two-step transplantation with adipose tissue-derived stem cells increases follicle survival by enhancing vascularization in xenografted frozen-thawed human ovarian tissue. Hum. Reprod. 2018, 33, 1107–1116. [Google Scholar] [CrossRef]
- Magen, R.; Shufaro, Y.; Daykan, Y.; Oron, G.; Tararashkina, E.; Levenberg, S.; Anuka, E.; Ben-Haroush, A.; Fisch, B.; Abir, R. Use of Simvastatin, Fibrin Clots, and Their Combination to Improve Human Ovarian Tissue Grafting for Fertility Restoration after Anti-Cancer Therapy. Front. Oncol. 2020, 10, 598026. [Google Scholar] [CrossRef]
- Son, W.Y.; Henderson, S.; Cohen, Y.; Dahan, M.; Buckett, W. Immature Oocyte for Fertility Preservation. Front. Endocrinol. 2019, 10, 464. [Google Scholar] [CrossRef]
- Shapira, M.; Raanani, H.; Barshack, I.; Amariglio, N.; Derech-Haim, S.; Marciano, M.N.; Schiff, E.; Orvieto, R.; Meirow, D. First delivery in a leukemia survivor after transplantation of cryopreserved ovarian tissue, evaluated for leukemia cells contamination. Fertil. Steril. 2018, 109, 48–53. [Google Scholar] [CrossRef]
- Loren, A.W.; Mangu, P.B.; Beck, L.N.; Brennan, L.; Magdalinski, A.J.; Partridge, A.H.; Quinn, G.; Wallace, W.H.; Oktay, K. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J. Clin. Oncol. 2013, 31, 2500–2510. [Google Scholar] [CrossRef]
- Moghadam, A.R.E.; Moghadam, M.T.; Hemadi, M.; Saki, G. Oocyte quality and aging. JBRA Assist. Reprod. 2021. [Google Scholar] [CrossRef]
- Vo, K.C.T.; Kawamura, K. In Vitro Activation Early Follicles: From the Basic Science to the Clinical Perspectives. Int. J. Mol. Sci. 2021, 22, 3785. [Google Scholar] [CrossRef]
- De Vos, M.; Devroey, P.; Fauser, B.C. Primary ovarian insufficiency. Lancet 2010, 376, 911–921. [Google Scholar] [CrossRef]
- Kawamura, K.; Kawamura, N.; Hsueh, A.J. Activation of dormant follicles: A new treatment for premature ovarian failure? Curr. Opin. Obstet. Gynecol. 2016, 28, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Cheng, Y.; Suzuki, N.; Deguchi, M.; Sato, Y.; Takae, S.; Ho, C.H.; Kawamura, N.; Tamura, M.; Hashimoto, S.; et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc. Natl. Acad. Sci. USA 2013, 110, 17474–17479. [Google Scholar] [CrossRef]
- Suzuki, N.; Yoshioka, N.; Takae, S.; Sugishita, Y.; Tamura, M.; Hashimoto, S.; Morimoto, Y.; Kawamura, K. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum. Reprod. 2015, 30, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, A.J.W.; Kawamura, K. Hippo signaling disruption and ovarian follicle activation in infertile patients. Fertil. Steril. 2020, 114, 458–464. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, M.; Albertini, D.F.; Wallace, W.H.B.; Anderson, R.A.; Telfer, E.E. Metaphase II oocytes from human unilaminar follicles grown in a multi-step culture system. Mol. Hum. Reprod. 2018, 24, 135–142. [Google Scholar] [CrossRef]
- O’Brien, M.J.; Pendola, J.K.; Eppig, J.J. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol. Reprod. 2003, 68, 1682–1686. [Google Scholar] [CrossRef]
- Woodruff, T.K. Oncofertility: A grand collaboration between reproductive medicine and oncology. Reproduction 2015, 150, S1–S10. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, J.; Romero, M.M.; Smith, K.N.; Shea, L.D.; Woodruff, T.K. In vitro follicle growth supports human oocyte meiotic maturation. Sci. Rep. 2015, 5, 17323. [Google Scholar] [CrossRef] [PubMed]
