Balloon Aortic Valvuloplasty for Severe Aortic Stenosis as Rescue or Bridge Therapy
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
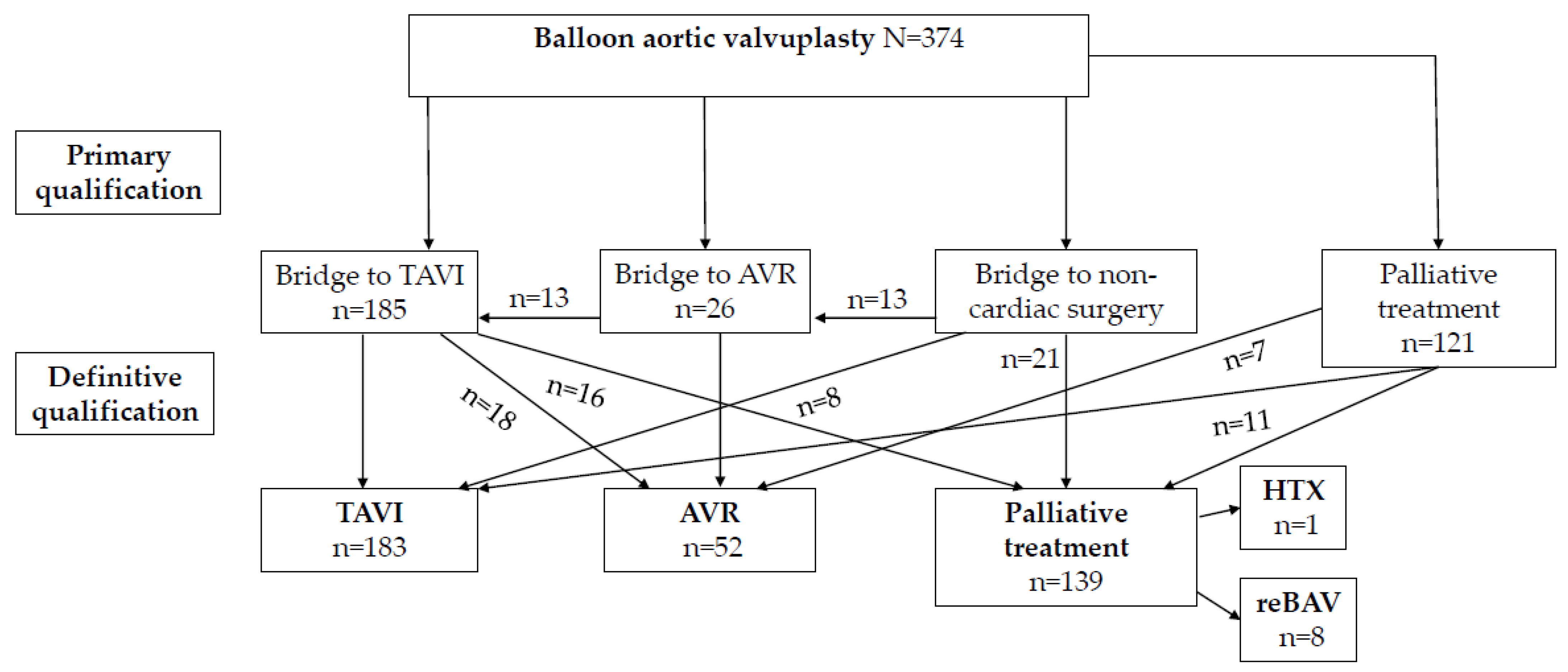
3. Results
3.1. Baseline Characteristics
3.2. Procedural Data
3.3. Echocardiographic Data
3.4. Complications
3.5. Outcomes
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Lansac, E.; Muñoz, D.R.; Rosenhek, R.; Sjögren, J.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, ehab395. [Google Scholar] [CrossRef]
- Daniec, M.; Nawrotek, B.; Sorysz, D.; Rakowski, T.; Dziewierz, A.; Rzeszutko, Ł.; Kleczyński, P.; Trębacz, J.; Tomala, M.; Żmudka, K.; et al. Acute and long-term outcomes of percutaneous balloon aortic valvuloplasty for the treatment of severe aortic stenosis. Catheter. Cardiovasc. Interv. 2016, 90, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Debry, N.; Altes, A.; Vincent, F.; Delhaye, C.; Schurtz, G.; Nedjari, F.; Legros, G.; Porouchani, S.; Coisne, A.; Richardson, M.; et al. Balloon Aortic Valvuloplasty for Severe Aortic Stenosis Before Urgent Noncardiac Surgery. EuroIntervention 2021, 17, e680–e687. [Google Scholar] [CrossRef] [PubMed]
- Percutaneous Balloon Aortic Valvuloplasty. Acute and 30-day follow-up results in 674 patients from the NHLBI Balloon Valvuloplasty Registry. Circulation 1991, 84, 2383–2397. [Google Scholar] [CrossRef] [Green Version]
- Lieberman, E.B.; Bashore, T.M.; Hermiller, J.B.; Wilson, J.S.; Pieper, K.S.; Keeler, G.P.; Pierce, C.H.; Kisslo, K.B.; Harrison, J.K.; Davidson, C.J. Balloon aortic valvuloplasty in adults: Failure of procedure to improve long-term survival. J. Am. Coll. Cardiol. 1995, 26, 1522–1528. [Google Scholar] [CrossRef] [Green Version]
- Otto, C.M.; Mickel, M.C.; Kennedy, J.W.; Alderman, E.L.; Bashore, T.M.; Block, P.C.; Brinker, J.A.; Diver, D.; Ferguson, J.; Holmes, D.R. Three-year outcome after balloon aortic valvuloplasty. Insights into prognosis of valvular aortic stenosis. Circulation 1994, 89, 642–650. [Google Scholar] [CrossRef] [Green Version]
- Wacławski, J.; Wilczek, K.; Hudzik, B.; Pres, D.; Hawranek, M.; Milewski, K.; Chodór, P.; Zembala, M.; Gąsior, M. Aortic balloon valvuloplasty as a bridge-to-decision in patients with aortic stenosis. Adv. Interv. Cardiol. 2019, 15, 195–202. [Google Scholar] [CrossRef]
- Kleczynski, P.; Dziewierz, A.; Socha, S.; Rakowski, T.; Daniec, M.; Zawislak, B.; Arif, S.; Wojtasik-Bakalarz, J.; Dudek, D.; Rzeszutko, L. Direct Rapid Left Ventricular Wire Pacing during Balloon Aortic Valvuloplasty. J. Clin. Med. 2020, 9, 1017. [Google Scholar] [CrossRef] [Green Version]
- Stąpór, M.; Trębacz, J.; Wiewiórka, Ł.; Ostrowska-Kaim, E.; Nawara-Skipirzepa, J.; Sobczyński, R.; Konstanty-Kalandyk, J.; Musiał, R.; Trębacz, O.; Kleczyński, P.; et al. Direct left ventricular wire pacing during transcatheter aortic valve implantation. Kardiol. Pol. 2020, 78, 882–888. [Google Scholar] [CrossRef]
