Risk of Non-Vertebral Fracture in Gout Compared to Rheumatoid Arthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Cohort
2.3. Outcome Definition
2.4. Covariate Assessment
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Risk of Non-Vertebral Fracture, Hip Fracture and All-Cause Death
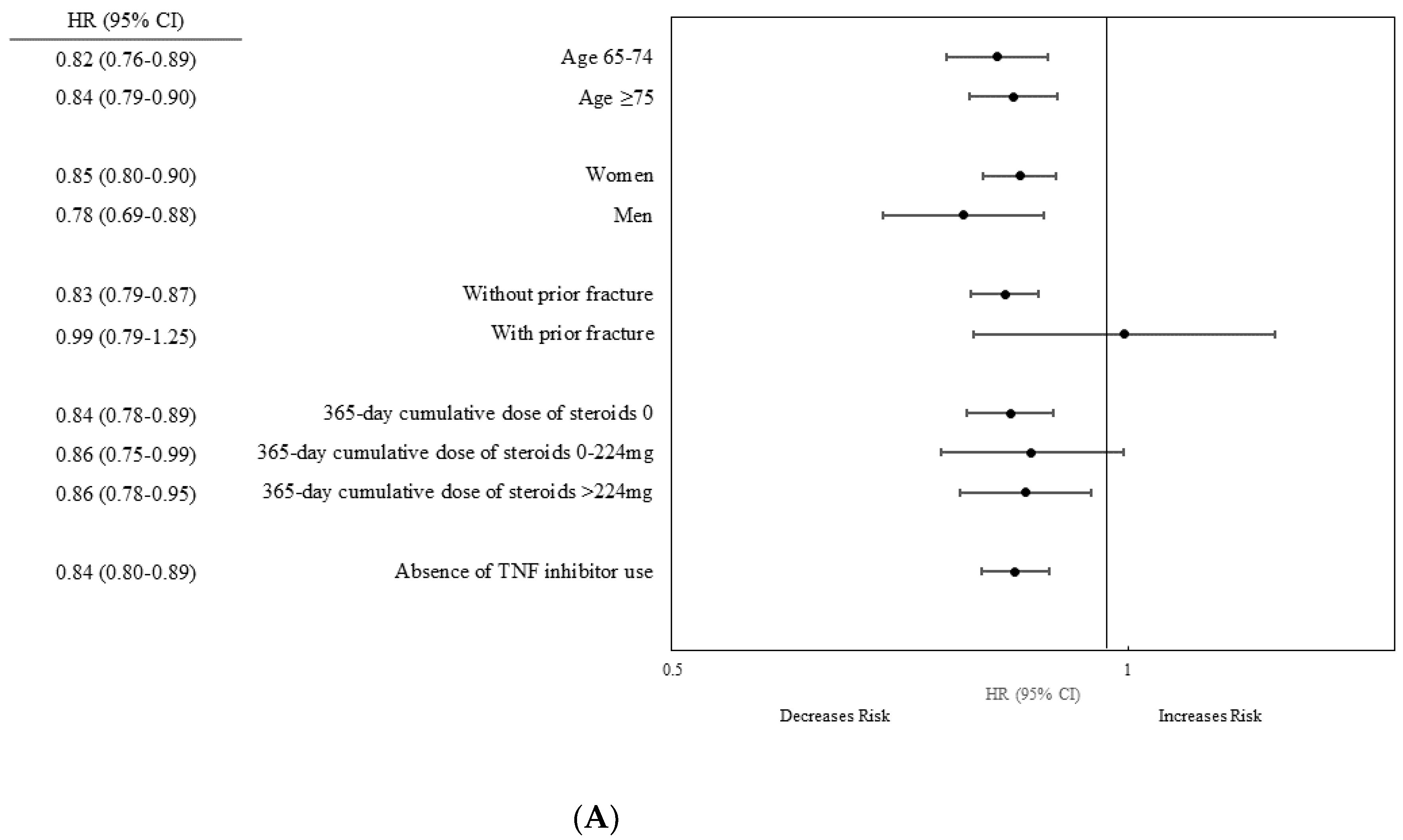
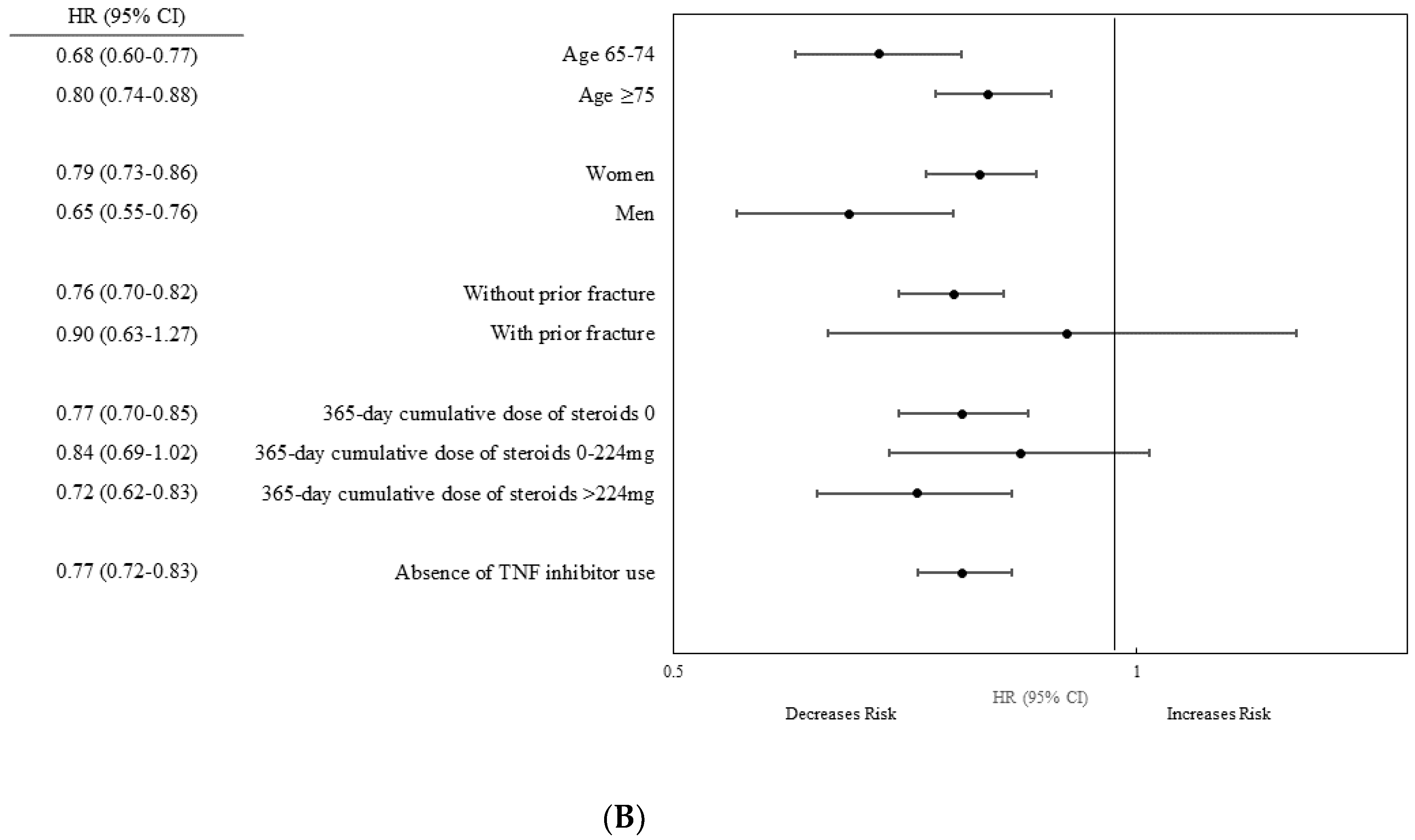
3.3. Stratified Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Terkeltaub, R.A. Gout. N. Engl. J. Med. 2003, 349, 1647–1655. [Google Scholar] [CrossRef]
- Inaba, S.; Sautin, Y.; Garcia, G.E.; Johnson, R.J. What can asymptomatic hyperuricaemia and systemic inflammation in the absence of gout tell us? Rheumatology 2013, 52, 963–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, C.F.; Grainge, M.J.; Zhang, W.; Doherty, M. Global epidemiology of gout: Prevalence, incidence and risk factors. Nat. Rev. Rheumatol. 2015, 11, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Hak, A.E.; Curhan, G.C.; Grodstein, F.; Choi, H.K. Menopause, postmenopausal hormone use and risk of incident gout. Ann. Rheum. Dis. 2010, 69, 1305–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, J.A.; Strand, V. Gout is associated with more comorbidities, poorer health-related quality of life and higher healthcare utilisation in US veterans. Ann. Rheum. Dis. 2008, 67, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Lottmann, K.; Chen, X.; Schädlich, P.K. Association between gout and all-cause as well as cardiovascular mortality: A systematic review. Curr. Rheumatol. Rep. 2012, 14, 195–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, D.H.; Ha, S.K. Uric acid puzzle: Dual role as anti-oxidantand pro-oxidant. Electrolyte Blood Press. 2014, 12, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.J.; Baek, S.; Ahn, S.H.; Kim, S.H.; Jo, M.W.; Bae, S.J.; Kim, H.K.; Choe, J.; Park, G.M.; Kim, Y.H.; et al. Higher serum uric acid as a protective factor against incident osteoporotic fractures in Korean men: A longitudinal study using the National Claim Registry. Osteoporos. Int. 2014, 25, 1837–1844. [Google Scholar] [CrossRef]
- Chang, I.; Gazeley, D. Crystalline arthropathy and bone health. Curr. Opin. Rheumatol. 2018, 30, 173–176. [Google Scholar] [CrossRef]