- Telfer, E.E.; Andersen, C.Y. In vitro growth and maturation of primordial follicles and immature oocytes. Fertil. Steril. 2021, 115, 1116–1125. [Google Scholar] [CrossRef]
- Tilly, J.L.; Telfer, E.E. Purification of germline stem cells from adult mammalian ovaries: A step closer towards control of the female biological clock? Mol. Hum. Reprod. 2009, 15, 393–398. [Google Scholar] [CrossRef] [PubMed]
- White, Y.A.; Woods, D.C.; Takai, Y.; Ishihara, O.; Seki, H.; Tilly, J.L. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat. Med. 2012, 18, 413–421. [Google Scholar] [CrossRef]
- Hikabe, O.; Hamazaki, N.; Nagamatsu, G.; Obata, Y.; Hirao, Y.; Hamada, N.; Shimamoto, S.; Imamura, T.; Nakashima, K.; Saitou, M.; et al. Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature 2016, 539, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Ogushi, S.; Kurimoto, K.; Shimamoto, S.; Ohta, H.; Saitou, M. Offspring from oocytes derived from in vitro primordial germ cell-like cells in mice. Science 2012, 338, 971–975. [Google Scholar] [CrossRef]
- Zhang, H.; Panula, S.; Petropoulos, S.; Edsgärd, D.; Busayavalasa, K.; Liu, L.; Li, X.; Risal, S.; Shen, Y.; Shao, J.; et al. Adult human and mouse ovaries lack DDX4-expressing functional oogonial stem cells. Nat. Med. 2015, 21, 1116–1118. [Google Scholar] [CrossRef]
- Horan, C.J.; Williams, S.A. Oocyte stem cells: Fact or fantasy? Reproduction 2017, 154, R23–R35. [Google Scholar] [CrossRef]
- Luyckx, V.; Dolmans, M.M.; Vanacker, J.; Scalercio, S.R.; Donnez, J.; Amorim, C.A. First step in developing a 3D biodegradable fibrin scaffold for an artificial ovary. J. Ovarian Res. 2013, 6, 83. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, V.; Dolmans, M.M.; Vanacker, J.; Legat, C.; Fortuño Moya, C.; Donnez, J.; Amorim, C.A. A new step toward the artificial ovary: Survival and proliferation of isolated murine follicles after autologous transplantation in a fibrin scaffold. Fertil. Steril. 2014, 101, 1149–1156. [Google Scholar] [CrossRef]
- Chiti, M.C.; Dolmans, M.M.; Mortiaux, L.; Zhuge, F.; Ouni, E.; Shahri, P.A.K.; Van Ruymbeke, E.; Champagne, S.D.; Donnez, J.; Amorim, C.A. A novel fibrin-based artificial ovary prototype resembling human ovarian tissue in terms of architecture and rigidity. J. Assist. Reprod. Genet. 2018, 35, 41–48. [Google Scholar] [CrossRef]
- Díaz-García, C.; Herraiz, S. The artificial ovary: Any new step is a step forward. Fertil. Steril. 2014, 101, 940. [Google Scholar] [CrossRef]
- Campo, H.; Cervelló, I.; Simón, C. Bioengineering the Uterus: An Overview of Recent Advances and Future Perspectives in Reproductive Medicine. Ann. Biomed. Eng. 2017, 45, 1710–1717. [Google Scholar] [CrossRef]
- Chiti, M.C.; Dolmans, M.M.; Orellana, O.; Soares, M.; Paulini, F.; Donnez, J.; Amorim, C.A. Influence of follicle stage on artificial ovary outcome using fibrin as a matrix. Hum. Reprod. 2016, 31, 2898. [Google Scholar] [CrossRef]
- Pors, S.E.; Ramløse, M.; Nikiforov, D.; Lundsgaard, K.; Cheng, J.; Andersen, C.Y.; Kristensen, S.G. Initial steps in reconstruction of the human ovary: Survival of pre-antral stage follicles in a decellularized human ovarian scaffold. Hum. Reprod. 2019, 34, 1523–1535. [Google Scholar] [CrossRef] [PubMed]
