Effect of Dosing Interval on Compliance of Osteoporosis Patients on Bisphosphonate Therapy: Observational Study Using Nationwide Insurance Claims Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
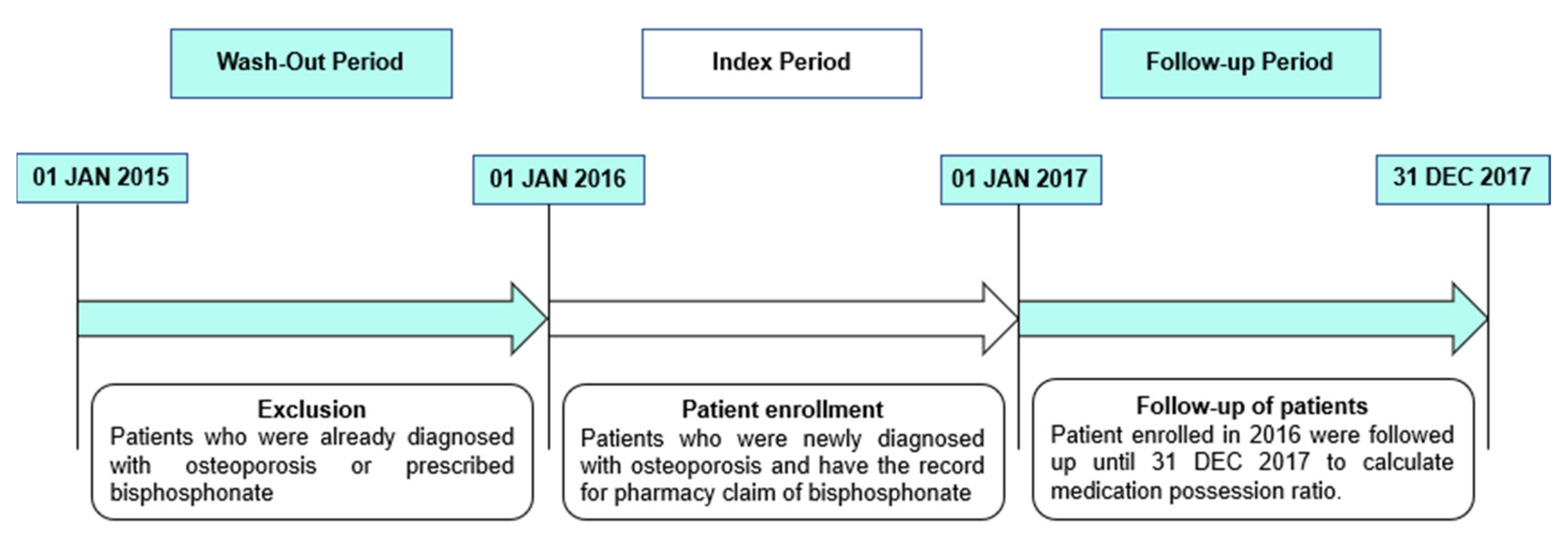
2.2. Study Population
2.3. Demographic Data
2.4. Measure of Compliance
2.5. Statistical Analyses
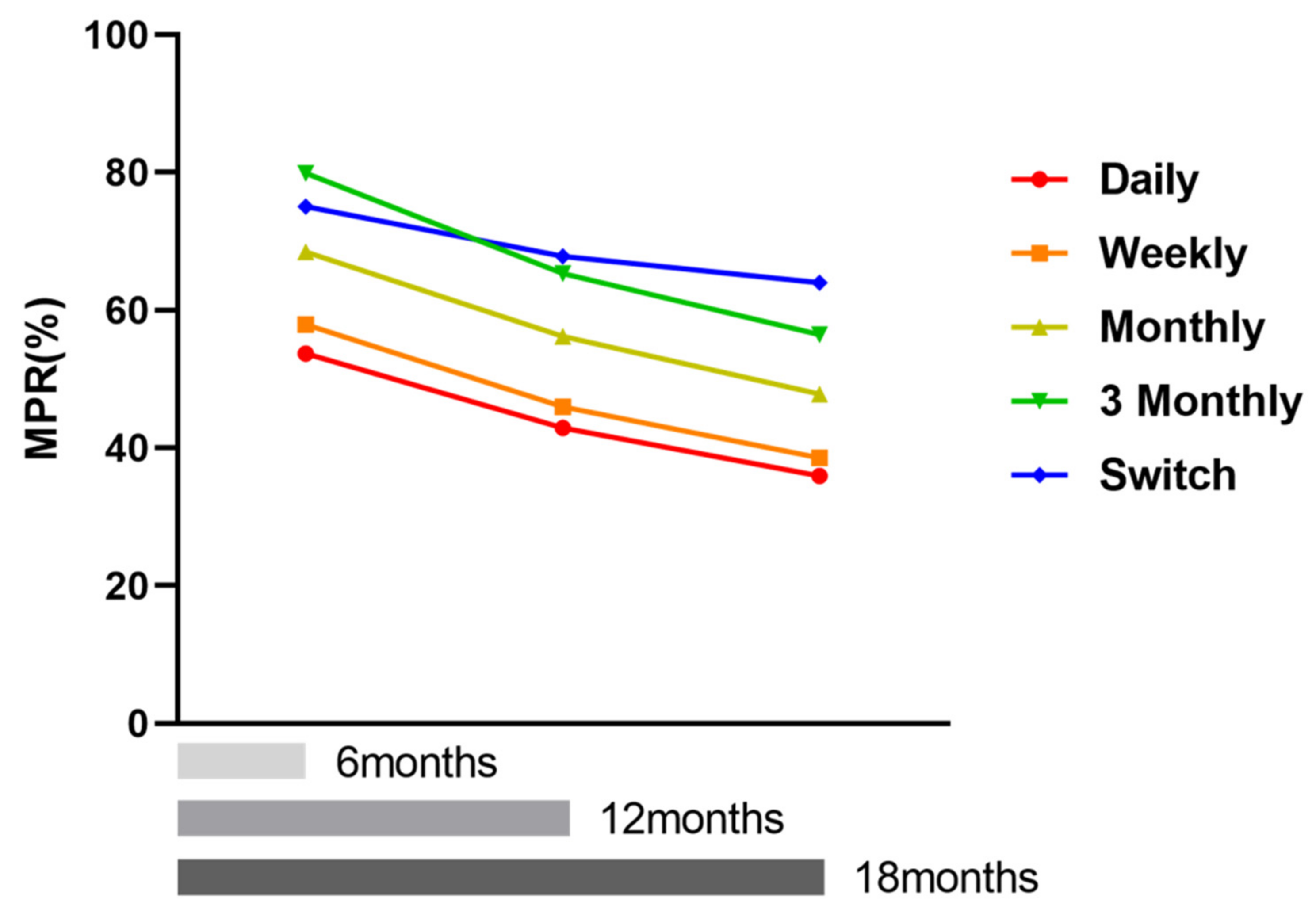
3. Additional Analyses with Longer Follow-Up
4. Results
4.1. Demographics of the Patients
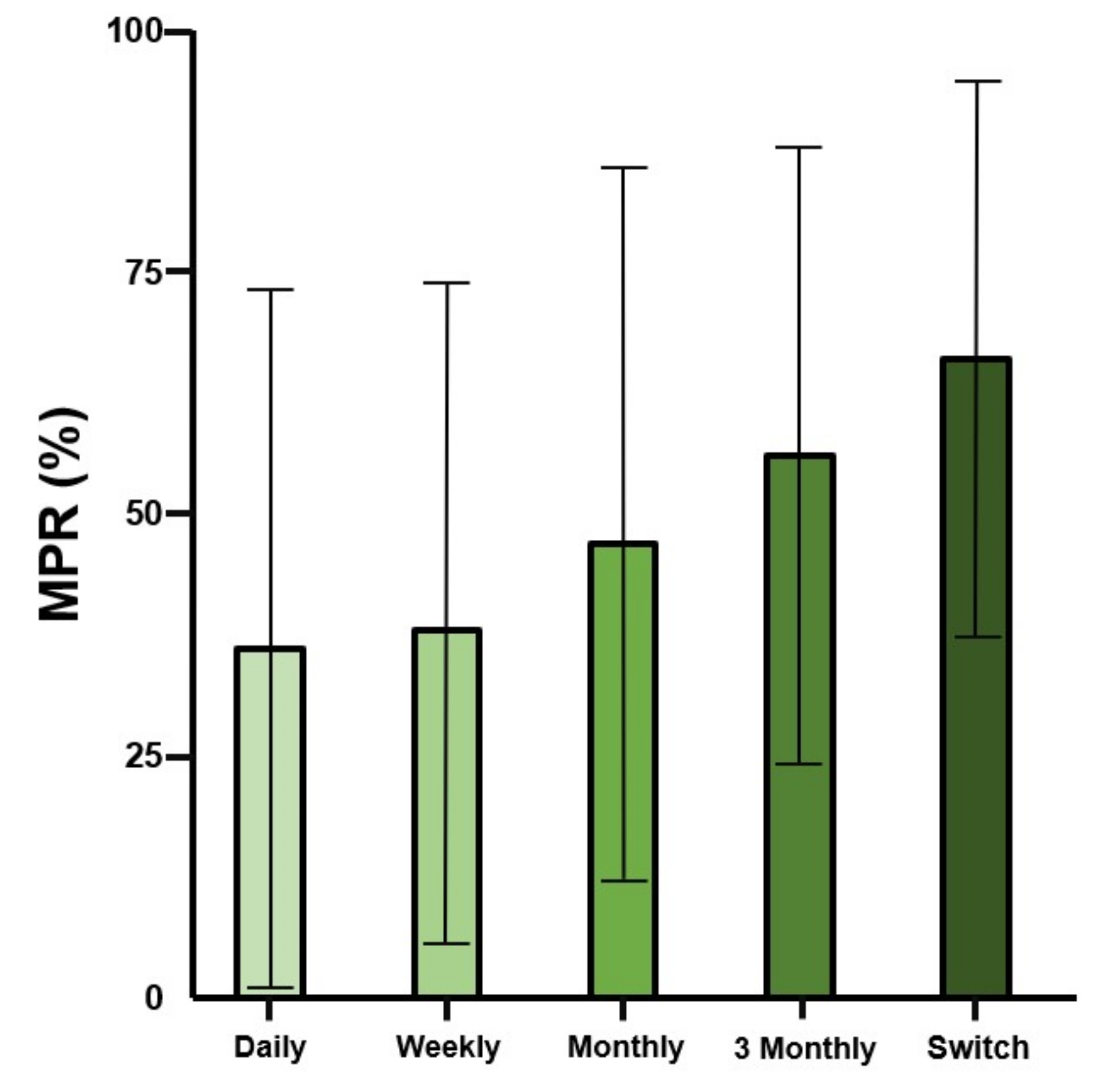
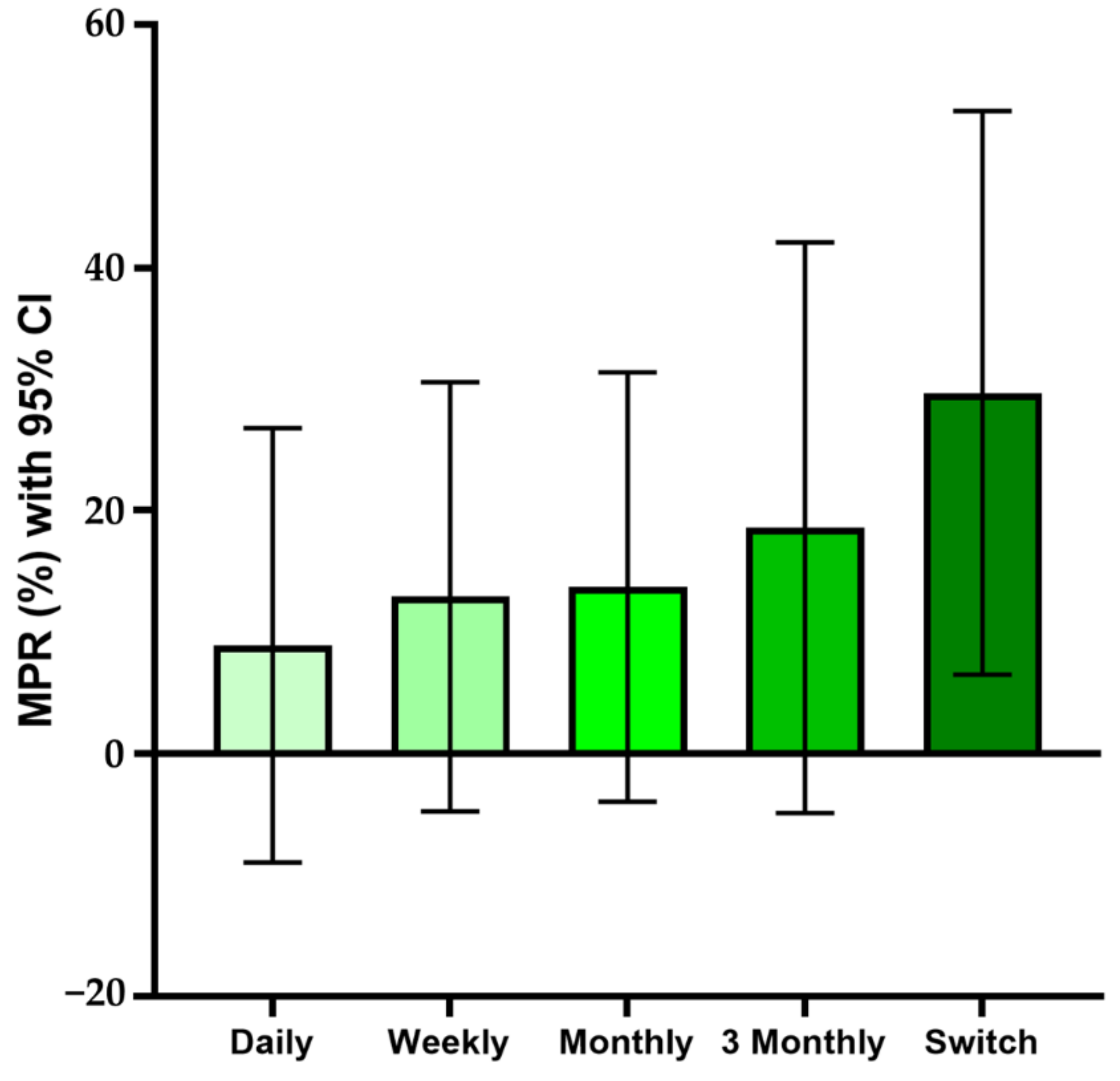
4.2. Drug Compliance
4.3. Factors Affecting Compliance Rate
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Johnell, O.; Kanis, J.A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006, 17, 1726–1733. [Google Scholar] [CrossRef]
- Burge, R.; Dawson-Hughes, B.; Solomon, D.H.; Wong, J.B.; King, A.; Tosteson, A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J. Bone Miner. Res. 2007, 22, 465–475. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, K.; Ko, Y.J.; Kim, S.J.; Oh, S.I.; Durrance, D.Y.; Yoo, D.; Park, S.M. Prevalence, awareness, and treatment of osteoporosis among Korean women: The Fourth Korea National Health and Nutrition Examination Survey. Bone 2012, 50, 1039–1047. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, Y.H.; Moon, S.H.; Lee, Y.S. Prevalence of Osteoporotic Vertebral Compression Fractures in Korean Post Menopausal Women Study, G. Influence of insurance benefit criteria on the administration rate of osteoporosis drugs in postmenopausal females. Clin. Orthop. Surg. 2014, 6, 56–61. [Google Scholar] [CrossRef]
- Siris, E.S.; Selby, P.L.; Saag, K.G.; Borgstrom, F.; Herings, R.M.; Silverman, S.L. Impact of osteoporosis treatment adherence on fracture rates in North America and Europe. Am. J. Med. 2009, 122, 3–13. [Google Scholar] [CrossRef]
- Solomon, D.H.; Avorn, J.; Katz, J.N.; Finkelstein, J.S.; Arnold, M.; Polinski, J.M.; Brookhart, M.A. Compliance with osteoporosis medications. Arch. Intern. Med. 2005, 165, 2414–2419. [Google Scholar] [CrossRef][Green Version]