- Yin, P.; Lv, H.; Li, Y.; Meng, Y.; Zhang, L.; Tang, P. The association between serum uric acid level and the risk of fractures: A systematic review and meta-analysis. Osteoporos. Int. 2017, 28, 2299–2307. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Montagnana, M.; Franchini, M.; Favaloro, E.J.; Targher, G. The paradoxical relationship between serum uric acid and cardiovascular disease. Clin. Chim. Acta Int. J. Clin. Chem. 2008, 392, 1–7. [Google Scholar] [CrossRef]
- Spiga, R.; Marini, M.A.; Mancuso, E.; Di Fatta, C.; Fuoco, A.; Perticone, F.; Andreozzi, F.; Mannino, G.C.; Sesti, G. Uric acid is associated with inflammatory biomarkers and induces inflammation via activating the NF-κB signaling pathway in HepG2 cells. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1241–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, T.; Schett, G. Pathways for bone loss in inflammatory disease. Curr. Osteoporos. Rep. 2012, 10, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Pietschmann, P.; Mechtcheriakova, D.; Meshcheryakova, A.; Foger-Samwald, U.; Ellinger, I. Immunology of Osteoporosis: A mini-review. Gerontology 2016, 62, 128–137. [Google Scholar] [CrossRef] [Green Version]
- Ruggiero, C.; Cherubini, A.; Ble, A.; Bos, A.J.; Maggio, M.; Dixit, V.D.; Lauretani, F.; Bandinelli, S.; Senin, U.; Ferrucci, L. Uric acid and inflammatory markers. Eur. Heart J. 2006, 27, 1174–1781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Straub, R.H.; Cutolo, M.; Pacifici, R. Evolutionary medicine and bone loss in chronic inflammatory diseases—A theory of inflammation-related osteopenia. Semin. Arthr. Rheum. 2015, 45, 220–228. [Google Scholar] [CrossRef] [Green Version]
- Le Goff, B.; Blanchard, F.; Berthelot, J.M.; Heymann, D.; Maugars, Y. Role for interleukin-6 in structural joint damage and systemic bone loss in rheumatoid arthritis. Jt. Bone Spine 2010, 77, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Polzer, K.; Joosten, L.; Gasser, J.; Distler, J.H.; Ruiz, G.; Baum, W.; Redlich, K.; Bobacz, K.; Smolen, J.S.; van den Berg, W.; et al. Interleukin-1 is essential for systemic inflammatory bone loss. Ann. Rheum. Dis. 2010, 69, 284–290. [Google Scholar] [CrossRef]
- Zerbini, C.A.; Clark, P.; Mendez-Sanchez, L.; Pereira, R.M.; Messina, O.D.; Uña, C.R.; Adachi, J.D.; Lems, W.F.; Cooper, C.; Lane, N.E. Biologic therapies and bone loss in rheumatoid arthritis. Osteoporos. Int. 2017, 28, 429–446. [Google Scholar] [CrossRef]
