Prognostic Factors at Admission for In-Hospital Mortality from COVID-19 Infection in an Older Rural Population in Central Spain
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Source and Selection of Variables
2.3. Data Quality Assessment
2.4. Statistical Analysis
3. Results
Factors Associated with Mortality at Hospital Admission
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet Lond. Engl. 2020, 395, 565–574. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet Lond. Engl. 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Chauhan, S. Comprehensive review of coronavirus disease 2019 (COVID-19). Biomed. J. 2020, 43, 334–340. [Google Scholar] [CrossRef]
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Niu, S.; Tian, S.; Lou, J.; Kang, X.; Zhang, L.; Lian, H.; Zhang, J. Clinical characteristics of older patients infected with COVID-19: A descriptive study. Arch. Gerontol. Geriatr. 2020, 89, 104058. [Google Scholar] [CrossRef]
- Casas-Rojo, J.M.; Antón-Santos, J.M.; Millán-Núñez-Cortés, J.; Lumbreras-Bermejo, C.; Ramos-Rincón, J.M.; Roy-Vallejo, E.; Artero-Mora, A.; Arnalich-Fernández, F.; García-Bruñén, J.M.; Vargas-Núñez, J.A.; et al. Clinical characteristics of patients hospitalized with COVID-19 in Spain: Results from the SEMI-COVID-19 Registry. Rev. Clin. Esp. 2020, 220, 480–494. [Google Scholar] [CrossRef] [PubMed]
- Mostaza, J.M.; García-Iglesias, F.; González-Alegre, T.; Blanco, F.; Varas, M.; Hernández-Blanco, C.; Hontañón, V.; Jaras-Hernández, M.J.; Martínez-Prieto, M.; Menéndez-Saldaña, A.; et al. Clinical course and prognostic factors of COVID-19 infection in an elderly hospitalized population. Arch. Gerontol. Geriatr. 2020, 91, 104204. [Google Scholar] [CrossRef]
- Rivera-Izquierdo, M.; Del Carmen Valero-Ubierna, M.; R-delAmo, J.L.; Fernández-García, M.Á.; Martínez-Diz, S.; Tahery-Mahmoud, A.; Rodríguez-Camacho, M.; Gámiz-Molina, A.B.; Barba-Gyengo, N.; Gámez-Baeza, P.; et al. Sociodemographic, clinical and laboratory factors on admission associated with COVID-19 mortality in hospitalized patients: A retrospective observational study. PLoS ONE 2020, 15, e0235107. [Google Scholar] [CrossRef] [PubMed]
- Boëlle, P.-Y.; Delory, T.; Maynadier, X.; Janssen, C.; Piarroux, R.; Pichenot, M.; Lemaire, X.; Baclet, N.; Weyrich, P.; Melliez, H.; et al. Trajectories of Hospitalization in COVID-19 Patients: An Observational Study in France. J. Clin. Med. 2020, 9, 3148. [Google Scholar] [CrossRef] [PubMed]
- Giorgi Rossi, P.; Marino, M.; Formisano, D.; Venturelli, F.; Vicentini, M.; Grilli, R. Reggio Emilia COVID-19 Working Group Characteristics and outcomes of a cohort of COVID-19 patients in the Province of Reggio Emilia, Italy. PLoS ONE 2020, 15, e0238281. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; the Northwell COVID-19 Research Consortium; Barnaby, D. P.; Becker, L.B.; Chelico, J.D.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Informe sobre la situación de COVID-19enEspaña. Available online: https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Documents/INFORMES/Informes%20COVID-19/Informe%20n%c2%ba%2030.%20Situaci%c3%b3n%20de%20COVID-19%20en%20Espa%c3%b1a%20a%2011%20de%20mayo%20de%202020.pdf (accessed on 29 October 2020).