- Barrett-Connor, E.; Wade, S.W.; Do, T.P.; Satram-Hoang, S.; Stewart, R.; Gao, G.; Macarios, D. Treatment satisfaction and persistence among postmenopausal women on osteoporosis medications: 12-month results from POSSIBLE US. Osteoporos Int. 2012, 23, 733–741. [Google Scholar] [CrossRef]
- Balkhi, B.; Seoane-Vazquez, E.; Rodriguez-Monguio, R. Changes in the utilization of osteoporosis drugs after the 2010 FDA bisphosphonate drug safety communication. Saudi Pharm. J. 2018, 26, 238–243. [Google Scholar] [CrossRef] [PubMed]
- McClung, M.R.; Benhamou, C.L.; Man, Z.; Tlustochowicz, W.; Zanchetta, J.R.; Eusebio, R.; Balske, A.M.; Matzkin, E.; Olszynski, W.P.; Recker, R.; et al. A novel monthly dosing regimen of risedronate for the treatment of postmenopausal osteoporosis: 2-year data. Calcif. Tissue Int. 2013, 92, 59–67. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cole, R.E.; Harris, S.T. Preventing nonvertebral osteoporotic fractures with extended-interval bisphosphonates: Regimen selection and clinical application. Medscape J. Med. 2009, 11, 12. [Google Scholar] [PubMed]
- Seong, J.M.; Kim, J.J.; Kim, H.J.; Sohn, H.S. Comparison of heart failure risk and medical costs between patients with type 2 diabetes mellitus treated with dapagliflozin and dipeptidyl peptidase-4 inhibitors: A nationwide population-based cohort study. Cardiovasc. Diabetol. 2020, 19, 95. [Google Scholar] [CrossRef]
- Kwon, J.W.; Ha, J.W.; Lee, T.S.; Moon, S.H.; Lee, H.M.; Park, Y. Comparison of the Prevalence of Low Back Pain and Related Spinal Diseases among Smokers and Nonsmokers: Using Korean National Health Insurance Database. Clin. Orthop. Surg. 2020, 12, 200–208. [Google Scholar] [CrossRef]
- Lee, Y.C.; Ju, H.J.; Kwon, J.W.; Bae, J.M. Seasonality of allergic diseases: Real-world evidence from a nationwide population-based study. Immun. Inflamm. Dis. 2020, 8, 360–362. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Park, S.Y.; Lee, P.H.; Park, H.R.; Park, S.Q.; Cho, S.J.; Chang, J.C. The Influence of Comorbidities on Reoperations Following Primary Surgery of Lumbar Degenerative Diseases: A Nationwide Population-Based Retrospective Cohort Study from 2009–2016. J. Korean Neurosurg. Soc. 2020, 63, 730–737. [Google Scholar] [CrossRef]