- Daniec, M.; Dziewierz, A.; Sorysz, D.; Kleczyński, P.; Rakowski, T.; Rzeszutko, Ł.; Trębacz, J.; Tomala, M.; Nawrotek, B.; Żmudka, K.; et al. Sex-Related Differences in Outcomes After Percutaneous Balloon Aortic Valvuloplasty. J. Invasive Cardiol. 2017, 29, 188–194. [Google Scholar] [PubMed]
- Daniec, M.; Sorysz, D.; Dziewierz, A.; Kleczyński, P.; Rzeszutko, Ł.; Krawczyk-Ożóg, A.; Dudek, D. In-hospital and long-term outcomes of percutaneous balloon aortic valvuloplasty with concomitant percutaneous coronary intervention in patients with severe aortic stenosis. J. Interv. Cardiol. 2018, 31, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Moretti, C.; Chandran, S.; Vervueren, P.; D’Ascenzo, F.; Barbanti, M.; Weerackody, R.; Boccuzzi, G.; Lee, D.-H.; de la Torre Hernandez, J.; Omedè, P.; et al. Outcomes of patients undergoing balloon aortic valvuloplasty in the TAVI Era: A multicenter registry. J. Invasive Cardiol. 2015, 27, 547–555. [Google Scholar]
- McKay, R.G. The Mansfield Scientific aortic valvuloplasty registry: Overview of acute hemodynamic results and procedural complications. J. Am. Coll. Cardiol. 1991, 17, 485–491. [Google Scholar] [CrossRef]
- Malkin, C.J.; Judd, J.; Chew, D.P.; Sinhal, A. Balloon aortic valvuloplasty to bridge and triage patients in the era of trans-catheter aortic valve implantation. Catheter. Cardiovasc. Interv. 2013, 81, 358–363. [Google Scholar] [CrossRef]
- Saia, F.; Marrozzini, C.; Moretti, C.; Ciuca, C.; Taglieri, N.; Bordoni, B.; Dall’ara, G.; Alessi, L.; Lanzillotti, V.; Bacchi-Reggiani, M.L.; et al. The role of percutaneous balloon aortic valvuloplasty as a bridge for transcatheter aortic valve implantation. EuroIntervention 2011, 7, 723–729. [Google Scholar] [CrossRef]
- Doguet, F.; Godin, M.; Lebreton, G.; Eltchaninoff, H.; Cribier, A.; Bessou, J.P.; Litzler, P.Y. Aortic valve replacement after percutaneous valvuloplasty—An approach in otherwise inoperable patients. Eur. J. Cardiothorac. Surg. 2010, 38, 394–399. [Google Scholar] [CrossRef] [Green Version]
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 2010, 363, 1597–1607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armario, X.; Rosseel, L.; McGrath, B.; Mylotte, D. Balloon aortic valvuloplasty with two simultaneous balloons via ipsilateral transradial and transbrachial access. EuroIntervention 2021, 17, 88–89. [Google Scholar] [CrossRef]
- Tumscitz, C.; Di Cesare, A.; Balducelli, M.; Piva, T.; Santarelli, A.; Saia, F.; Tarantino, F.; Preti, G.; Picchi, A.; Rolfo, C.; et al. Safety, efficacy and impact on frailty of mini-invasive radial balloon aortic valvuloplasty. Heart 2021, 107, 874–880. [Google Scholar] [CrossRef]