- Xue, A.L.; Wu, S.Y.; Jiang, L.; Feng, A.M.; Guo, H.F.; Zhao, P. Bone fracture risk in patients with rheumatoid arthritis: A meta-analysis. Medicine 2017, 96, e6983. [Google Scholar] [CrossRef]
- Wright, N.C.; Lisse, J.R.; Walitt, B.T.; Eaton, C.B.; Chen, Z. Arthritis increases the risk for fractures—Results from the Women’s Health Initiative. J. Rheumatol. 2011, 38, 1680–1688. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Cho, S.K.; Choi, C.B.; Jun, J.B.; Kim, T.H.; Lee, H.S.; Lee, J.; Lee, S.S.; Yoo, D.H.; Yoo, W.H.; et al. Incidence and risk factors of fractures in patients with rheumatoid arthritis: An Asian prospective cohort study. Rheumatol. Int. 2016, 36, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Li, Y.H.; Chang, C.H.; Hu, C.C.; Chen, D.W.; Hsieh, P.H.; Lee, M.S.; Ueng, S.N.; Chang, Y. Rheumatoid arthritis patients with hip fracture: A nationwide study. Osteoporos. Int. 2015, 26, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, H.E.; Lin, C.C.; Wang, I.K.; Huang, P.H.; Tsai, C.H. Gout increases risk of fracture: A nationwide population-based cohort study. Medicine 2016, 95, e4669. [Google Scholar] [CrossRef] [PubMed]
- Paik, J.M.; Kim, S.C.; Feskanich, D.; Choi, H.K.; Solomon, D.H.; Curhan, G.C. Gout and risk of fracture in women: A prospective cohort study. Arthritis Rheumatol. 2017, 69, 422–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhou, R.; Zhong, W.; Hu, C.; Lu, S.; Chai, Y. Association of gout with osteoporotic fractures. Int. Orthop. 2018, 42, 2041–2047. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Paik, J.M.; Liu, J.; Curhan, G.C.; Solomon, D.H. Gout and the Risk of Non-vertebral Fracture. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2017, 32, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Dong, J.; Zhou, D.; Kang, Q.; Xiong, F. Gout is not associated with the risk of fracture: A meta-analysis. J. Orthop. Surg. Res. 2019, 14, 272. [Google Scholar] [CrossRef] [Green Version]
- Zong, Q.; Hu, Y.; Zhang, Q.; Zhang, X.; Huang, J.; Wang, T. Associations of hyperuricemia, gout, and UA-lowering therapy with the risk of fractures: A meta-analysis of observational studies. Jt. Bone Spine 2019, 86, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Hudson, M.; Avina-Zubieta, A.; Lacaille, D.; Bernatsky, S.; Lix, L.; Jean, S. The validity of administrative data to identify hip fractures is high—A systematic review. J. Clin. Epidemiol. 2013, 66, 278–285. [Google Scholar] [CrossRef]