- Cramer, J.A.; Roy, A.; Burrell, A.; Fairchild, C.J.; Fuldeore, M.J.; Ollendorf, D.A.; Wong, P.K. Medication compliance and persistence: Terminology and definitions. Value Health 2008, 11, 44–47. [Google Scholar] [CrossRef]
- Peterson, A.M.; Nau, D.P.; Cramer, J.A.; Benner, J.; Gwadry-Sridhar, F.; Nichol, M. A checklist for medication compliance and persistence studies using retrospective databases. Value Health 2007, 10, 3–12. [Google Scholar] [CrossRef]
- Briesacher, B.A.; Andrade, S.E.; Fouayzi, H.; Chan, K.A. Medication adherence and use of generic drug therapies. Am. J. Manag. Care. 2009, 15, 450–456. [Google Scholar]
- Karve, S.; Cleves, M.A.; Helm, M.; Hudson, T.J.; West, D.S.; Martin, B.C. Good and poor adherence: Optimal cut-point for adherence measures using administrative claims data. Curr. Med. Res. Opin. 2009, 25, 2303–2310. [Google Scholar] [CrossRef] [PubMed]
- Demidenko, E. The p-Value You Can’t Buy. Am. Stat. 2016, 70, 33–38. [Google Scholar] [CrossRef]
- Rabenda, V.; Hiligsmann, M.; Reginster, J.Y. Poor adherence to oral bisphosphonate treatment and its consequences: A review of the evidence. Expert Opin. Pharmacother. 2009, 10, 2303–2315. [Google Scholar] [CrossRef]
- Kishimoto, H.; Maehara, M. Compliance and persistence with daily, weekly, and monthly bisphosphonates for osteoporosis in Japan: Analysis of data from the CISA. Arch. Osteoporosis 2015, 10, 231. [Google Scholar] [CrossRef] [PubMed]
- Recker, R.R.; Gallagher, R.; MacCosbe, P.E. Effect of dosing frequency on bisphosphonate medication adherence in a large longitudinal cohort of women. Mayo Clin. Proc. 2005, 80, 856–861. [Google Scholar] [CrossRef]
- Cramer, J.A.; Amonkar, M.M.; Hebborn, A.; Altman, R. Compliance and persistence with bisphosphonate dosing regimens among women with postmenopausal osteoporosis. Curr. Med. Res. Opin. 2005, 21, 1453–1460. [Google Scholar] [CrossRef]