- Laronda, M.M.; Rutz, A.L.; Xiao, S.; Whelan, K.A.; Duncan, F.E.; Roth, E.W.; Woodruff, T.K.; Shah, R.N. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat. Commun. 2017, 8, 15261. [Google Scholar] [CrossRef] [PubMed]
- Laronda, M.M. Engineering a bioprosthetic ovary for fertility and hormone restoration. Theriogenology 2020, 150, 8–14. [Google Scholar] [CrossRef]
- Dath, C.; Dethy, A.; Van Langendonckt, A.; Van Eyck, A.S.; Amorim, C.A.; Luyckx, V.; Donnez, J.; Dolmans, M.M. Endothelial cells are essential for ovarian stromal tissue restructuring after xenotransplantation of isolated ovarian stromal cells. Hum. Reprod. 2011, 26, 1431–1439. [Google Scholar] [CrossRef]
- Sittadjody, S.; Saul, J.M.; Joo, S.; Yoo, J.J.; Atala, A.; Opara, E.C. Engineered multilayer ovarian tissue that secretes sex steroids and peptide hormones in response to gonadotropins. Biomaterials 2013, 34, 2412–2420. [Google Scholar] [CrossRef]
- Laronda, M.M.; Jakus, A.E.; Whelan, K.A.; Wertheim, J.A.; Shah, R.N.; Woodruff, T.K. Initiation of puberty in mice following decellularized ovary transplant. Biomaterials 2015, 50, 20–29. [Google Scholar] [CrossRef]
- Woodard, T.L.; Bolcun-Filas, E. Prolonging Reproductive Life after Cancer: The Need for Fertoprotective Therapies. Trends Cancer 2016, 2, 222–233. [Google Scholar] [CrossRef]
- Spears, N.; Lopes, F.; Stefansdottir, A.; Rossi, V.; De Felici, M.; Anderson, R.A.; Klinger, F.G. Ovarian damage from chemotherapy and current approaches to its protection. Hum. Reprod. Update 2019, 25, 673–693. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Anastácio, A.; Liu, K.; Rodriguez-Wallberg, K.A. Ovarian Follicle Depletion Induced by Chemotherapy and the Investigational Stages of Potential Fertility-Protective Treatments-A Review. Int. J. Mol. Sci. 2019, 20, 4720. [Google Scholar] [CrossRef] [PubMed]
- Guzel, Y.; Bildik, G.; Oktem, O. Sphingosine-1-phosphate protects human ovarian follicles from apoptosis in vitro. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 222, 19–24. [Google Scholar] [CrossRef]
- Meng, Y.; Xu, Z.; Wu, F.; Chen, W.; Xie, S.; Liu, J.; Huang, X.; Zhou, Y. Sphingosine-1-phosphate suppresses cyclophosphamide induced follicle apoptosis in human fetal ovarian xenografts in nude mice. Fertil. Steril. 2014, 102, 871–877.e873. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, B.; Sánchez, D.I.; González-Gallego, J.; Tuñón, M.J. Sphingosine 1-Phosphate Signaling as a Target in Hepatic Fibrosis Therapy. Front. Pharmacol. 2017, 8, 579. [Google Scholar] [CrossRef]
- Wang, E.; He, X.; Zeng, M. The Role of S1P and the Related Signaling Pathway in the Development of Tissue Fibrosis. Front. Pharmacol. 2018, 9, 1504. [Google Scholar] [CrossRef] [PubMed]
- Hancke, K.; Strauch, O.; Kissel, C.; Göbel, H.; Schäfer, W.; Denschlag, D. Sphingosine 1-phosphate protects ovaries from chemotherapy-induced damage in vivo. Fertil. Steril. 2007, 87, 172–177. [Google Scholar] [CrossRef]
- Zelinski, M.B.; Murphy, M.K.; Lawson, M.S.; Jurisicova, A.; Pau, K.Y.; Toscano, N.P.; Jacob, D.S.; Fanton, J.K.; Casper, R.F.; Dertinger, S.D.; et al. In vivo delivery of FTY720 prevents radiation-induced ovarian failure and infertility in adult female nonhuman primates. Fertil. Steril. 2011, 95, 1440–1445.e7. [Google Scholar] [CrossRef]
- Kim, S.Y.; Cordeiro, M.H.; Serna, V.A.; Ebbert, K.; Butler, L.M.; Sinha, S.; Mills, A.A.; Woodruff, T.K.; Kurita, T. Rescue of platinum-damaged oocytes from programmed cell death through inactivation of the p53 family signaling network. Cell Death Differ. 2013, 20, 987–997. [Google Scholar] [CrossRef]