- Ray, W.A.; Griffin, M.R.; Fought, R.L.; Adams, M.L. Identification of fractures from computerized Medicare files. J. Clin. Epidemiol. 1992, 45, 703–714. [Google Scholar] [CrossRef]
- Gagne, J.J.; Glynn, R.J.; Avorn, J.; Levin, R.; Schneeweiss, S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J. Clin. Epidemiol. 2011, 64, 749–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.H.; Schneeweiss, S.; Glynn, R.J.; Lipsitz, L.A.; Rockwood, K.; Avorn, J. Measuring frailty in medicare data: Development and validation of a claims-based frailty index. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018, 73, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun. Stat. Simul. Comput. 2009, 38, 1228–1234. [Google Scholar] [CrossRef]
- Kanis, J.; Johnell, O.; Odén, A.; Johansson, H.; McCloskey, E. FRAX™ and the assessment of fracture probability in men and women from the UK. Osteoporos. Int. 2008, 19, 385–397. [Google Scholar] [CrossRef] [Green Version]
- Straub, R.; Cutolo, M.; Buttgereit, F.; Pongratz, G. Energy regulation and neuroendocrine–immune control in chronic inflammatory diseases. J. Internal Med. 2010, 267, 543–560. [Google Scholar] [CrossRef]

| Variable | Gout (n = 134,157) | RA (n = 134,157) | Standardized Difference * (%) |
|---|---|---|---|
| Demographics † | |||
| Age, year, mean ± SD | 73.69 ± 6.52 | 73.69 ± 6.52 | 0.0 |
| Female | 94,456 (70.41) | 94,456 (70.41) | 0.0 |
| White race | 99,924 (74.48) | 115,625 (86.19) | 29.8 |
| Osteoporotic fracture related comorbidities | |||
| BMD test | 13,922 (10.38) | 33,274 (24.80) | 38.6 |
| Prior fall | 5953 (4.44) | 5810 (4.33) | −0.5 |
| Osteoporosis | 16,654 (12.41) | 44,135(32.90) | 50.5 |
| Obesity | 23,598 (17.59) | 9802 (7.31) | −31.5 |
| Prior fracture ‡ | 2649 (1.97) | 3426 (2.55) | 3.9 |
| Prior hip fracture ‡ | 753 (0.56) | 1246 (0.93) | 4.3 |
| Other comorbidities | |||
| Comorbidity score, mean ± SD | 2.03 ± 2.66 | 1.03 ± 1.93 | −0.4 |
| Smoking | 13,539 (10.09) | 13,277 (9.90) | −0.6 |
| Alcoholism | 581 (0.43) | 278 (0.21) | −3.9 |
| Dementia | 8879 (6.62) | 8172 (6.09) | −2.2 |
| Parkinson | 1602 (1.19) | 1772 (1.32) | 1.2 |
