High-Resolution CT Findings as Predictive Factors for Recurrent Nontuberculous Mycobacterial Pulmonary Disease after Successful Treatment
Abstract
1. Introduction
2. Materials and Methods
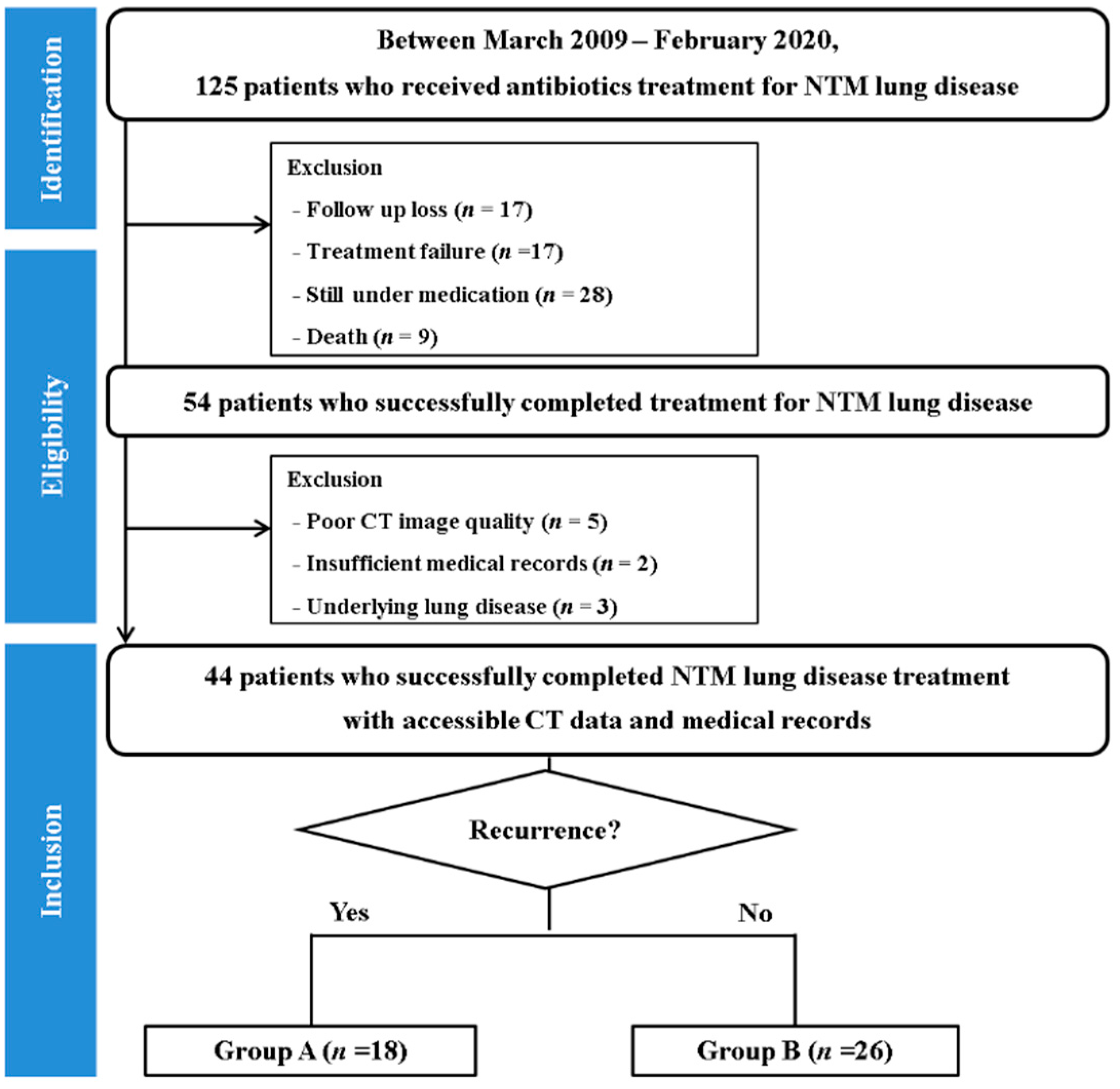
2.1. Study Population
2.2. CT Acquisition
2.3. CT Interpretation
2.4. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Comparison of CT Scores at the Time of Treatment Termination between Patients with and without NTM-PD Recurrence
3.3. Difference in Treatment Response between Patients with and without NTM-PD Recurrence
3.4. Reproducibility
3.5. Predictors of Recurrent NTM-PD at the Time of Treatment Termination
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stout, J.E.; Koh, W.-J.; Yew, W.W. Update on pulmonary disease due to non-tuberculous mycobacteria. Int. J. Infect. Dis. 2016, 45, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Winthrop, K.L.; Marras, T.K.; Adjemian, J.; Zhang, H.; Wang, P.; Zhang, Q. Incidence and Prevalence of Nontuberculous Mycobacterial Lung Disease in a Large US Managed Care Health Plan, 2008–2015. Ann. Am. Thorac. Soc. 2020, 17, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Griffith, D.E.; Aksamit, T.; Brown-Elliott, B.A.; Catanzaro, A.; Daley, C.; Gordin, F.; Holland, S.M.; Horsburg, R.; Huitt, G.; Lademarco, M.F.; et al. An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 2007, 175, 367–416. [Google Scholar] [CrossRef]
- Koh, W.-J.; Lee, K.S.; Kwon, O.J.; Jeong, Y.J.; Kwak, S.-H.; Kim, T.S. Bilateral bronchiectasis and bronchiolitis at thin-section CT: Diagnostic implications in nontuberculous mycobacterial pulmonary infection. Radiology 2005, 235, 282–288. [Google Scholar] [CrossRef]
- Haworth, C.S.; Banks, J.; Capstick, T.; Fisher, A.J.; Gorsuch, T.; Laurenson, I.F.; Leitch, A.; Loebinger, M.R.; Milburn, H.J.; Nightingale, M.; et al. British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax 2017, 72 (Suppl. 2), ii1–ii64. [Google Scholar] [CrossRef]
- Lee, B.Y.; Kim, S.; Hong, Y.; Lee, S.-D.; Kim, W.S.; Kim, D.S.; Shim, T.S.; Jo, K.-W. Risk factors for recurrence after successful treatment of Mycobacterium avium complex lung disease. Antimicrob. Agents Chemother. 2015, 59, 2972–2977. [Google Scholar] [CrossRef]
- Lee, G.; Lee, K.S.; Moon, J.W.; Koh, W.-J.; Jeong, B.-H.; Jeong, Y.J.; Kim, H.J.; Woo, S. Nodular bronchiectatic Mycobacterium avium complex pulmonary disease. Natural course on serial computed tomographic scans. Ann. Am. Thorac. Soc. 2013, 10, 299–306. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, K.S.; Koh, W.-J.; Jeon, K.; Lee, E.J.; Kang, H.; Ahn, J. Serial CT findings of Mycobacterium massiliense pulmonary disease compared with Mycobacterium abscessus disease after treatment with antibiotic therapy. Radiology 2012, 263, 260–270. [Google Scholar] [CrossRef]