- Theodoropoulos, K.C.; Kouparanis, A.; Didagelos, M.; Kassimis, G.; Karvounis, H.; Ziakas, A. Balloon rupture during aortic valvuloplasty: A severe complication or a well-tolerated event? Kardiol. Pol. 2021, 79, 201–202. [Google Scholar] [CrossRef]
- Bularga, A.; Bing, R.; Shah, A.S.; Adamson, P.D.; Behan, M.; Newby, D.E.; Flapan, A.; Uren, N.; Cruden, N. Clinical outcomes following balloon aortic valvuloplasty. Open Heart 2020, 7, e001330. [Google Scholar] [CrossRef]
- Kleczynski, P.; Dziewierz, A.; Bagienski, M.; Rzeszutko, L.; Sorysz, D.; Trebacz, J.; Sobczynski, R.; Tomala, M.; Stapor, M.; Dudek, D. Impact of frailty on mortality after transcatheter aortic valve implantation. Am. Heart J. 2017, 185, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Kleczynski, P.; Tokarek, T.; Dziewierz, A.; Sorysz, D.; Bagienski, M.; Rzeszutko, L.; Dudek, D. Usefulness of Psoas Muscle Area and Volume and Frailty Scoring to Predict Outcomes After Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2018, 122, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Padmini, V.; Nikhil, K.; Ramesh, C.B.; Ramdas, G.P. Clinical profile and natural history of 453 nonsurgically managed patients with severe aortic stenosis. Ann. Thorac. Surg. 2006, 82, 2111–2115. [Google Scholar]
- Dworakowski, R.; Bhan, A.; Brickham, B.; Monaghan, M.; Maccarthy, P. Effectiveness of balloon aortic valvuloplasty is greater in patients with impaired left ventricular function. Int. J. Cardiol. 2011, 150, 103–105. [Google Scholar] [CrossRef]
- Kefer, J.; Gapira, J.M.; Pierard, S.; De Meester, C.; Gurne, O.; Chenu, P.; Renkin, J. Recovery after balloon aortic valvuloplasty in patients with aortic stenosis and impaired left ventricular function: Predictors and prognostic implications. J. Invasive Cardiol. 2013, 25, 235–241. [Google Scholar]
- Husaini, M.; Soyama, Y.; Kagiyama, N.; Thakker, P.; Thangam, M.; Haque, N.; Deych, E.; Sintek, M.; Lasala, J.; Gorcsan, J., 3rd; et al. Clinical and Echocardiographic Features Associated With Improved Survival in Patients With Severe Aortic Stenosis Undergoing Balloon Aortic Valvuloplasty (BAV). J. Invasive Cardiol. 2020, 32, E277–E285. [Google Scholar]
- Tashiro, T.; Pislaru, S.V.; Blustin, J.M.; Nkomo, V.T.; Abel, M.D.; Scott, C.G.; Pellikka, P.A. Perioperative risk of major non-cardiac surgery in patients with severe aortic stenosis: A reappraisal in contemporary practice. Eur. Heart J. 2014, 35, 2372–2381. [Google Scholar] [CrossRef] [Green Version]
- Leibowitz, D.; Rivkin, G.; Schiffman, J.; Rott, D.; Weiss, A.T.; Mattan, Y.; Kandel, L. Effect of severe aortic stenosis on the outcome in elderly patients undergoing repair of hip fracture. Gerontology 2009, 55, 303–306. [Google Scholar] [CrossRef]
- Calleja, A.M.; Dommaraju, S.; Gaddam, R.; Cha, S.; Khandheria, B.K.; Chaliki, H.P. Cardiac risk in patients aged >75 years with asymptomatic, severe aortic stenosis undergoing noncardiac surgery. Am. J. Cardiol. 2010, 105, 1159–1163. [Google Scholar] [CrossRef] [PubMed]