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet Lond. Engl. 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Zheng, Z.; Peng, F.; Xu, B.; Zhao, J.; Liu, H.; Peng, J.; Li, Q.; Jiang, C.; Zhou, Y.; Liu, S.; et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J. Infect. 2020, 81, e16–e25. [Google Scholar] [CrossRef]
- Leung, C. Risk factors for predicting mortality in elderly patients with COVID-19: A review of clinical data in China. Mech. Ageing Dev. 2020, 188, 111255. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Jia, X.; Li, J.; Hu, K.; Chen, G.; Wei, J.; Gong, Z.; Zhou, C.; Yu, H.; et al. Risk factors for disease severity, unimprovement, and mortality in COVID-19 patients in Wuhan, China. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2020, 26, 767–772. [Google Scholar] [CrossRef]
- Cheng, Y.; Luo, R.; Wang, K.; Zhang, M.; Wang, Z.; Dong, L.; Li, J.; Yao, Y.; Ge, S.; Xu, G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020, 97, 829–838. [Google Scholar] [CrossRef]
- Liu, Y.; Du, X.; Chen, J.; Jin, Y.; Peng, L.; Wang, H.H.X.; Luo, M.; Chen, L.; Zhao, Y. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J. Infect. 2020, 81, e6–e12. [Google Scholar] [CrossRef]
- Wang, L.; He, W.; Yu, X.; Hu, D.; Bao, M.; Liu, H.; Zhou, J.; Jiang, H. Coronavirus disease 2019 in elderly patients: Characteristics and prognostic factors based on 4-week follow-up. J. Infect. 2020, 80, 639–645. [Google Scholar] [CrossRef]
- Smith, A.A.; Fridling, J.; Ibhrahim, D.; Porter, P.S. Identifying Patients at Greatest Risk of Mortality due to COVID-19: A New England Perspective. West. J. Emerg. Med. 2020, 21, 785–789. [Google Scholar] [CrossRef]
- Chilimuri, S.; Sun, H.; Alemam, A.; Mantri, N.; Shehi, E.; Tejada, J.; Yugay, A.; Nayudu, S.K. Predictors of Mortality in Adults Admitted with COVID-19: Retrospective Cohort Study from New York City. West. J. Emerg. Med. 2020, 21, 779–784. [Google Scholar] [CrossRef]
- Sisó-Almirall, A.; Kostov, B.; Mas-Heredia, M.; Vilanova-Rotllan, S.; Sequeira-Aymar, E.; Sans-Corrales, M.; Sant-Arderiu, E.; Cayuelas-Redondo, L.; Martínez-Pérez, A.; García-Plana, N.; et al. Prognostic factors in Spanish COVID-19 patients: A case series from Barcelona. PLoS ONE 2020, 15, e0237960. [Google Scholar] [CrossRef]
