Potentially Detrimental Effects of Hyperosmolality in Patients Treated for Traumatic Brain Injury
Abstract
1. Introduction
2. The Most Popular Hyperosmotic Agents
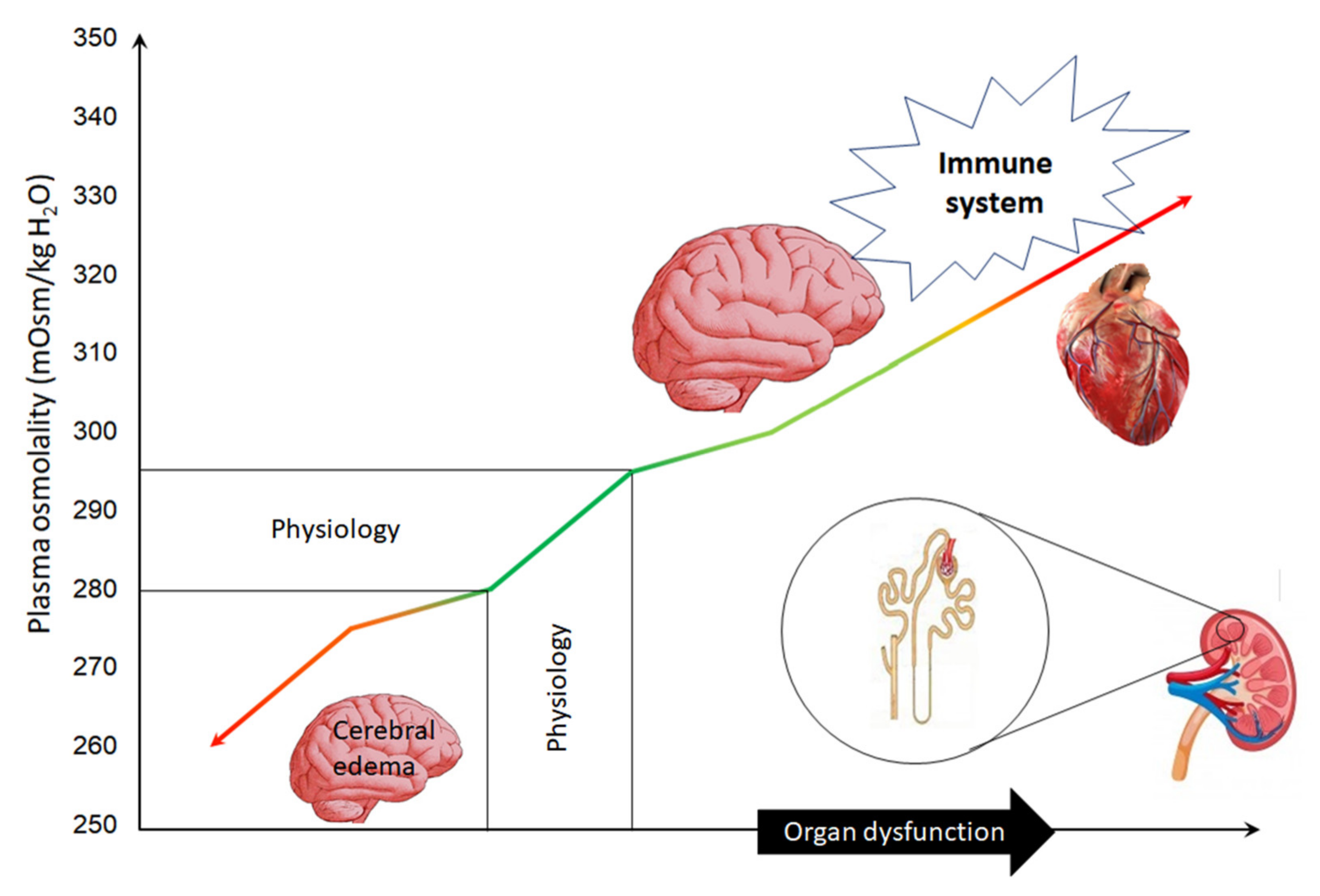
3. Basic Knowledge
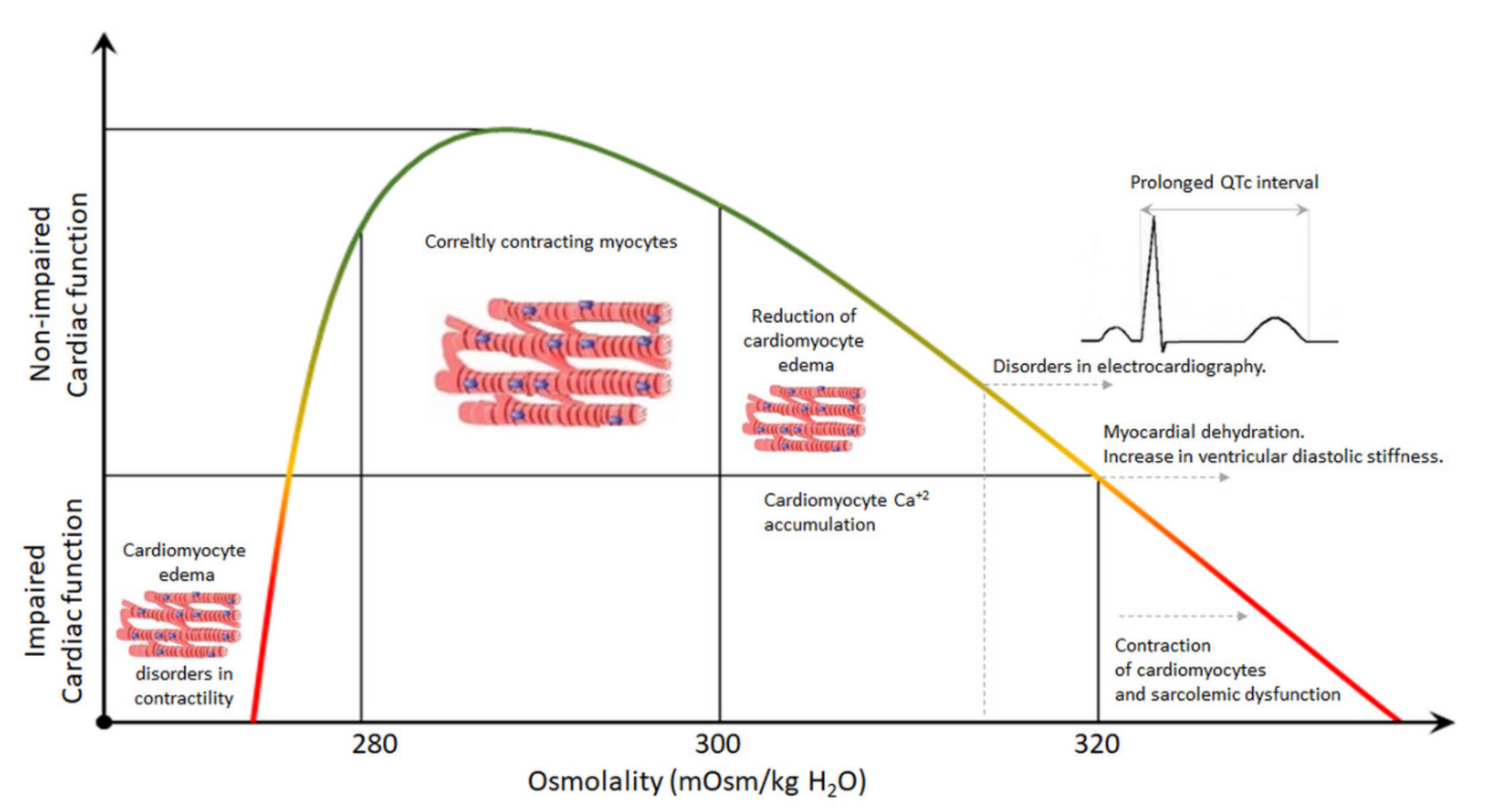
4. Plasma Hyperosmolality and the Heart
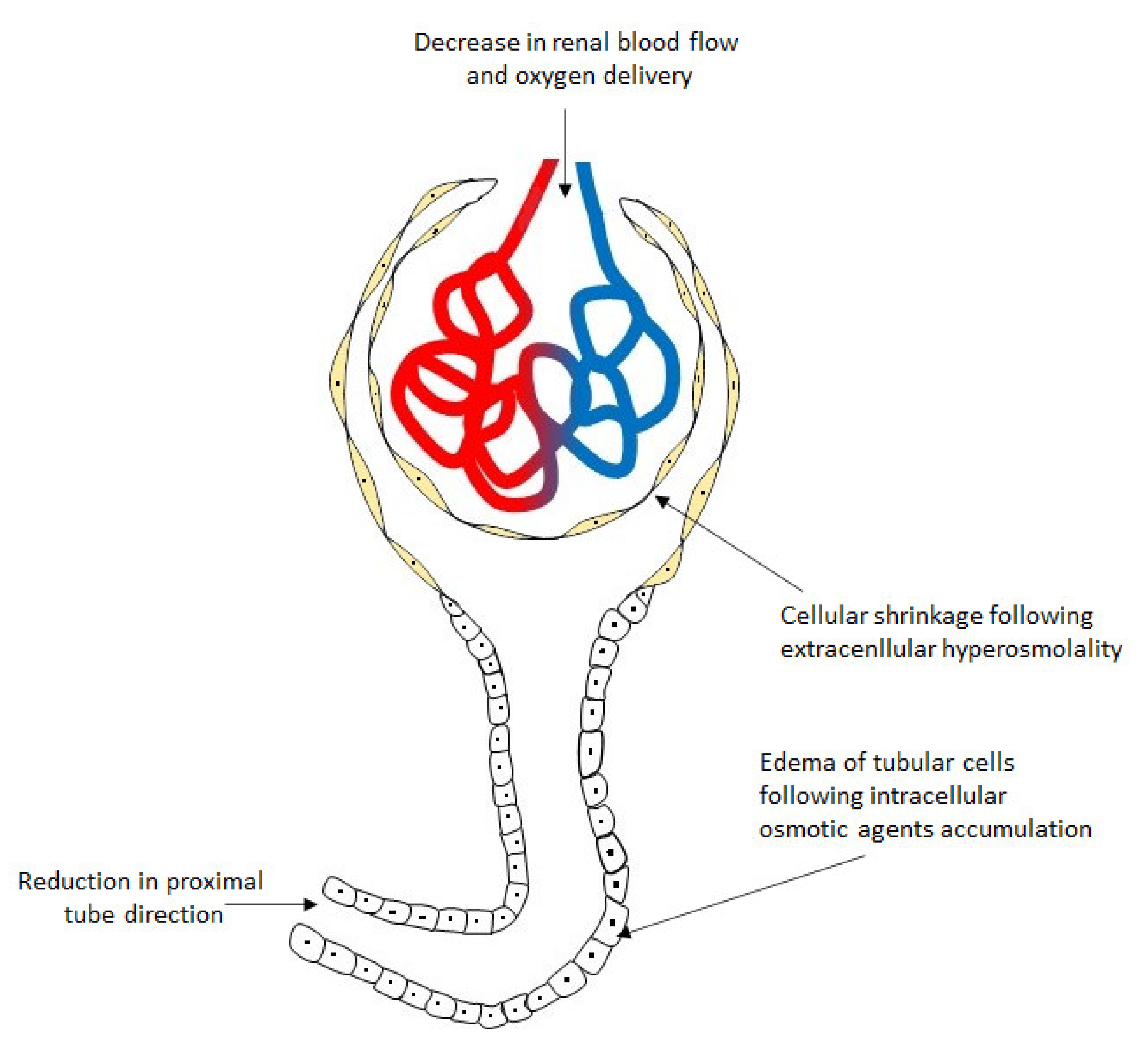
5. Plasma Hyperosmolality and the Kidney
6. Plasma Hyperosmolality and Immune System
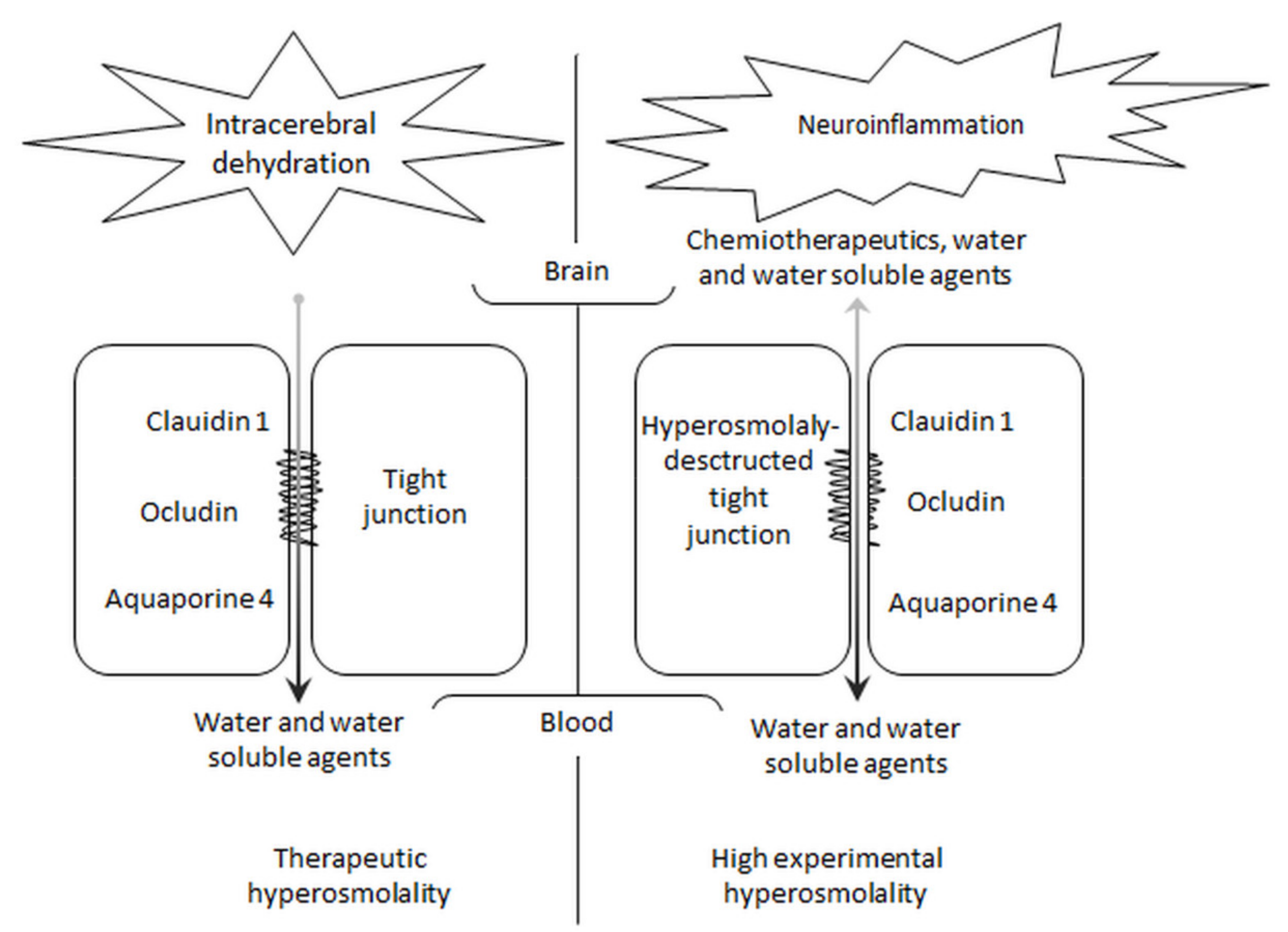
7. Plasma Hyperosmolality and the Blood–Brain Barrier
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Raslan, A.; Bhardwaj, A. Medical management of cerebral edema. Neurosurg. Focus 2007, 22, E12. [Google Scholar] [CrossRef]
- Chesnut, R.; Aguilera, S.; Buki, A.; Bulger, E.; Citerio, G.; Cooper, D.J.; Arradtia, R.D.; Diringer, M.; Figaji, A.; Gao, G.; et al. A management algorithm for patients with intracranial pressure monitoring: The Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med. 2020, 46, 1783–1794. [Google Scholar] [CrossRef]