- Rabenda, V.; Mertens, R.; Fabri, V.; Vanoverloop, J.; Sumkay, F.; Vannecke, C.; Deswaef, A.; Verpooten, G.A.; Reginster, J.Y. Adherence to bisphosphonates therapy and hip fracture risk in osteoporotic women. Osteoporos Int. 2008, 19, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Brankin, E.; Walker, M.; Lynch, N.; Aspray, T.; Lis, Y.; Cowell, W. The impact of dosing frequency on compliance and persistence with bisphosphonates among postmenopausal women in the UK: Evidence from three databases. Curr. Med. Res. Opin. 2006, 22, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Akarirmak, U.; Kocyigit, H.; Eskiyurt, N.; Esmaeilzadeh, S.; Kuru, Ö.; Yalçinkaya, E.Y.; Peker, Ö.; Ekim, A.A.; Özgirgin, N.; Çalış, M.; et al. Influence of patient training on persistence, compliance, and tolerability of different dosing frequency regimens of bisphosphonate therapy: An observational study in Turkish patients with postmenopausal osteoporosis. Acta Orthop. Traumatol. Turc. 2016, 50, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.; Drake, J.; Brankin, E.; Investigators, P. Treatment persistence with once-monthly ibandronate and patient support vs. once-weekly alendronate: Results from the PERSIST study. Int. J. Clin. Pract. 2006, 60, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Devine, J.; Trice, S.; Finney, Z.; Yarger, S.; Nwokeji, E.; Linton, A.; Davies, W. A retrospective analysis of extended-interval dosing and the impact on bisphosphonate compliance in the US Military Health System. Osteoporos Int. 2012, 23, 1415–1424. [Google Scholar] [CrossRef]
- Briesacher, B.A.; Andrade, S.E.; Harrold, L.R.; Fouayzi, H.; Yood, R.A. Adoption of once-monthly oral bisphosphonates and the impact on adherence. Am. J. Med. 2010, 123, 275–280. [Google Scholar] [CrossRef]
- Weiss, T.W.; Henderson, S.C.; McHorney, C.A.; Cramer, J.A. Persistence across weekly and monthly bisphosphonates: Analysis of US retail pharmacy prescription refills. Curr. Med. Res. Opin. 2007, 23, 2193–2203. [Google Scholar] [CrossRef]
- Wade, S.W.; Curtis, J.R.; Yu, J.; White, J.; Stolshek, B.S.; Merinar, C.; Balasubramanian, A.; Kallich, J.D.; Adams, J.L.; Viswanathan, H.N. Medication adherence and fracture risk among patients on bisphosphonate therapy in a large United States health plan. Bone 2012, 50, 870–875. [Google Scholar] [CrossRef]