- Roti, E.C.R.; Salih, S.M. Dexrazoxane ameliorates doxorubicin-induced injury in mouse ovarian cells. Biol. Reprod. 2012, 86, 96. [Google Scholar] [CrossRef]
- Salih, S.M.; Ringelstetter, A.K.; Elsarrag, M.Z.; Abbott, D.H.; Roti, E.C. Dexrazoxane abrogates acute doxorubicin toxicity in marmoset ovary. Biol. Reprod. 2015, 92, 73. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Keating, A.F. The ovarian DNA damage repair response is induced prior to phosphoramide mustard-induced follicle depletion, and ataxia telangiectasia mutated inhibition prevents PM-induced follicle depletion. Toxicol. Appl. Pharmacol. 2016, 292, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Majidinia, M.; Sadeghpour, A.; Mehrzadi, S.; Reiter, R.J.; Khatami, N.; Yousefi, B. Melatonin: A pleiotropic molecule that modulates DNA damage response and repair pathways. J. Pineal Res. 2017, 63, e12416. [Google Scholar] [CrossRef]
- Ting, A.Y.; Petroff, B.K. Tamoxifen decreases ovarian follicular loss from experimental toxicant DMBA and chemotherapy agents cyclophosphamide and doxorubicin in the rat. J. Assist. Reprod. Genet. 2010, 27, 591–597. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kimura, F.; Zheng, L.; Kaku, S.; Takebayashi, A.; Kasahara, K.; Tsuji, S.; Murakami, T. Protective effect of a mechanistic target of rapamycin inhibitor on an in vivo model ofcisplatin-induced ovarian gonadotoxicity. Exp. Anim. 2018, 67, 493–500. [Google Scholar] [CrossRef]
- Madden, J.A.; Thomas, P.Q.; Keating, A.F. Phosphoramide mustard induces autophagy markers and mTOR inhibition prevents follicle loss due to phosphoramide mustard exposure. Reprod. Toxicol. 2017, 67, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Lee, O.H.; Lee, Y.; Yoon, H.; Chang, E.M.; Park, M.; Lee, J.W.; Hong, K.; Kim, J.O.; Kim, N.K.; et al. Melatonin prevents cisplatin-induced primordial follicle loss via suppression of PTEN/AKT/FOXO3a pathway activation in the mouse ovary. J. Pineal Res. 2016, 60, 336–347. [Google Scholar] [CrossRef]
- Hayun, M.; Naor, Y.; Weil, M.; Albeck, M.; Peled, A.; Don, J.; Haran-Ghera, N.; Sredni, B. The immunomodulator AS101 induces growth arrest and apoptosis in multiple myeloma: Association with the Akt/survivin pathway. Biochem. Pharmacol. 2006, 72, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Kano, M.; Sosulski, A.E.; Zhang, L.; Saatcioglu, H.D.; Wang, D.; Nagykery, N.; Sabatini, M.E.; Gao, G.; Donahoe, P.K.; Pépin, D. AMH/MIS as a contraceptive that protects the ovarian reserve during chemotherapy. Proc. Natl. Acad. Sci. USA 2017, 114, E1688–E1697. [Google Scholar] [CrossRef]
- Mauri, D.; Gazouli, I.; Zarkavelis, G.; Papadaki, A.; Mavroeidis, L.; Gkoura, S.; Ntellas, P.; Amylidi, A.L.; Tsali, L.; Kampletsas, E. Chemotherapy Associated Ovarian Failure. Front. Endocrinol. 2020, 11, 572388. [Google Scholar] [CrossRef]
- Findeklee, S.; Radosa, J.C.; Takacs, Z.; Hamza, A.; Sima, R.; Solomayer, E.; Sklavounos, P. Fertility preservation in female cancer patients: Current knowledge and future perspectives. Minerva Ginecol. 2019, 71, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Lambertini, M.; Peccatori, F.A.; Demeestere, I.; Amant, F.; Wyns, C.; Stukenborg, J.B.; Paluch-Shimon, S.; Halaska, M.J.; Uzan, C.; Meissner, J.; et al. Fertility preservation and post-treatment pregnancies in post-pubertal cancer patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2020, 31, 1664–1678. [Google Scholar] [CrossRef]