- Macintyre, I. A hotbed of medical innovation: George Kellie (1770–1829), his colleagues at Leith and the Monro-Kellie doctrine. J. Med. Biogr. 2014, 22, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Mokri, B. The Monro-Kellie hypothesis: Applications in CSF volume depletion. Neurology 2001, 56, 1746–1748. [Google Scholar] [CrossRef] [PubMed]
- Schreibman, D.L.; Hong, C.M.; Keledjian, K.; Ivanowa, S.; Tsymbalyuk, S.; Gerzanich, V.; Simard, J.M. Mannitol and hypertonic saline reduce swelling and modulate inflammatory markers in a rat model of intracerebral haemorrhage. Neurocrit. Care 2018, 29, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Pascual, J.L.; Ferri, L.E.; Seely, A.J.E.; Campisi, G.; Chaudhury, P.; Giannias, B.; Evans, D.C.; Razek, T.; Michel, R.P.; Cgristou, N.V. Hypertonic saline resuscitation of hemorrhagic shock diminishes neutrophil rolling and adherence to endothelium and reduces in vivo vascular leakage. Ann. Surg. 2002, 236, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Oddo, M.; Poole, D.; Helbok, R.; Meyfroidt, G.; Stocchetti, N.; Bouzat, P.; Cecconi, M.; Geeraets, T.; Martin-Loeches, I.; Quintard, H.; et al. Fluid therapy in neurointensive care patients: ESCIM consensus and clinical practice recommendations. Intenvive Care Med. 2018, 44, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Toung, T.J.; Chen, C.H.; Lin, C.; Bhardwaj, A. Osmotherapy with hypertonic saline attenuates water content in brain and extracerebral organs. Crit. Care Med. 2007, 35, 526–531. [Google Scholar] [CrossRef]
- Hays, A.N.; Lazaridis, C.; Neyens, R.; Nicholas, J.; Gay, S.; Chalela, J.A. Osmotherapy: Use among neurointensivists. Neurocrit Care 2011, 14, 222–228. [Google Scholar] [CrossRef]
- Bhardwaj, A. Osmotherapy in neurocritical care. Curr. Neurol. Neurosci. Rep. 2007, 7, 513–521. [Google Scholar] [CrossRef]