- Galloway, J.B.; Norton, S.; Barker, R.D.; Brookes, A.; Carey, I.; Clarke, B.D.; Jina, R.; Reid, C.; Russell, M.D.; Sneep, R.; et al. A clinical risk score to identify patients with COVID-19 at high risk of critical care admission or death: An observational cohort study. J. Infect. 2020, 81, 282–288. [Google Scholar] [CrossRef]
- Figliozzi, S.; Masci, P.G.; Ahmadi, N.; Tondi, L.; Koutli, E.; Aimo, A.; Stamatelopoulos, K.; Dimopoulos, M.-A.; Caforio, A.L.P.; Georgiopoulos, G. Predictors of adverse prognosis in COVID-19: A systematic review and meta-analysis. Eur. J. Clin. Invest. 2020, 50, e13362. [Google Scholar] [CrossRef]
- Turcotte, J.J.; Meisenberg, B.R.; MacDonald, J.H.; Menon, N.; Fowler, M.B.; West, M.; Rhule, J.; Qureshi, S.S.; MacDonald, E.B. Risk factors for severe illness in hospitalized Covid-19 patients at a regional hospital. PLoS ONE 2020, 15, e0237558. [Google Scholar] [CrossRef]
- Pascual Gómez, N.F.; Monge Lobo, I.; Granero Cremades, I.; Figuerola Tejerina, A.; Ramasco Rueda, F.; von Wernitz Teleki, A.; Arrabal Campos, F.M.; Sanz de Benito, M.A. Potential biomarkers predictors of mortality in COVID-19 patients in the Emergency Department. Rev. Esp. Quimioter. Publ. Of. Soc. Esp. Quimioter. 2020, 33, 267–273. [Google Scholar] [CrossRef]
- Wortham, J.M.; Lee, J.T.; Althomsons, S.; Latash, J.; Davidson, A.; Guerra, K.; Murray, K.; McGibbon, E.; Pichardo, C.; Toro, B.; et al. Characteristics of Persons Who Died with COVID-19–United States, February 12-May 18, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 923–929. [Google Scholar] [CrossRef]
- Gómez Sáenz, J.T.; Quintano Jiménez, J.A.; Hidalgo Requena, A.; González Béjar, M.; Gérez Callejas, M.J.; Zangróniz Uruñuela, M.R.; Moreno Vilaseca, A.; Hernández García, R. Chronic obstructive pulmonary disease: Morbimortality and healthcare burden. Semergen 2014, 40, 198–204. [Google Scholar] [CrossRef]
- Gao, Q.; Hu, Y.; Dai, Z.; Xiao, F.; Wang, J.; Wu, J. The epidemiological characteristics of 2019 novel coronavirus diseases (COVID-19) in Jingmen, Hubei, China. Medicine 2020, 99, e20605. [Google Scholar] [CrossRef]
- Klang, E.; Kassim, G.; Soffer, S.; Freeman, R.; Levin, M.A.; Reich, D.L. Severe Obesity as an Independent Risk Factor for COVID-19 Mortality in Hospitalized Patients Younger than 50. Obesity 2020, 28, 1595–1599. [Google Scholar] [CrossRef]