| Coronary heart diseases | 55,635 (41.47) | 39,839 (29.70) | −24.8 |
| COPD | 38,012 (28.33) | 35,979 (26.82) | −3.4 |
| Diabetes mellitus | 67,909 (50.62) | 37,643 (28.06) | −47.5 |
| Heart failure | 33,290 (24.81) | 14,758 (11.00) | −36.6 |
| Hypertension | 126,632 (94.39) | 101,379 (75.57) | −54.6 |
| Hyperlipidemia | 115,472 (86.07) | 95,978 (71.54) | −36.1 |
| Chronic kidney diseases | 46,420 (34.60) | 13,829 (10.31) | −60.8 |
| Stroke | 24,349 (18.15) | 18,855 (14.05) | −11.2 |
| Frailty index | |||
| Robust | 35,902 (26.76) | 41,942 (31.26) | 9.9 |
| Prefrail | 81,216 (60.54) | 81,327 (60.62) | 0.2 |
| Mildly frail | 15,975 (11.91) | 10,389 (7.74) | −14.0 |
| Moderately to severely frail | 1064 (0.79) | 499 (0.37) | −5.5 |
| Osteoporosis medication | |||
| Bisphosphonate | 10,061 (7.50) | 29,866 (22.26) | 42.4 |
| PTH | 191 (0.14) | 941 (0.70) | 8.7 |
| Calcitonin | 573 (0.43) | 1183 (0.88) | 5.6 |
| Denosumab | 411 (0.31) | 1401 (1.04) | 8.9 |
| Raloxifene | 1640 (1.22) | 2294 (1.71) | 4.1 |
| Teriparatide | 191 (0.14) | 941 (0.70) | 8.7 |
| Other medications | |||
| Prior use of steroids | 41,047 (30.60) | 74,294 (55.38) | −51.7 |
| Prior cumulative dose of prednisolone equivalent, mg, mean ± SD | 160.57 ± 540.52 | 673.19 ± 1096.69 | 0.59 |
| 0 | 93,110 (69.40) | 59,863 (44.62) | −51.7 |
| >0 and ≤224 | 20,503 (15.28) | 12,786 (9.53) | −17.5 |
| >224 | 20,544 (15.31) | 61,508 (45.85) | 70.3 |
| Steroid injection | 10,357 (7.72) | 18,893 (14.08) | 20.5 |
| TNF inhibitor | 0(0) | 13,061 (9.74) | 46.5 |
| Methotrexate | 0(0) | 83,428 (62.19) | 181.4 |
| Anticonvulsants | 21,499 (16.03) | 22,030 (16.42) | 1.1 |
| Antipsychotic | 32,739 (24.40) | 32,007 (23.86) | −1.3 |
| Benzodiazepines | 15,823 (11.79) | 17,571 (13.10) | 4.0 |
| Beta blocker | 62,397 (46.51) | 44,015 (32.81) | −28.3 |
| ACE inhibitor | 91,489 (68.20) | 63,783 (47.54) | −42.8 |
| Calcium channel blocker | 54,211 (40.41) | 37,065 (27.63) | −27.2 |
| Diuretics | 97,994 (73.04) | 58,219 (43.40) | −63.0 |
| Statin | 80,794 (60.22) | 61,059 (45.51) | −29.8 |
| NSAIDs | 64,465 (48.05) | 58,786 (43.82) | −8.5 |
| Opioids | 58,529 (43.63) | 56,567 (42.16) | −3.0 |
| Proton pump inhibitor | 44,834 (33.42) | 48,896 (36.45) | 6.4 |
| SSRI | 21,975 (16.38) | 26,365 (19.65) | 8.5 |
| Health care utilization, mean ± SD | |||
| No. of all prescription drugs | 13.60 ± 6.49 | 12.08 ± 6.11 | −0.2 |