- Keswani, A.; Lovy, A.; Khalid, M.; Blaufarb, I.; Moucha, C.; Forsh, D.; Chen, D. The effect of aortic stenosis on elderly hip fracture outcomes: A case control study. Injury 2016, 47, 413–418. [Google Scholar] [CrossRef] [PubMed]
- MacIntyre, P.A.; Scott, M.; Seigne, R.; Clark, A.; Deveer, F.; Minchin, I. An observational study of perioperative risk associated with aortic stenosis in non-cardiac surgery. Anaesth. Intensive Care 2018, 46, 207–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleczynski, P.; Trebacz, J.; Stapor, M.; Sobczynski, R.; Konstanty-Kalandyk, J.; Kapelak, B.; Zmudka, K.; Legutko, J. Inpatient Cardiac Rehabilitation after Transcatheter Aortic Valve Replacement Is Associated with Improved Clinical Performance and Quality of Life. J. Clin. Med. 2021, 10, 2125. [Google Scholar] [CrossRef]
- Rogers, P.; Al-Aidrous, S.; Banya, W.; Haley, S.R.; Mittal, T.; Kabir, T.; Panoulas, V.; Raja, S.; Bhudia, S.; Probert, H.; et al. Cardiac rehabilitation to improve health-related quality of life following trans-catheter aortic valve implantation: A randomised controlled feasibility study: RECOVER-TAVI Pilot, ORCA 4, For the Optimal Restoration of Cardiac Activity Group. Pilot Feasibility Stud. 2018, 4, 185. [Google Scholar] [CrossRef] [Green Version]
- Tarro Genta, F.; Tidu, M.; Corbo, P.; Bertolin, F.; Salvetti, I.; Bouslenko, Z.; Giordano, A.; Dalla Vecchia, L. Predictors of survival in patients undergoing cardiac rehabilitation after transcatheter aortic valve implantation. J. Cardiovasc. Med. 2019, 20, 606–615. [Google Scholar] [CrossRef] [PubMed]
| All (n = 374) | |
|---|---|
| Age, median (IQR) (years) | 84 (81.3–89.5) |
| Men, n (%) | 176 (47.0) |
| Body mass index, median (IQR) (kg/m2) | 24.7 (22.4–28.4) |
| Glomerular filtration rate, median (IQR) (mL/min/1.73 m2) | 48 (37.5–75.2) |
| CCS class, n (%) I + II III IV | 23 (6.1) 289 (77.3) 62 (16.6) |
| NYHA class, n (%) | |
| I + II | 0 |
| III | 228 (60.9) |
| IV | 146 (39.0) |
| Coronary artery disease, n (%) | 324 (79.1) |
| Arterial hypertension, n (%) | 192 (86.6) |
| Diabetes mellitus, n (%) | 158 (42.2) |
| Atrial fibrillation, n (%) | 103 (27.5) |
| History of myocardial infarction, n (%) | 89 (23.8) |
| History of percutaneous coronary intervention, n (%) | 106 (28.3) |
| History of coronary artery bypass grafting, n (%) | 72 (19.2) |
| Chronic obstructive pulmonary disease, n (%) | 79 (21.1) |
| Peripheral artery disease, n (%) | 83 (22.1) |
| Stroke/transient ischemic attack, n (%) | 49 (13.1) |
| Syncope, n (%) | 57 (15.2) |
| Previous heart failure deterioration, n (%) | 226 (60.4) |
| Cardiogenic shock, n (%) | 22 (5.8) |
| Previous pacemaker, n (%) | 43 (11.4) |
| Neoplasm, n (%) | 41 (10.9) |
| Previous radiotherapy, n (%) | 29 (7.7) |