- Palaiodimos, L.; Kokkinidis, D.G.; Li, W.; Karamanis, D.; Ognibene, J.; Arora, S.; Southern, W.N.; Mantzoros, C.S. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism 2020, 108, 154262. [Google Scholar] [CrossRef]
- Giacomelli, A.; Ridolfo, A.L.; Milazzo, L.; Oreni, L.; Bernacchia, D.; Siano, M.; Bonazzetti, C.; Covizzi, A.; Schiuma, M.; Passerini, M.; et al. 30-day mortality in patients hospitalized with COVID-19 during the first wave of the Italian epidemic: A prospective cohort study. Pharmacol. Res. 2020, 158, 104931. [Google Scholar] [CrossRef]
- Gil-Rodrigo, A.; Miró, Ò.; Piñera, P.; Burillo-Putze, G.; Jiménez, S.; Martín, A.; Martín-Sánchez, F.J.; Jacob, J.; Guardiola, J.M.; García-Lamberechts, E.J.; et al. Analysis of clinical characteristics and outcomes in patients with COVID-19 based on a series of 1000 patients treated in Spanish emergency departments. Emerg. Rev. Soc. Espanola Med. Emerg. 2020, 32, 233–241. [Google Scholar]
- Wu, T.; Zuo, Z.; Kang, S.; Jiang, L.; Luo, X.; Xia, Z.; Liu, J.; Xiao, X.; Ye, M.; Deng, M. Multi-organ Dysfunction in Patients with COVID-19: A Systematic Review and Meta-analysis. Aging Dis. 2020, 11, 874–894. [Google Scholar] [CrossRef]
- Xie, J.; Covassin, N.; Fan, Z.; Singh, P.; Gao, W.; Li, G.; Kara, T.; Somers, V.K. Association Between Hypoxemia and Mortality in Patients With COVID-19. Mayo Clin. Proc. 2020, 95, 1138–1147. [Google Scholar] [CrossRef]
- Laguna-Goya, R.; Utrero-Rico, A.; Talayero, P.; Lasa-Lazaro, M.; Ramirez-Fernandez, A.; Naranjo, L.; Segura-Tudela, A.; Cabrera-Marante, O.; de Frias, E.R.; Garcia-Garcia, R.; et al. IL-6-based mortality risk model for hospitalized patients with COVID-19. J. Allergy Clin. Immunol. 2020, 146, 799–807.e9. [Google Scholar] [CrossRef]
- Yang, W.; Sirajuddin, A.; Zhang, X.; Liu, G.; Teng, Z.; Zhao, S.; Lu, M. The role of imaging in 2019 novel coronavirus pneumonia (COVID-19). Eur. Radiol. 2020, 30, 4874–4882. [Google Scholar] [CrossRef]
- Bashash, D.; Hosseini-Baharanchi, F.S.; Rezaie-Tavirani, M.; Safa, M.; Akbari Dilmaghani, N.; Faranoush, M.; Abolghasemi, H. The Prognostic Value of Thrombocytopenia in COVID-19 Patients; a Systematic Review and Meta-Analysis. Arch. Acad. Emerg. Med. 2020, 8, e75. [Google Scholar]