- Dolmans, M.M.; Taylor, H.S.; Rodriguez-Wallberg, K.A.; Blumenfeld, Z.; Lambertini, M.; von Wolff, M.; Donnez, J. Utility of gonadotropin-releasing hormone agonists for fertility preservation in women receiving chemotherapy: Pros and cons. Fertil. Steril. 2020, 114, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Oktay, K.; Harvey, B.E.; Partridge, A.H.; Quinn, G.P.; Reinecke, J.; Taylor, H.S.; Wallace, W.H.; Wang, E.T.; Loren, A.W. Fertility Preservation in Patients With Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 1994–2001. [Google Scholar] [CrossRef]
- Deindl, E.; Zaruba, M.M.; Brunner, S.; Huber, B.; Mehl, U.; Assmann, G.; Hoefer, I.E.; Mueller-Hoecker, J.; Franz, W.M. G-CSF administration after myocardial infarction in mice attenuates late ischemic cardiomyopathy by enhanced arteriogenesis. Faseb. J. 2006, 20, 956–958. [Google Scholar] [CrossRef]
- Skaznik-Wikiel, M.E.; McGuire, M.M.; Sukhwani, M.; Donohue, J.; Chu, T.; Krivak, T.C.; Rajkovic, A.; Orwig, K.E. Granulocyte colony-stimulating factor with or without stem cell factor extends time to premature ovarian insufficiency in female mice treated with alkylating chemotherapy. Fertil. Steril. 2013, 99, 2045–2054.e2043. [Google Scholar] [CrossRef] [PubMed]
- Akdemir, A.; Zeybek, B.; Akman, L.; Ergenoglu, A.M.; Yeniel, A.O.; Erbas, O.; Yavasoglu, A.; Terek, M.C.; Taskiran, D. Granulocyte-colony stimulating factor decreases the extent of ovarian damage caused by cisplatin in an experimental rat model. J. Gynecol. Oncol. 2014, 25, 328–333. [Google Scholar] [CrossRef]
- Winarto, H.; Febia, E.; Purwoto, G.; Nuranna, L. The need for laparoscopic ovarian transposition in young patients with cervical cancer undergoing radiotherapy. Int. J. Reprod. Med. 2013, 2013, 173568. [Google Scholar] [CrossRef]
- Morgan, R.; Mimoun, C.; Lo Dico, R. Ovarian transposition. J. Visc. Surg. 2021, 158, 420–424. [Google Scholar] [CrossRef]
- Buonomo, B.; Multinu, F.; Casarin, J.; Betella, I.; Zanagnolo, V.; Aletti, G.; Peccatori, F. Ovarian transposition in patients with cervical cancer prior to pelvic radiotherapy: A systematic review. Int. J. Gynecol. Cancer 2021, 31, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Finch, A.; Valentini, A.; Greenblatt, E.; Lynch, H.T.; Ghadirian, P.; Armel, S.; Neuhausen, S.L.; Kim-Sing, C.; Tung, N.; Karlan, B.; et al. Frequency of premature menopause in women who carry a BRCA1 or BRCA2 mutation. Fertil. Steril. 2013, 99, 1724–1728. [Google Scholar] [CrossRef] [PubMed]
- Weinberg-Shukron, A.; Rachmiel, M.; Renbaum, P.; Gulsuner, S.; Walsh, T.; Lobel, O.; Dreifuss, A.; Ben-Moshe, A.; Zeligson, S.; Segel, R.; et al. Essential Role of BRCA2 in Ovarian Development and Function. N. Engl. J. Med. 2018, 379, 1042–1049. [Google Scholar] [CrossRef]
- Dou, X.; Guo, T.; Li, G.; Zhou, L.; Qin, Y.; Chen, Z.J. Minichromosome maintenance complex component 8 mutations cause primary ovarian insufficiency. Fertil. Steril. 2016, 106, 1485–1489.e1482. [Google Scholar] [CrossRef] [PubMed]
- Zhe, J.; Chen, S.; Chen, X.; Liu, Y.; Li, Y.; Zhou, X.; Zhang, J. A novel heterozygous splice-altering mutation in HFM1 may be a cause of premature ovarian insufficiency. J. Ovarian Res. 2019, 12, 61. [Google Scholar] [CrossRef]