| Porcelain aorta, n (%) | 19 (5.0) |
| Logistic EuroSCORE II (%), median (IQR) | 7.8 (5.6–14.2) |
| The Society of Thoracic Surgeons score (%), median (IQR) | 11.1 (8.1–13.9) |
| All (n = 374) | |
|---|---|
| Concomitant coronary angiography, n (%) | 355 (94.9) |
| Concomitant PCI, n (%) | 81 (21.6) |
| Size of femoral arterial sheath, median (IQR) (Fr) | 9 (8.0–10.0) |
| Size of femoral venous sheath if used, median (IQR) (Fr) | 6 (6.0–7.0) |
| Unfractionated heparin dose, median (IQR) (units) | 5000 (4000.0–6500.0) |
| Wire pacing, n (%) | 196 (52.4) |
| Number of inflations, median (IQR) | 1 (1–3) |
| Vascular closure device, n (%) | 215 (57.4) |
| Manual compression after sheath(s) removal, n (%) | 159 (42.5) |
| Balloon size, median (IQR) (mm) | 22 (18–24.5) |
| Radiation dose (BAV alone), median (IQR) (Gy) | 0.26 (0.15–0.45) |
| Contrast media volume (BAV alone), median (IQR) (mL) | 10 (5.0–24.0) |
| Fluoroscopy time (BAV alone), median (IQR) (min) | 7.4 (5.2–15.4) |
| Duration (BAV alone), median (IQR) (min) | 26 (17.9–35.5) |
| Baseline (n = 374) | After BAV (n = 365) | 30 Days (n = 335) | 6 Months (n = 293) | 12 Months (n = 46) ^ | |
|---|---|---|---|---|---|
| Maximal transaortic gradient, median (IQR) (mmHg) | 93.8 (81.2–104.82) | 64 (47.2–73.5) * | 67 (48.4–76.4) * | 72.5 (55.3–85.3) *# | 86.3 (58.6–97.4) # |
| Mean transaortic gradient, median (IQR) (mmHg) | 41.1 (40.4–55.2) | 31.3 (21.4–38.2) * | 32.5 (22.4–39.4) * | 38.4 (30.3–49.6) *# | 40.6 (39.5–54.7) # |
| Aortic valve area, median (IQR) (cm2) | 0.52 (0.42–0.61) | 0.79 (0.65–0.92) | 0.77 (0.66–0.90) * | 0.71 (0.63–0.88) *# | 0.53 (0.44–0.63) # |
| Left ventricle ejection fraction, median (IQR) (%) | 41.2 (33.5–52.0) | 44.2 (38.5–54.8) | 48.2 (42.6–58.3) *# | 46.7 (40.1–55.3) | 42.9 (39.1–53.1) # |
| Right ventricular systolic pressure, median (IQR) (mm Hg) | 53 (36.0–68.5) | 45.2 (32.2–56.2) * | 46.4 (33.2–57.6) * | 49.3(35.2–62.2) # | 52 (37.1–65.2) *# |
| Aortic regurgitation None/trivial, n (%) Mild, n (%) Moderate, n (%) Severe, n (%) | 145 (38.7) 157 (41.9) 52 (13.9) 0 (0.0) | 93 (24.8) 183 (48.9) 82 (21.9) * 8 (2.1) | 86 (22.9) 191 (51.0) * 75 (20.0) * 0 (0.0) | 105 (28.0) 206 (55.0) * 67 (17.9) 0 (0.0) | 18 (39.1) 12 (26.0) 16 (34.7) 0 (0.0) |
| All (n = 374) | |
|---|---|
| Severe aortic regurgitation after BAV, n (%) | 8 (2.1) |
| Balloon rupture, n (%) | 23 (6.1) |
| Cardiac tamponade, n (%) | 9 (2.4) |
| Severe cardiac arrythmias, n (%) | 18 (4.8) |
| Cerebrovascular incident, n (%) | 6 (1.6) |
| Vascular access site complications, n (%) | 47 (12.5) |
| hematoma, n (%) | 18 (4.8) |
| pseudoaneurysm, n (%) | 17 (4.5) |
| arteriovenous fistula, n (%) | 4 (1.0) |
| retroperitoneal bleeding, n (%) | 8 (2.1) |
| Blood transfusion, n (%) | 31 (8.2) |
| Baseline creatinine level, median (IQR) (g/dL) | 107 (87.0–147.6) |