- Pan, F.; Yang, L.; Li, Y.; Liang, B.; Li, L.; Ye, T.; Li, L.; Liu, D.; Gui, S.; Hu, Y.; et al. Factors associated with death outcome in patients with severe coronavirus disease-19 (COVID-19): A case-control study. Int. J. Med. Sci. 2020, 17, 1281–1292. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Rad, S.; Rostami, T.; Rostami, M.; Mousavi, S.A.; Mirhoseini, S.A.; Kiumarsi, A. Hematologic predictors of mortality in hospitalized patients with COVID-19: A comparative study. Hematol. Amst. Neth. 2020, 25, 383–388. [Google Scholar] [CrossRef]
- Azoulay, E.; Fartoukh, M.; Darmon, M.; Géri, G.; Voiriot, G.; Dupont, T.; Zafrani, L.; Girodias, L.; Labbé, V.; Dres, M.; et al. Increased mortality in patients with severe SARS-CoV-2 infection admitted within seven days of disease onset. Intensive Care Med. 2020, 46, 1714–1722. [Google Scholar] [CrossRef]
- Lang, M.; Buch, K.; Li, M.D.; Mehan, W.A.; Lang, A.L.; Leslie-Mazwi, T.M.; Rincon, S.P. Leukoencephalopathy Associated with Severe COVID-19 Infection: Sequela of Hypoxemia? AJNR Am. J. Neuroradiol. 2020, 41, 1641–1645. [Google Scholar] [CrossRef] [PubMed]
- Marengoni, A.; Zucchelli, A.; Grande, G.; Fratiglioni, L.; Rizzuto, D. The impact of delirium on outcomes for older adults hospitalised with COVID-19. Age Ageing 2020, 49, 923–926. [Google Scholar] [CrossRef] [PubMed]
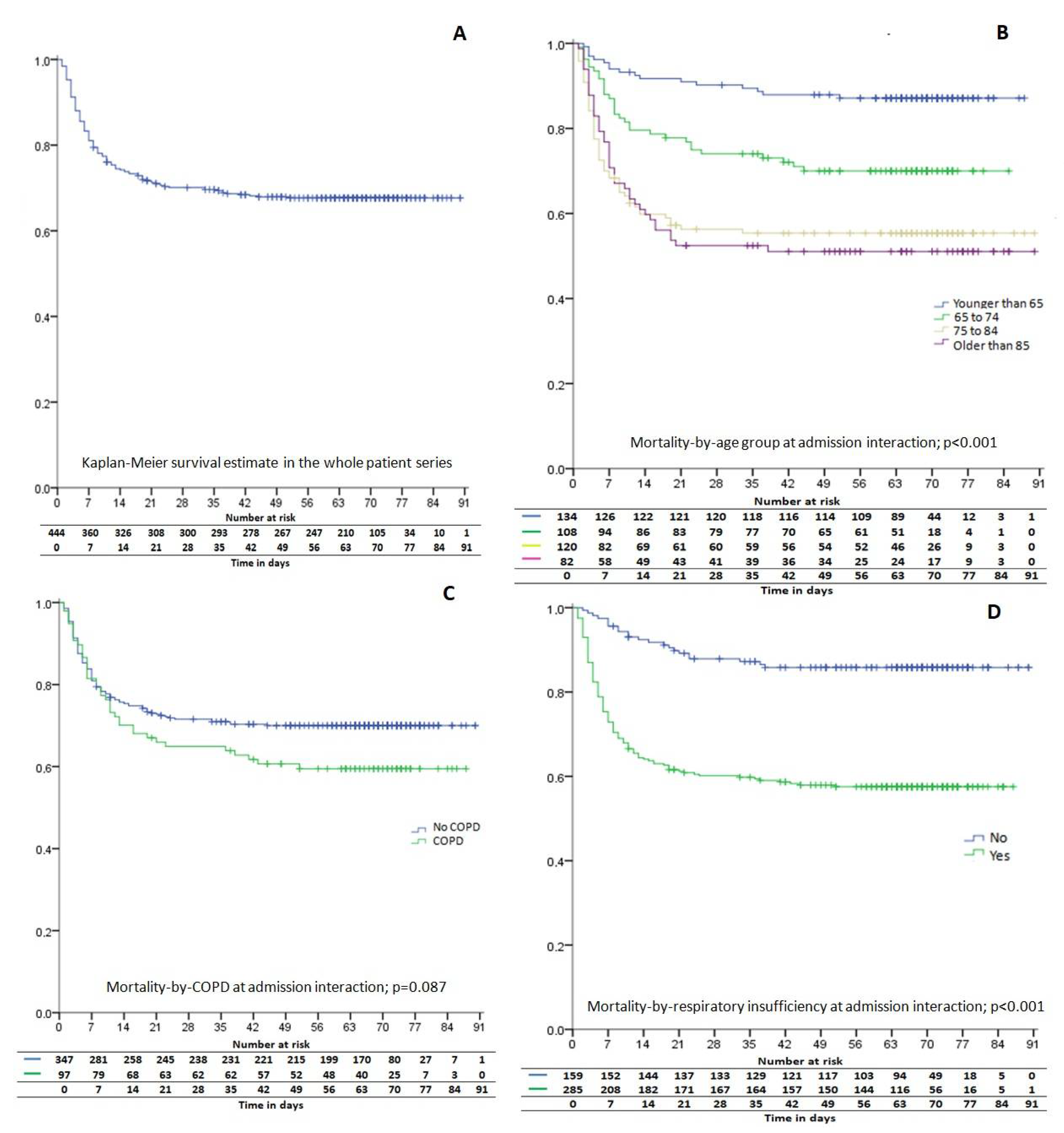
| Total (n = 444) | Survivors (n = 302) | Dead (n = 142) | p | |||
|---|---|---|---|---|---|---|
| Mean age at admission, years (SD; rank) | 71.2 (14.6; 22–98) | 68.2 (15.1) | 77.4 (10.9) | <0.001 | ||
| bellow 65, n (%) | 134 (30.2%) | 117 (87.3%) | 17 (12.7%) | <0.001 | ||
| 65 to 74, n (%) | 108 (24.3%) | 76 (70.4%) | 32 (29.6%) | |||
| 75 to 84, n (%) | 120 (27%) | 67 (55.8%) | 53 (44.2%) | |||
| over 85, n (%) | 82 (18.5%) | 42 (51.2%) | 40 (48.8%) | |||
| Sex | Male, n (%) | 251 (56.5%) | 172 (68.5%) | 79 (31.5%) | 0.794 | |
| Female, n (%) | 193 (43.5%) | 130 (67.4%) | 63 (32.6%) | |||
| Smoker | No, n (%) | 317 (71.4%) | 210 (66.2%) | 107 (33.8%) | 0.296 | |
| Yes, n (%) | 21 (4.7%) | 17 (81%) | 4 (19%) | |||
| Former, n (%) | 106 (23.9%) | 75 (70.8%) | 31 (29.2%) | |||
| BMI | Normal weight, n (%) | 138 (31.1%) | 97 (70.3%) | 41 (29.7%) | 0.057 | |
| Overweight, n (%) | 102 (23%) | 73 (71.6%) | 29 (28.4%) | |||
| Obesity class 1, n (%) | 110 (24.8%) | 66 (60%) | 44 (40%) | |||
| Obesity class 2, n (%) | 74 (16.7%) | 56 (75.7%) | 18 (24.3%) | |||
| Obesity class 3, n (%) | 20 (4.5%) | 10 (50%) | 10 (50%) | |||
| Other comorbidities | Immunosuppression, n (%) | Yes | 26 (5.9%) | 14 (53.8%) | 12 (46.2%) | 0.110 |