- Weinberg-Shukron, A.; Renbaum, P.; Kalifa, R.; Zeligson, S.; Ben-Neriah, Z.; Dreifuss, A.; Abu-Rayyan, A.; Maatuk, N.; Fardian, N.; Rekler, D.; et al. A mutation in the nucleoporin-107 gene causes XX gonadal dysgenesis. J. Clin. Investig. 2015, 125, 4295–4304. [Google Scholar] [CrossRef]
- de Vries, L.; Behar, D.M.; Smirin-Yosef, P.; Lagovsky, I.; Tzur, S.; Basel-Vanagaite, L. Exome sequencing reveals SYCE1 mutation associated with autosomal recessive primary ovarian insufficiency. J. Clin. Endocrinol. Metab. 2014, 99, E2129–E2132. [Google Scholar] [CrossRef] [PubMed]
- Islam, N.; Ugwoke, S.P.; Alhamdan, R.; Medrano, J.H.; Campbell, B.K.; Marsters, P.; Maalouf, W.E. Steroids and miRNAs in assessment of ovarian tissue damage following cryopreservation. J. Mol. Endocrinol. 2019, 62, 207–216. [Google Scholar] [CrossRef]
- Rodríguez-Iglesias, B.; Novella-Maestre, E.; Herraiz, S.; Díaz-García, C.; Pellicer, N.; Pellicer, A. New methods to improve the safety assessment of cryopreserved ovarian tissue for fertility preservation in breast cancer patients. Fertil. Steril. 2015, 104, 1493–1502.e2. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gudlevičienė, Ž.; Žilinskas, K.; Kundrotas, G.; Grubliauskaitė, M.; Baltriukienė, D.; Bukelskienė, V. Slow-Freezing Cryopreservation Ensures High Ovarian Tissue Quality Followed by In Vivo and In Vitro Methods and Is Safe for Fertility Preservation. Medicina 2020, 56, 547. [Google Scholar] [CrossRef] [PubMed]
- Ayuandari, S.; Khasanah, N.; Riyanti, I.W.; Dewanto, A.; Sangun, D.I.E.; Wiweko, B. Current Awareness and Attitude toward Fertility Preservation in Indonesia: A Nationwide Survey Among Health-care Providers. J. Hum. Reprod. Sci. 2021, 14, 81–86. [Google Scholar] [CrossRef]
- Karim, A.K.A.; Ahmad, M.F.; Hamid, H.A. Fertility preservation opportunities for cancer patients in Malaysia. Med. J. Malays. 2021, 76, 417–418. [Google Scholar]
- Akisada, N.; Monden, N.; Kishino, T.; Aoi, J.; Hayashi, Y.; Takahashi, S.; Nakamura, M.; Ishihara, H.; Nishizaki, K. Otorhinolaryngologists/head and neck surgeons’ knowledge, attitudes, and practices regarding fertility preservation in young cancer patients treated with chemotherapy: An anonymous questionnaire survey. Int. J. Clin. Oncol. 2021, 26, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.; Crocker, L.; Mathur, A.; Holman, D.; Weston, J.; Campbell, S.; Housten, A.; Bradford, A.; Agrawala, S.; Woodard, T.L. Patients’ and Providers’ Needs and Preferences When Considering Fertility Preservation Before Cancer Treatment: Decision-Making Needs Assessment. JMIR Form. Res. 2021, 5, e25083. [Google Scholar] [CrossRef] [PubMed]
| Authors | Number of CCS | Age | Exposure Agent a | Radiation | Effects | |
|---|---|---|---|---|---|---|
| Clinical | Laboratory Test | |||||
| Berjeb et al. (2021) [20] | 66 | 15–40 (26.7 ± 6.8) | Bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, doxorubicin, vinblastine, dacarbazine | No | N/A | ↓ AMH |
| Filippi et al. (2021) [21] | 90 | 21.3 ± 5.4 | Bleomycin, cisplatin, bleomycin, dacarbazine-vinblastine | Yes/No b | ↑ POI rate (21% of treated women) | |
| Gini et al. (2019) [22] | 97 | 16–50 (median: 28) | Doxorubicin, cyclophosphamide, vincristine, bleomycin | Yes | ↑ Amenorrhea | N/A |