- Kuroishi, S.; Nakamura, Y.; Hayakawa, H.; Shirai, M.; Nakano, Y.; Yasuda, K.; Suda, T.; Nakamura, H.; Chida, K. Mycobacterium avium complex disease: Prognostic implication of high-resolution computed tomography findings. Eur. Respir. J. 2008, 32, 147–152. [Google Scholar] [CrossRef]
- Park, T.Y.; Chong, S.; Jung, J.-W.; Park, I.W.; Choi, B.W.; Lim, C.; Lee, C.U.; Kim, Y.S.; Choi, H.W.; Choi, J.C. Natural course of the nodular bronchiectatic form of Mycobacterium Avium complex lung disease: Long-term radiologic change without treatment. PLoS ONE 2017, 12, e0185774. [Google Scholar] [CrossRef]
- Lee, G.; Kim, H.S.; Lee, K.S.; Koh, W.-J.; Jeon, K.; Jeong, B.-H.; Ahn, J. Serial CT findings of nodular bronchiectatic Mycobacterium avium complex pulmonary disease with antibiotic treatment. AJR Am. J. Roentgenol. 2013, 201, 764–772. [Google Scholar] [CrossRef]
- Koh, W.-J.; Mon, S.M.; Kim, S.-Y.; Woo, M.-A.; Kim, S.; Jhun, B.W.; Park, H.Y.; Jeon, K.; Huh, H.J.; Ki, C.-S.; et al. Outcomes of Mycobacterium avium complex lung disease based on clinical phenotype. Eur. Respir. J. 2017, 50, 1602503. [Google Scholar] [CrossRef] [PubMed]
- van Ingen, J.; Aksamit, T.; Andrejak, C.; Bottger, E.C.; Cambau, E.; Daley, C.L.; Griffith, D.E.; Guglielmetti, L.; Holland, S.M.; Huitt, G.A.; et al. Treatment outcome definitions in nontuberculous mycobacterial pulmonary disease: An NTM-NET consensus statement. Eur. Respir. J. 2018, 51, 1800170. [Google Scholar] [CrossRef]
- Hayashi, M.; Takayanagi, N.; Kanauchi, T.; Miyahara, Y.; Yanagisawa, T.; Sugita, Y. Prognostic factors of 634 HIV-negative patients with Mycobacterium avium complex lung disease. Am. J. Respir. Crit. Care Med. 2012, 185, 575–583. [Google Scholar] [CrossRef]
- Hansell, D.M.; Bankier, A.A.; MacMahon, H.; McLoud, T.C.; Muller, N.L.; Remy, J. Fleischner Society: Glossary of terms for thoracic imaging. Radiology 2008, 246, 697–722. [Google Scholar] [CrossRef]
- Tanaka, E.; Kimoto, T.; Tsuyuguchi, K.; Watanabe, I.; Matsumoto, H.; Niimi, A.; Suzuki, K.; Murayama, T.; Amitani, R.; Kuze, F. Effect of clarithromycin regimen for Mycobacterium avium complex pulmonary disease. Am. J. Respir. Crit. Care Med. 1999, 160, 866–872. [Google Scholar] [CrossRef]