| No | 418 (94.1%) | 288 (68.9%) | 130 (31.1%) | |||
| Arterial hypertension, n (%) | Yes | 303 (68.2%) | 194 (64%) | 109 (36%) | 0.008 | |
| No | 141 (31.8%) | 108 (76.6%) | 33 (23.4%) | |||
| Diabetes, n (%) | Yes | 143 (32.2%) | 94 (65.7%) | 49 (34.3%) | 0.477 | |
| No | 301 (67.8%) | 208 (69.1%) | 93 (30.9%) | |||
| Hypothyroidism, n (%) | Yes | 58 (13.1%) | 37 (63.8%) | 21 (36.2%) | 0.459 | |
| No | 386 (86.9%) | 265 (68.7%) | 121 (31.3%) | |||
| Oncological disease, n (%) | Yes | 45 (10.1%) | 25 (55.6%) | 20 (44.4%) | 0.059 | |
| No | 399 (89.8%) | 277 (69.4%) | 122 (30.6%) | |||
| Asthma, n (%) | Yes | 31 (7%) | 22 (71%) | 9 (29%) | 0.715 | |
| No | 413 (93%) | 280 (67.8%) | 133 (32.2%) | |||
| COPD, n (%) | Yes | 97 (21.8%) | 58 (59.8%) | 39 (40.2%) | 0.049 | |
| No | 347 (78.2%) | 244 (70.3%) | 103 (29.7%) | |||
| CKD > stage II, n (%) | Yes | 59 (13.3%) | 31 (52.5%) | 28 (47.5%) | 0.006 | |
| No | 385 (86.7%) | 271 (70.4%) | 114 (29.6%) | |||
| CHF > Class II, n (%) | Yes | 57 (12.8%) | 30 (52.6%) | 27 (47.4%) | 0.008 | |
| No | 387 (87.2%) | 272 (70.3%) | 115 (29.7%) | |||
| Ischemic heart disease, n (%) | Yes | 57 (12.8%) | 32 (56.1%) | 25 (43.9%) | 0.039 | |
| No | 387 (87.2%) | 270 (69.8%) | 117 (30.2%) | |||
| Chronic liver disease, n (%) | Yes | 31 (7%) | 19 (61.3%) | 12 (38.7%) | 0.405 | |
| No | 413 (93%) | 283 (68.5%) | 130 (31.5%) | |||
| Mean (SD) number of comorbidities, n (%) | 3.1 (1.9) | 2.7 (1.8) | 3.6 (1.9) | <0.001 | ||
| Nursing-home residents, n (%) | Yes | 71 (16%) | 40 (56.3%) | 31 (43.7%) | 0.021 | |
| No | 373 (84%) | 262 (70.2%) | 111 (29.8%) | |||
| Presenting Symptoms | Dyspnea, n (%) | 351 (79.1%) | 229 (65.2%) | 122 (34.8%) | 0.015 | |
| Cough, n (%) | 280 (63.1%) | 204 (72.9%) | 76 (27.1%) | 0.004 | ||
| Fever, n (%) | 286 (64.4%) | 202 (70.6%) | 84 (29.4%) | 0.112 | ||
| Days from first symptoms to admission, mean (SD) | 7 (5.3; 0–37) | 5.37 (4.08) | 7.76 (5.65) | <0.001 | ||
| Respiratory insufficiency | Yes | 285 (64.2%) | 165 (57.9%) | 120 (42.1%) | <0.001 | |
| No | 159 (35.8%) | 137 (86.2%) | 22 (13.8%) | |||
| FIO2 to maintain O2 saturation >90%, mean (SD, rank) | 42.4 (30.9; 21–100) | 36.27 (26.3) | 55.37 (35.6) | <0.001 | ||
| Pneumonia | Unilateral | 51 (11.55) | 39 (76.5%) | 12 (23.5%) | 0.238 | |
| Bilateral | 358 (80.6%) | 237 (66.2%) | 121 (33.8%) | |||