| No. of ER visit | 0.86 ± 1.62 | 0.52 ± 1.12 | −24.1 |
| No. of visits | 12.95 ± 9.16 | 14.21 ± 9.17 | 0.1 |
| No. of hospitalization | 0.41 ± 0.87 | 0.24 ± 0.61 | −20.4 |
| Gout Group | RA Group | IRR (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subgroup | No. of Patients | Cases | Person-Years | IR (95% CI) | No. of Patients | Cases | Person-Years | IR (95% CI) | ||
| Non-vertebral fracture | ||||||||||
| All patients | 134,157 | 3891 | 373,336 | 10.42 (10.10–10.75) | 134,157 | 5785 | 385,306 | 15.01 (14.63–15.40) | 0.69 (0.66–0.72) | |
| Age, years | 65–74 | 81,439 | 1642 | 235,612 | 6.97 (6.64–7.32) | 81,439 | 2570 | 246,103 | 10.44 (10.04–10.85) | 0.67 (0.63–0.71) |
| ≥75 | 52,718 | 2249 | 137,723 | 16.33 (15.67–17.02) | 52,718 | 3215 | 139,202 | 23.10 (22.32–23.91) | 0.71 (0.67–0.75) | |
| Sex | Women | 94,456 | 3258 | 254,914 | 12.78 (12.35–13.23) | 94,456 | 4854 | 263,895 | 18.39 (17.88–18.91) | 0.69 (0.66–0.72) |
| Men | 39,701 | 633 | 118,421 | 5.35 (4.95–5.78) | 39,701 | 931 | 121,410 | 7.67 (7.19–8.18) | 0.70 (0.63–0.77) | |
| Prior fracture | No | 131,508 | 3701 | 368,057 | 10.06 (9.74–10.39) | 130,731 | 5501 | 378,326 | 14.54 (14.16–14.93) | 0.69 (0.66–0.72) |
| Yes | 2649 | 190 | 5278 | 35.99 (31.22–41.49) | 3426 | 284 | 6979 | 40.69 (36.22–45.71) | 0.88 (0.73–1.06) | |
| 365-d cumulative dose of steroids | 0 | 93,110 | 2561 | 264,162 | 9.69 (9.32–10.07) | 59,863 | 2473 | 177,812 | 13.91 (13.37–14.47) | 0.70 (0.66–0.74) |
| 0–224 mg | 20,503 | 634 | 56,134 | 11.29 (10.44–12.20) | 12,786 | 527 | 36,930 | 14.27 (13.10–15.54) | 0.79 (0.70–0.89) | |
| >224 mg | 20,544 | 696 | 53,039 | 13.12 (12.18–14.13) | 61,508 | 2785 | 170,563 | 16.33 (15.73–16.95) | 0.80 (0.74–0.87) | |
| TNF inhibitor | No | 134,157 | 3891 | 373,336 | 10.42 (10.10–10.75) | 121,096 | 5270 | 351,257 | 15.00 (14.60–15.41) | 0.69 (0.66–0.72) |
| Yes | 13,061 | 515 | 34,048 | 15.13 (13.88–16.49) | NA | |||||
| Hip fracture | ||||||||||
| All patients | 134,157 | 1831 | 376,535 | 4.86 (4.64–5.09) | 134,157 | 3013 | 389,675 | 7.73 (7.46–8.01) | 0.63 (0.59–0.67) | |
| Age, years | 65–74 | 81,439 | 588 | 237,454 | 2.48 (2.29–2.69) | 81,439 | 1142 | 248,645 | 4.59 (4.33–4.86) | 0.54 (0.49–0.60) |
| ≥75 | 52,718 | 1243 | 139,081 | 8.94 (8.46–9.45) | 52,718 | 1871 | 141,029 | 13.27 (12.68–13.89) | 0.67 (0.62–0.72) | |