| Lehmann et al. (2019) [23] | 444 | ≤40 | N/A | Yes/No | N/A | ↑ LH ↑ FSH ↓ E2 |
| Anderson et al. (2018) [4] | 23,201 | ≤39 | N/A | N/A | ↓ Pregnancy rate (↓ 38%) | |
| Shandley et al. (2018) [24] | 1090 | 20–35 (median: 26) | N/A | No | N/A | ↓ AFC↓ AMH |
| Sinha et al. (2018) [25] | 88 | 24–43 (median: 35) | Taxotere, cyclophosphamide, carboplatin, fluorouracil, epirubicin | No | N/A | ↓ AFC |
| Al-Rawi et al. (2018) [26] | 58 | 25–45 (38.83 ± 4.74) | Anthracycline, cyclophosphamide | No | N/A | ↓ AFC↓ E2 ↑ LH |
| Aderson et al. (2018) [27] | 67 | 18–45 | Doxorubicin, bleomycin, vinblastine, and dacarbazine | No | N/A | ↓ AMH ↑ FSH ↓ E2 |
| Levine et al. (2018) [28] | 2930 | 18–58 (median: 32) | Alkylating agent, procarbazine | Yes/No | ↑ POI rate (9.1% of treated women) | N/A |
| Armuand et al. (2017) [29] | 552 | ≥13 | N/A | N/A | ↓ The probability of having a first live birth | N/A |
| Chemaitilly et al. (2017) [30] | 988 | 18–45 (median: 31.7) | Alkylating agents | Yes | ↑ POI rate (10.9% of treated women) | N/A |
| D’Avila et al. (2017) [31] | 52 | 27–40 (35.3 ± 3.8) | Cyclophosphamide | No | ↑ Amenorrhea | ↓ AFC↓ AMH ↑ FSH |
| Abir et al. (2016) [32] | 20 | 5–18 | Alkylating agents, bleomycin, cisplatin, vincristine, etoposide, carboplatin, doxorubicin, etopside, doxorubicin, bleomycin, vinblastine, dacarbazine. | No | ↑ Atretic follicles↓ Oocyte maturation | N/A |
| Hamy et al. (2016) [33] | 134 | 26–43 (median: 36) | Anthracyclines, taxane | No | N/A | ↓ AMH |
| Even-Or et al. (2016) [34] | 35 | 13–36 (median: 25.5) | Melphalan | No | N/A | ↓ AMH ↑ FSH ↓ LH |
| Gupta et al. (2016) [35] | 16 | 11–18 (median: 14.3) | Doxorubicin, cyclophosphamide, cisplatin | No | ↑ Amenorrhea | ↓ AMH |
| Chow et al. (2016) [5] | 5298 | 15–44 | Busulfan, carboplatin, carmustine, chlorambucil, chlormethine, cisplatin, cyclophosphamide, dacarbazine, ifosfamide, lomustine, melphalan, procarbazine, temozolomide | Yes/No | ↓ Pregnancy rate | N/A |
| Thomas-Teinturier et al. (2015) [36] | 105 | 18–39 (median: 21.5) | Cyclophosphamide, ifosfamide | Yes | N/A | ↓AMH ↑ FSH |
| Behringer et al. (2012) [37] | 106 | 18–39 (28 ± 7) | Bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, doxorubicin, bleomycin, vinblastine, dacarbazine | N/A | N/A | ↓ AMH ↑ FSH |
| Green et al. (2009) [38] | 5149 | 15–44 | Alkylating agents | Yes/No | ↓ Pregnancy rate | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vo, K.C.T.; Kawamura, K. Female Oncofertility: Current Understandings, Therapeutic Approaches, Controversies, and Future Perspectives. J. Clin. Med. 2021, 10, 5690. https://doi.org/10.3390/jcm10235690
Vo KCT, Kawamura K. Female Oncofertility: Current Understandings, Therapeutic Approaches, Controversies, and Future Perspectives. Journal of Clinical Medicine. 2021; 10(23):5690. https://doi.org/10.3390/jcm10235690
Chicago/Turabian StyleVo, Kim Cat Tuyen, and Kazuhiro Kawamura. 2021. "Female Oncofertility: Current Understandings, Therapeutic Approaches, Controversies, and Future Perspectives" Journal of Clinical Medicine 10, no. 23: 5690. https://doi.org/10.3390/jcm10235690
APA StyleVo, K. C. T., & Kawamura, K. (2021). Female Oncofertility: Current Understandings, Therapeutic Approaches, Controversies, and Future Perspectives. Journal of Clinical Medicine, 10(23), 5690. https://doi.org/10.3390/jcm10235690