- Wallace, R.J., Jr.; Brown-Elliott, B.A.; McNulty, S.; Philley, J.V.; Killingley, J.; Wilson, R.W.; York, D.S.; Shepherd, S.; Griffith, D.E. Macrolide/azalide therapy for nodular/bronchiectatic Mycobacterium avium complex lung disease. Chest 2014, 146, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.-L.; Hasker, E.; Deun, A.V.; Roberfroid, D.; Boelaert, M.; der Stuyft, P.V. Recurrence in tuberculosis: Relapse or reinfection? Lancet Infect. Dis. 2003, 3, 282–287. [Google Scholar] [CrossRef]
- Gennaro, F.D.; Vittozzi, P.; Gualano, G.; Musso, M.; Mosti, S.; Mencarini, P.; Pareo, C.; Caro, A.D.; Schinina, V.; Girardi, E.; et al. Active Pulmonary Tuberculosis in Eldery Patients: A 2016–2019 Retrospective Anlysis from an Intalian Referral Hospital. Antibiotics 2020, 9, 489. [Google Scholar] [CrossRef]
- Kim, T.S.; Koh, W.-J.; Han, J.; Chung, M.J.; Lee, J.H.; Lee, K.S.; Kwon, O.J. Hypothesis on the evolution of cavitary lesions in nontuberculous mycobacterial pulmonary infection: Thin-section CT and histopathologic correlation. AJR Am. J. Roentgenol. 2005, 184, 1247–1252. [Google Scholar] [CrossRef]
- Lake, M.A.; Ambrose, L.R.; Lipman, M.C.; Lowe, D.M. “Why me, why now?” Using clinical immunology and epidemiology to explain who gets nontuberculous mycobacterial infection. BMC Med. 2016, 14, 54. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Non-Recurrent Group (n = 26) | Recurrent Group (n = 18) | p-Value |
|---|---|---|---|
| Age, y * | 58.7 ± 11.8 | 62.3 ± 10.1 | 0.55 |
| Sex (male: female) † | |||
| Height (m) * | 1.61 ± 0.09 | 1.62 ± 0.07 | 0.73 |
| Weight (kg) * | 51.1 ± 8.1 | 53.7 ± 8.8 | 0.18 |
| Body mass index (kg/m2) * | 19.8 ± 2.6 | 20.4 ± 2.9 | 0.12 |
| Pathogen, n (%) | 0.74 | ||
| M. avium complex | 16 (61.5) | 15 (83.4) | |
| M. abscessus subsp. abscessus | 3 (11.5) | 1 (5.5) | |
| M. abscessus subsp. massiliense | 2 (7.6) | 2 (11.1) | |
| Others | 3 (11.5) | ||
| Mixed pathogen | 2 (7.6) | ||
| Duration of treatment (days) * | 585.5 ± 160.8 | 683.6 ± 123.3 | 0.17 |
| CT acquisition to treatment initiation (median, day) | 21 | 14.5 | 0.42 |
| Treatment termination to CT acquisition (median, day) | 21.5 | 28.2 | 0.61 |
| Initial CT presentation † n (%) | 0.76 | ||
| Nodular bronchiectatic form | 14 (53.8) | 11 (61.1) | |
| Fibrocavitary form | 5 (19.2) | 2 (11.1) | |
| Unclassifiable form | 7 (26.9) | 5 (27.8) |
| CT Scores | Non-Recurrent Group (n = 26) | Recurrent Group (n = 18) | p-Value |
|---|---|---|---|
| At the time of treatment initiation | |||
| Bronchiectasis (9 points) | 4.69 ± 1.72 | 5.50 ± 1.72 | 0.119 |
| Cellular bronchiolitis (6 points) | 4.35 ± 1.57 | 4.89 ± 1.49 | 0.204 |
| Cavity (9 points) | 2.96 ± 3.04 | 2.89 ± 2.85 | 0.960 |
| Nodules (3 points) | 0.58 ± 0.51 | 1.17 ± 0.86 | 0.014 |