- Durden, E.; Pinto, L.; Lopez-Gonzalez, L.; Juneau, P.; Barron, R. Two-year persistence and compliance with osteoporosis therapies among postmenopausal women in a commercially insured population in the United States. Arch. Osteoporos. 2017, 12, 22. [Google Scholar] [CrossRef]
- Claxton, A.J.; Cramer, J.; Pierce, C. A systematic review of the associations between dose regimens and medication compliance. Clin. Ther. 2001, 23, 1296–1310. [Google Scholar] [CrossRef]
- Raebel, M.A.; Schmittdiel, J.; Karter, A.J.; Konieczny, J.L.; Steiner, J.F. Standardizing terminology and definitions of medication adherence and persistence in research employing electronic databases. Med. Care 2013, 51, 11–21. [Google Scholar] [CrossRef]
- Andrade, S.E.; Kahler, K.H.; Frech, F.; Chan, K.A. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol. Drug Saf. 2006, 15, 565–574. [Google Scholar] [CrossRef]
- Krass, I.; Schieback, P.; Dhippayom, T. Adherence to diabetes medication: A systematic review. Diabet Med. 2015, 32, 725–737. [Google Scholar] [CrossRef]
- Klop, C.; Welsing, P.M.; Elders, P.J.; Overbeek, J.A.; Souverein, P.C.; Burden, A.M.; van Onzenoort, H.A.; Leufkens, H.G.; Bijlsma, J.W.; de Vries, F. Long-term persistence with anti-osteoporosis drugs after fracture. Osteoporos. Int. 2015, 26, 1831–1840. [Google Scholar] [CrossRef]
- Martin-Merino, E.; Huerta-Alvarez, C.; Prieto-Alhambra, D.; Montero-Corominas, D. Cessation rate of anti-osteoporosis treatments and risk factors in Spanish primary care settings: A population-based cohort analysis. Arch. Osteoporos. 2017, 12, 39. [Google Scholar] [CrossRef]
- McCarney, R.; Warner, J.; Iliffe, S.; van Haselen, R.; Griffin, M.; Fisher, P. The Hawthorne Effect: A randomised, controlled trial. BMC Med. Res. Methodol. 2007, 7, 30. [Google Scholar] [CrossRef]
- Manteuffel, M.; Williams, S.; Chen, W.; Verbrugge, R.R.; Pittman, D.G.; Steinkellner, A. Influence of patient sex and gender on medication use, adherence, and prescribing alignment with guidelines. J. Womens Health 2014, 23, 112–119. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, J.H.; Cho, D.S. Gender difference in osteoporosis prevalence, awareness and treatment: Based on the Korea national health and nutrition examination survey 2008–2011. J. Korean Acad. Nurs. 2015, 45, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Cadarette, S.M.; Katz, J.N.; Brookhart, M.A.; Levin, R.; Stedman, M.R.; Choudhry, N.K.; Solomon, D.H. Trends in drug prescribing for osteoporosis after hip fracture, 1995–2004. J. Rheumatol. 2008, 35, 319–326. [Google Scholar]
- Jung, Y.; Ko, Y.; Kim, H.Y.; Ha, Y.C.; Lee, Y.K.; Kim, T.Y.; Choo, D.S.; Jang, S. Gender differences in anti-osteoporosis drug treatment after osteoporotic fractures. J. Bone Miner. Metab. 2019, 37, 134–141. [Google Scholar] [CrossRef]
- Roerholt, C.; Eiken, P.; Abrahamsen, B. Initiation of anti-osteoporotic therapy in patients with recent fractures: A nationwide analysis of prescription rates and persistence. Osteoporos Int. 2009, 20, 299–307. [Google Scholar] [CrossRef]
- Solomon, D.H.; Morris, C.; Cheng, H.; Cabral, D.; Katz, J.N.; Finkelstein, J.S.; Avorn, J. Medication use patterns for osteoporosis: An assessment of guidelines, treatment rates, and quality improvement interventions. Mayo Clin. Proc. 2005, 80, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Shanthi Johnson, C.; McLeod, W.; Kennedy, L.; McLeod, K. Osteoporosis health beliefs among younger and older men and women. Health Educ Behav. 2008, 35, 721–733. [Google Scholar] [CrossRef]
- Sedlak, C.A.; Doheny, M.O.; Estok, P.J. Osteoporosis in older men: Knowledge and health beliefs. Orthop Nurs. 2000, 19, 38–42, 44–46. [Google Scholar] [CrossRef]
- Center, J.R.; Nguyen, T.V.; Schneider, D.; Sambrook, P.N.; Eisman, J.A. Mortality after all major types of osteoporotic fracture in men and women: An observational study. Lancet 1999, 353, 878–882. [Google Scholar] [CrossRef]
- Rabenda, V.; Vanoverloop, J.; Fabri, V.; Mertens, R.; Sumkay, F.; Vannecke, C.; Deswaef, A.; Verpooten, G.A.; Reginster, J.Y. Low incidence of anti-osteoporosis treatment after hip fracture. J. Bone Jt. Surg. Am. 2008, 90, 2142–2148. [Google Scholar] [CrossRef]
- von Friesendorff, M.; Besjakov, J.; Akesson, K. Long-term survival and fracture risk after hip fracture: A 22-year follow-up in women. J. Bone Miner. Res. 2008, 23, 1832–1841. [Google Scholar] [CrossRef] [PubMed]