| Lobar | 4 (0.9%) | 4 (100%) | 0 | |||
| Mean (SD) days from admission to discharge or dead | 11.2 (10.3) | 11.9 (10.4) | 9.8 (10.1) | 0.045 | ||
| Total (n = 444) N (%) | Survivors (n = 302) N (%) | Dead (n = 142) N (%) | p | |
|---|---|---|---|---|
| Hemoglobin, g/dL | 13.6 (1.9) | 13.8 (1.8) | 13.3 (2) | 0.018 |
| Leukocytes, ×106, per L | 8326 (3866.2) | 8223.4 (3646.8) | 8544.5 (4301.8) | 0.444 |
| Lymphocytes, ×106, per L | 1050.5 (839.4) | 1099.4 (775.9) | 945.7 (956.2) | 0.074 |
| Platelets, ×109, per L | 219.84 (96.09) | 231.40 (102.50) | 195.80 (74.28) | <0.001 |
| Fibrinogen (n = 183) | 636 (179) | 628.7 (187.8) | 654.4 (154.7) | 0.382 |
| D Dimer, ng/mL (n = 266) | 2.8 (4.4) | 2.75 (4.4) | 3.03 (4.4) | 0.650 |
| C-reactive protein, mg/L | 138 (102) | 126 (102) | 164 (99) | <0.001 |
| Urea, mg/dL | 57.7 (40.4) | 48.8 (32.4) | 76.5 (48.5) | <0.001 |
| Creatinine, mg/dL | 1.29 (0.8) | 1.11 (0.5) | 1.66 (1.05) | <0.001 |
| AST, U/L (n = 331) | 42.3 (28.4) | 40.7 (27) | 46.2 (31.6) | 0.117 |
| ALT, U/L (n = 351) | 35.5 (33.7) | 37.6 (37.6) | 30.4 (20.6) | 0.023 |
| GGT, U/L (n = 96) | 80.3 (107.5) | 84.1 (109.6) | 65.9 (100.8) | 0.506 |
| LDH, U/L (n = 337) | 651.49 (275.24) | 594.5 (246.70) | 825.78 (286.07) | <0.001 |
| Ferritin, ng/mL (n = 91) | 1042.3 (770.3) | 1043.8 (799.6) | 1036 (658.2) | 0.970 |
| TSH, mU/L (n = 78) | 1.2 (1.2) | 1.25 (1.37) | 1.06 (0.7) | 0.569 |
| T4 libre, μg/dL (n = 27) | 1.3 (0.3) | 1.36 (0.4) | 1.29 (0.19) | 0.643 |
| Ultra-sensitive troponin, ng/dL (n = 87) | 66.1 (178.7) | 28.23 (45.3) | 134.6 (283.2) | 0.046 |
| Interleukine 6, pg/mL (n = 19) | 170.8 (302.3) | 81.8 (75.6) | 420.1 (535.9) | 0.231 |
| Partial arterial pressure of oxygen (paO2), mmHg | 62.6 (24.6) | 65.6 (26) | 55.7 (19.5) | <0.001 |
| Partial arterial pressure of carbon dioxide (paCO2), mmHg | 35.5 (7.6) | 35 (6.8) | 36.6 (9) | 0.077 |
| HCO3, mEq/L | 23.7 (4.1) | 23.9 (3.7) | 3.7) | 0.122 |
| Lactate, mmol/L | 1.87 (4.5) | 1.8 (5.3) | 2 (1.4) | 0.753 |
| Total (n = 444) | Survivors (n = 302) | Dead (n = 142) | p | ||
|---|---|---|---|---|---|
| No treatment, n (%) | Yes | 74 (16.7%) | 25 (33.8%) | 49 (66.2%) | <0.001 |
| No | 370 (83.3%) | 277 (74.9%) | 93 (25.1%) | ||
| Antiviral drugs, n (%) | Yes | 357 (80.4%) | 269 (75.4%) | 88 (24.6%) | <0.001 |
| No | 87 (19.6%) | 33 (37.9%) | 54 (62.1%) | ||