| Consolidation (3 points) | 0.69 ± 0.84 | 0.72 ± 0.83 | 0.876 |
| Total score (30 points) | 13.27 ± 4.21 | 15.17 ± 4.63 | 0.187 |
| At the time of treatment termination | |||
| Bronchiectasis (9 points) | 4.31 ± 1.62 | 5.61 ± 1.54 | 0.008 |
| Cellular bronchiolitis (6 points) | 2.73 ± 1.46 | 3.61 ± 1.29 | 0.112 |
| Cavity (9 points) | 1.65 ± 2.45 | 1.56 ± 2.71 | 0.784 |
| Nodules (3 points) | 0.27 ± 0.45 | 0.78 ± 0.65 | 0.006 |
| Consolidation (3 points) | 0.00 | 0.17 ± 0.38 | 0.033 |
| Total score (30 points) | 9.00 ± 3.82 | 11.72 ± 3.83 | 0.017 |
| CT Scores | ICC | 95% CI | p-Value |
|---|---|---|---|
| Bronchiectasis (9 points) | 0.876 | 0.729–0.944 | <0.001 |
| Cellular bronchiolitis (6 points) | 0.937 | 0.861–0.971 | <0.001 |
| Cavity (9 points) | 0.902 | 0.785–0.955 | <0.001 |
| Nodules (3 points) | 0.857 | 0.687–0.935 | <0.001 |
| Consolidation (3 points) | 0.531 | 0.063–0.794 | 0.035 |
| Total score (30 points) | 0.841 | 0.651–0.928 | <0.001 |
| Univariable Regression | Multivariable Regression | ||||
|---|---|---|---|---|---|
| Variables | Odds Ratio (95% CI) | p-Value | Odds Ratio (95% CI) | p-Value | Beta Coefficient |
| Initial nodule score | 4.344 (1.255, 15.038) | 0.020 | |||
| Last nodule score | 5.268 (1.543, 17.983) | 0.008 | 5.246 (1.370–20.087) | 0.016 | 1.657 |
| Last bronchiectasis score | 1.653 (1.075, 2.541) | 0.022 | 1.638 (1.049–2.558) | 0.030 | 0.494 |
| Last total score | 1.209 (1.012, 1.443) | 0.036 | |||
| Model constant | 0.004 | −3.627 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.; Cha, M.J.; Kim, Y.S.; Choi, J.C. High-Resolution CT Findings as Predictive Factors for Recurrent Nontuberculous Mycobacterial Pulmonary Disease after Successful Treatment. J. Clin. Med. 2021, 10, 172. https://doi.org/10.3390/jcm10020172
Choi H, Cha MJ, Kim YS, Choi JC. High-Resolution CT Findings as Predictive Factors for Recurrent Nontuberculous Mycobacterial Pulmonary Disease after Successful Treatment. Journal of Clinical Medicine. 2021; 10(2):172. https://doi.org/10.3390/jcm10020172
Chicago/Turabian StyleChoi, Hyewon, Min Jae Cha, Yang Soo Kim, and Jae Chol Choi. 2021. "High-Resolution CT Findings as Predictive Factors for Recurrent Nontuberculous Mycobacterial Pulmonary Disease after Successful Treatment" Journal of Clinical Medicine 10, no. 2: 172. https://doi.org/10.3390/jcm10020172
APA StyleChoi, H., Cha, M. J., Kim, Y. S., & Choi, J. C. (2021). High-Resolution CT Findings as Predictive Factors for Recurrent Nontuberculous Mycobacterial Pulmonary Disease after Successful Treatment. Journal of Clinical Medicine, 10(2), 172. https://doi.org/10.3390/jcm10020172