| Sex | Women | 94,456 | 1492 | 257,622 | 5.79 (5.50–6.09) | 94,456 | 2439 | 267,678 | 9.11 (8.76–9.48) | 0.64 (0.60–0.68) |
| Men | 39,701 | 339 | 118,913 | 2.85 (2.56–3.17) | 39,701 | 574 | 121,996 | 4.71 (4.34–5.11) | 0.61 (0.53–0.70) | |
| Prior fracture | No | 131,508 | 1755 | 371,107 | 4.73 (4.51–4.96) | 130,731 | 2880 | 382,493 | 7.53 (7.26–7.81) | 0.63 (0.59–0.67) |
| Yes | 2649 | 76 | 5428 | 14.00 (11.18–17.53) | 3426 | 133 | 7181 | 18.52 (15.63–21.95) | 0.76 (0.57–1.01) | |
| 365-d cumulative dose of steroids * | 0 | 93,110 | 1218 | 266,342 | 4.57 (4.32–4.83) | 59,863 | 1257 | 179,791 | 6.99 (6.61–7.39) | 0.65 (0.60–0.70) |
| 0–224 mg | 20,503 | 304 | 56,640 | 5.37 (4.80–6.01) | 12,786 | 263 | 37,300 | 7.05 (6.25–7.96) | 0.76 (0.64–0.90) | |
| >224 mg | 20,544 | 309 | 53,552 | 5.77 (5.16–6.45) | 61,508 | 1493 | 172,583 | 8.65 (8.22–9.10) | 0.67 (0.59–0.76) | |
| TNF inhibitor | No | 134,157 | 1831 | 376,535 | 4.86 (4.64–5.09) | 121,096 | 2750 | 355,227 | 7.74 (7.46–8.03) | 0.63 (0.59–0.67) |
| Yes | 13,061 | 263 | 34,447 | 7.63 (6.76–8.61) | NA | |||||
| Adjustment | Hazard Ratio (95% CI) |
|---|---|
| Non-vertebral fracture | |
| Model 1 | 0.69 (0.66–0.72) |
| Model 2 | 0.78 (0.75–0.82) |
| Model 3 | 0.75 (0.72–0.79) |
| Final model | 0.84 (0.80–0.88) |
| Hip fracture | |
| Model 1 | 0.62 (0.59–0.66) |
| Model 2 | 0.72 (0.67–0.76) |
| Model 3 | 0.68 (0.64–0.73) |
| Final model | 0.76 (0.71–0.82) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, S.-K.; Liu, J.; Jin, Y.; Kim, S.C. Risk of Non-Vertebral Fracture in Gout Compared to Rheumatoid Arthritis. J. Clin. Med. 2021, 10, 4655. https://doi.org/10.3390/jcm10204655
Cho S-K, Liu J, Jin Y, Kim SC. Risk of Non-Vertebral Fracture in Gout Compared to Rheumatoid Arthritis. Journal of Clinical Medicine. 2021; 10(20):4655. https://doi.org/10.3390/jcm10204655
Chicago/Turabian StyleCho, Soo-Kyung, Jun Liu, Yinzhu Jin, and Seoyoung C. Kim. 2021. "Risk of Non-Vertebral Fracture in Gout Compared to Rheumatoid Arthritis" Journal of Clinical Medicine 10, no. 20: 4655. https://doi.org/10.3390/jcm10204655
APA StyleCho, S.-K., Liu, J., Jin, Y., & Kim, S. C. (2021). Risk of Non-Vertebral Fracture in Gout Compared to Rheumatoid Arthritis. Journal of Clinical Medicine, 10(20), 4655. https://doi.org/10.3390/jcm10204655