- Warriner, A.H.; Patkar, N.M.; Yun, H.; Delzell, E. Minor, major, low-trauma, and high-trauma fractures: What are the subsequent fracture risks and how do they vary? Curr. Osteoporos. Rep. 2011, 9, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Silverman, S.L.; Siris, E.; Kendler, D.L.; Belazi, D.; Brown, J.P.; Gold, D.T.; Lewiecki, E.M.; Papaioannou, A.; Simonelli, C.; Ferreira, I.; et al. Persistence at 12 months with denosumab in postmenopausal women with osteoporosis: Interim results from a prospective observational study. Osteoporos. Int. 2015, 26, 361–372. [Google Scholar] [CrossRef] [PubMed]


| Variable | Noncompliance (MPR < 80) | Compliance (MPR ≥ 80) | p-Value * | |
|---|---|---|---|---|
| Sex | Men, n (%) | 20,218 (9.65) | 4573 (5.53) | <0.001 |
| Women, n (%) | 189,205 (90.35) | 78,156 (94.47) | ||
| Age | 50–59, n (%) | 38,595 (18.43) | 14,613 (17.66) | <0.001 |
| 60–69, n (%) | 70,608 (33.72) | 32,243 (38.97) | ||
| 70–79, n (%) | 67,436 (32.2) | 26,823 (32.42) | ||
| ≥80, n (%) | 32,784 (15.65) | 9050 (10.94) | ||
| Insurance fee | 1–5q | 57,599 (27.5) | 22,767 (27.52) | <0.001 |
| 6–10q | 31,751 (15.16) | 12,022 (14.53) | ||
| 11–15q | 44,891 (21.44) | 17,614 (21.29) | ||
| 16–20q | 75,182 (35.9) | 30,326 (36.66) | ||
| Area of residence | Metropolis | 81,012 (38.68) | 33,054 (39.95) | <0.001 |
| City | 94,760 (45.25) | 37,410 (45.22) | ||
| Rural area | 33,648 (16.07) | 12,263 (14.82) | ||
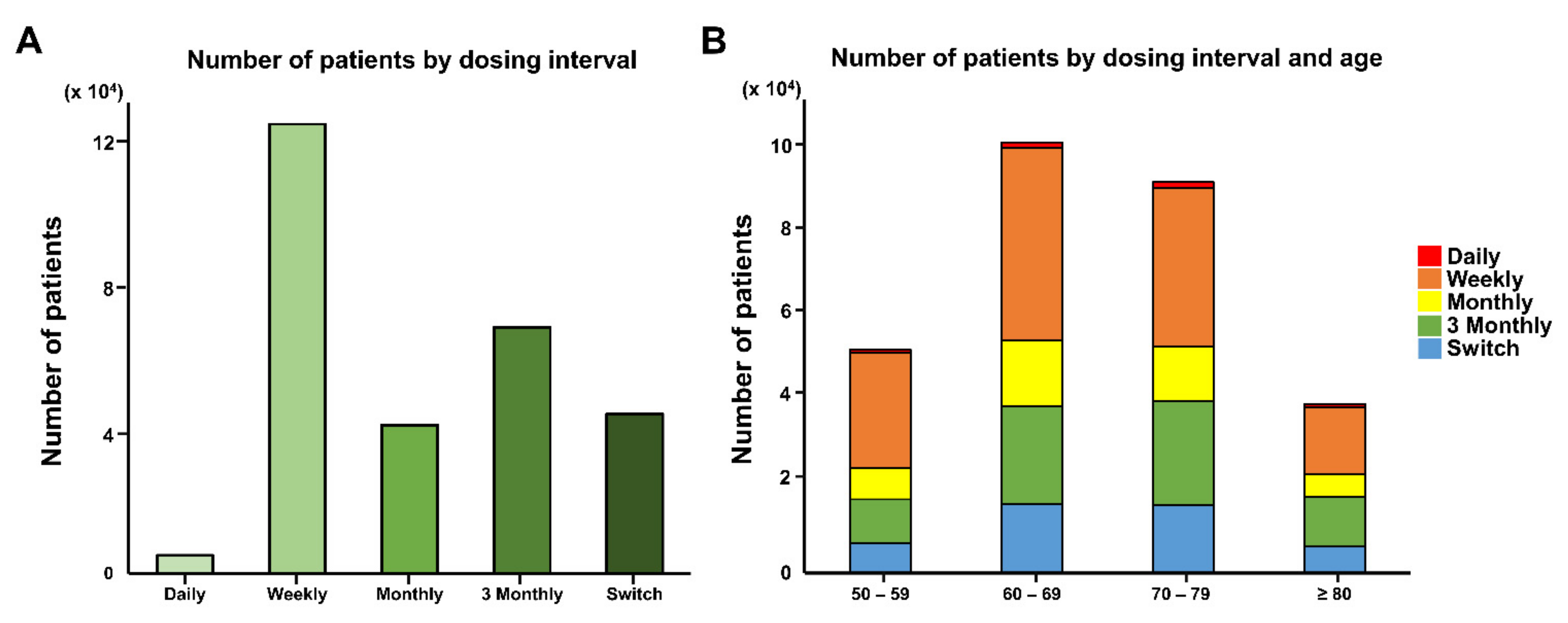
| Dosing interval | Daily, n (%) | 3445 (1.70) | 922 (1.17) | <0.001 |
| Weekly, n (%) | 98,431 (48.51) | 24,974 (31.57) | ||
| Monthly, n (%) | 29,365 (14.47) | 11,513 (14.56) | ||
| 3 Monthly, n (%) | 45,063 (22.21) | 23,512 (29.73) | ||
| Switch, n (%) | 26,594 (13.11) | 18,177 (22.98) | ||
| Fracture history | No, n (%) | 193,661 (92.47) | 76,122 (92.01) | <0.001 |
| Yes, n (%) | 15,762 (7.53) | 6607 (7.99) | ||
| Surgery history | No, n (%) | 203,474 (97.16) | 80,331 (97.1) | 0.4038 |
| Yes, n (%) | 5949 (2.84) | 2398 (2.9) |
| Variable | Odd Ratio (95% CI) * | p-Value | |
|---|---|---|---|