| Creatinine level after procedures, median (IQR) (g/dL) | 104 (91.0–149.2) |
| Urgent cardiac surgery, n (%) | 13 (3.5) |
| Permanent pacemaker implantation, n (%) | 3 (0.8) |
| Hospital stay duration, median (IQR) (days) | 5.5 (4.0–11.5) |
| Intraprocedural mortality, n (%) | 9 (2.4) |
| All (n = 374) | |
|---|---|
| In-hospital mortality, n (%) | 19 (5.1) |
| 30-day mortality rate, n (%) | 39 (10.4) |
| 6-month mortality rate, n (%) | 81 (21.6) |
| 12-month morality rate, n (%) | 106 (28.3) |
| 12-month mortality in palliative group, n (%) | 93 (66.9) * |
| HR (95% CI) | p | HR (95% CI) Adjusted for Age/Gender | p | |
|---|---|---|---|---|
| Age | 0.87 (0.68–1.15) | 0.25 | - | - |
| Sex (female) | 0.94 (0.72–2.01) | 0.14 | - | - |
| Hypertension | 1.12 (0.79–1.79) | 0.29 | 1.05 (0.81–1.63) | 0.31 |
| Coronary artery disease | 1.06 (0.71–1.56) | 0.37 | 1.03 (0.73–1.43) | 0.43 |
| Diabetes | 0.82 (0.75–1.34) | 0.23 | 0.83 (0.87–1.29) | 0.54 |
| Atrial fibrillation | 1.03 (0.86–1.52) | 0.30 | 1.01 (0.90–1.42) | 0.47 |
| Cerebrovascular event | 1.23 (0.75–2.13) | 0.16 | 1.18 (0.79–2.04) | 0.24 |
| Chronic obstructive pulmonary disease | 1.19 (0.76–1.99) | 0.14 | 1.14 (0.77–1.87) | 0.28 |
| Chronic kidney disease | 1.27 (0.80–2.15) | 0.19 | 1.23 (0.78–1.92) | 0.32 |
| STS score (per 1%) | 1.25 (1.08–2.46) | 0.001 | 1.42 (1.34–2.88) | <0.0001 |
| LVEF < 20% at baseline | 1.65 (1.04–2.67) | 0.02 | 1.89 (1.55–2.83) | <0.0001 |
| LVEF < 30% at 1 month | 1.87 (1.35–3.43) | 0.001 | 1.97 (1.62–3.67) | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kleczynski, P.; Kulbat, A.; Brzychczy, P.; Dziewierz, A.; Trebacz, J.; Stapor, M.; Sorysz, D.; Rzeszutko, L.; Bartus, S.; Dudek, D.; et al. Balloon Aortic Valvuloplasty for Severe Aortic Stenosis as Rescue or Bridge Therapy. J. Clin. Med. 2021, 10, 4657. https://doi.org/10.3390/jcm10204657
Kleczynski P, Kulbat A, Brzychczy P, Dziewierz A, Trebacz J, Stapor M, Sorysz D, Rzeszutko L, Bartus S, Dudek D, et al. Balloon Aortic Valvuloplasty for Severe Aortic Stenosis as Rescue or Bridge Therapy. Journal of Clinical Medicine. 2021; 10(20):4657. https://doi.org/10.3390/jcm10204657
Chicago/Turabian StyleKleczynski, Pawel, Aleksandra Kulbat, Piotr Brzychczy, Artur Dziewierz, Jaroslaw Trebacz, Maciej Stapor, Danuta Sorysz, Lukasz Rzeszutko, Stanislaw Bartus, Dariusz Dudek, and et al. 2021. "Balloon Aortic Valvuloplasty for Severe Aortic Stenosis as Rescue or Bridge Therapy" Journal of Clinical Medicine 10, no. 20: 4657. https://doi.org/10.3390/jcm10204657
APA StyleKleczynski, P., Kulbat, A., Brzychczy, P., Dziewierz, A., Trebacz, J., Stapor, M., Sorysz, D., Rzeszutko, L., Bartus, S., Dudek, D., & Legutko, J. (2021). Balloon Aortic Valvuloplasty for Severe Aortic Stenosis as Rescue or Bridge Therapy. Journal of Clinical Medicine, 10(20), 4657. https://doi.org/10.3390/jcm10204657