| Corticosteroids, n (%) | Yes | 134 (30.2%) | 111 (82.8%) | 23 (17.2%) | <0.001 |
| No | 310 (69.8%) | 191 (61.6%) | 119 (38.4%) | ||
| Doxycyclin and/or acetylcysteine, n (%) | Yes | 41 (9.2%) | 38 (92.7%) | 3 (7.3%) | <0.001 |
| No | 403 (90.8%) | 264 (65.5%) | 139 (34.5%) | ||
| Heparins of low molecular weight, n (%) | Yes | 99 (22.3%) | 74 (74.7%) | 25 (25.3%) | 0.103 |
| No | 345 (77.7%) | 228 (66.1%) | 117 (33.9%) | ||
| Predictor | Odds Ratio (95% Confidence Interval) | p | |
|---|---|---|---|
| Age (years) | Below 65 | Ref | - |
| 65 to 74 | 3.04 (1.21–7.66) | 0.019 | |
| 75 to 84 | 4.22 (1.67–10.66) | 0.002 | |
| Over 85 | 8.16 (2.91–22.86) | <0.001 | |
| Chronic Obstructive Pulmonary Disease (COPD) | 2.01 (1.01–4.02) | 0.048 | |
| Respiratory insufficiency at admission | 2.31 (1.16–4.62) | 0.018 | |
| Analytical values at admission | Creatinine (each 1 mg/dL increase) | 3.12 (1.95–5.01) | <0.001 |
| LDH (>500 U/L) | 4.61 (1.95–10.91) | <0.001 | |
| Platelets (<150 × 109, per L) | 2.84 (1.39–5.79) | 0.004 | |
| Lymphocytes (<1000 cells per µL) | 1.75 (0.94–3.25) | 0.080 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maestre-Muñiz, M.M.; Arias, Á.; Arias-González, L.; Angulo-Lara, B.; Lucendo, A.J. Prognostic Factors at Admission for In-Hospital Mortality from COVID-19 Infection in an Older Rural Population in Central Spain. J. Clin. Med. 2021, 10, 318. https://doi.org/10.3390/jcm10020318
Maestre-Muñiz MM, Arias Á, Arias-González L, Angulo-Lara B, Lucendo AJ. Prognostic Factors at Admission for In-Hospital Mortality from COVID-19 Infection in an Older Rural Population in Central Spain. Journal of Clinical Medicine. 2021; 10(2):318. https://doi.org/10.3390/jcm10020318
Chicago/Turabian StyleMaestre-Muñiz, Modesto M., Ángel Arias, Laura Arias-González, Basilio Angulo-Lara, and Alfredo J. Lucendo. 2021. "Prognostic Factors at Admission for In-Hospital Mortality from COVID-19 Infection in an Older Rural Population in Central Spain" Journal of Clinical Medicine 10, no. 2: 318. https://doi.org/10.3390/jcm10020318
APA StyleMaestre-Muñiz, M. M., Arias, Á., Arias-González, L., Angulo-Lara, B., & Lucendo, A. J. (2021). Prognostic Factors at Admission for In-Hospital Mortality from COVID-19 Infection in an Older Rural Population in Central Spain. Journal of Clinical Medicine, 10(2), 318. https://doi.org/10.3390/jcm10020318