| Sex | Male vs. Female | 1.389 (1.342, 1.439) | <0.001 |
| Age | 50–59 vs. 80+ | 0.645 (0.625, 0.665) | <0.001 |
| 60–69 vs. 80+ | 0.565 (0.549, 0.581) | <0.001 | |
| 70–79 vs. 80+ | 0.674 (0.655, 0.693) | <0.001 | |
| Insurance fee | 2qu vs. 1qu | 1.051 (1.023, 1.079) | <0.001 |
| 3qu vs. 1qu | 1.024 (1, 1.048) | 0.0539 | |
| 4qu vs. 1qu | 0.966 (0.946, 0.987) | 0.0012 | |
| Area of residence | Small city vs. Rural area | 0.909 (0.887, 0.931) | <0.001 |
| Metropolis vs. Rural area | 0.877 (0.855, 0.899) | <0.001 | |
| Dosing interval | Weekly vs. Daily | 1.1 (1.02, 1.184) | 0.0122 |
| Monthly vs. Daily | 0.737 (0.682, 0.795) | <0.001 | |
| 3 Monthly vs. Daily | 0.54 (0.501, 0.582) | <0.001 | |
| Switch vs. Daily | 0.415 (0.385, 0.447) | <0.001 | |
| Fracture history | Yes vs. No | 0.885 (0.856, 0.914) | <0.001 |
| Surgery history | Yes vs. No | 0.945 (0.897, 0.996) | 0.0334 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Lee, S.; Kim, D.; Cho, W.; Cho, S.; Yoon, S.; Lee, S. Effect of Dosing Interval on Compliance of Osteoporosis Patients on Bisphosphonate Therapy: Observational Study Using Nationwide Insurance Claims Data. J. Clin. Med. 2021, 10, 4350. https://doi.org/10.3390/jcm10194350
Lee H, Lee S, Kim D, Cho W, Cho S, Yoon S, Lee S. Effect of Dosing Interval on Compliance of Osteoporosis Patients on Bisphosphonate Therapy: Observational Study Using Nationwide Insurance Claims Data. Journal of Clinical Medicine. 2021; 10(19):4350. https://doi.org/10.3390/jcm10194350
Chicago/Turabian StyleLee, Hyunil, Sangcheol Lee, Dokyung Kim, Weonmin Cho, Sungtan Cho, Siyeong Yoon, and Soonchul Lee. 2021. "Effect of Dosing Interval on Compliance of Osteoporosis Patients on Bisphosphonate Therapy: Observational Study Using Nationwide Insurance Claims Data" Journal of Clinical Medicine 10, no. 19: 4350. https://doi.org/10.3390/jcm10194350
APA StyleLee, H., Lee, S., Kim, D., Cho, W., Cho, S., Yoon, S., & Lee, S. (2021). Effect of Dosing Interval on Compliance of Osteoporosis Patients on Bisphosphonate Therapy: Observational Study Using Nationwide Insurance Claims Data. Journal of Clinical Medicine, 10(19), 4350. https://doi.org/10.3390/jcm10194350