- FDA Approved Drug Products: BRONCHITOL (mannitol) Oral Inhalation Power. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/202049s000lbl.pdf (accessed on 30 October 2020).
- Southern, K.W.; Clancy, J.P.; Ranganathan, S. Aerosolized agents for airway clearance in cystic fibrosis. Pediatr. Pulmonol. 2019, 54, 858–864. [Google Scholar] [CrossRef]
- Georgidis, A.L.; Suarez, J.I. Hypertonic saline for cerebral edema. Curr. Neurol. Neurosci. Rep. 2003, 6, 524–530. [Google Scholar] [CrossRef]
- Kheirbek, T.; Pascual, J.L. Hypertonic saline for the treatment of intracranial hypertension. Curr. Neurol. Neurosci. Rep. 2014, 14, 482. [Google Scholar] [CrossRef] [PubMed]
- Badaut, J.; Ashwal, S.; Obenaus, A. Aquaporins in cerebrovascular disease: A target for treatment of brain edema? Cerebrovasc Dis. 2011, 31, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Badenes, R.; Hutton, B.; Citerio, G.; Robba, C.; Aguilar, G.; Alonso-Arroyo, A.; Taccone, F.S.; Tornero, C.; Catala-Lopez, F. Hyperosmolar therapy for acute brain injury: Study protocol for an umbrella rewie of meta-analyses and evidence mapping. BMJ Open 2020, 10, e033913. [Google Scholar] [CrossRef] [PubMed]
- Himmelsehr, S. Hypertonic saline solutions for treatment of intracranial hypertension. Curr. Opin. Anaesthesiol. 2007, 20, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Garrard, A.; Sollee, D.R.; Butterfiels, R.C.; Johannsen, L.; Wood, A.; Bertholf, R.L. Validation of pre-existing formula to calculate the contribution of ethanol to the osmolar gap. Clin. Toxicol. 2012, 50, 562–566. [Google Scholar] [CrossRef]
- Cochrane, T.T. Improvements in the equation for calculating the contribution to osmotic potential of the separate solutes of water solutions. Med. Phys. 1984, 11, 338–340. [Google Scholar] [CrossRef] [PubMed]
- Moucka, F.; Nezbeda, I.; Smith, W.R. Molecular simulation of aqueous electrocytes: Water chemical potential results and Gibbs-Duhem equation consistency tests. J. Chem. Phys. 2013, 139, 124505. [Google Scholar] [CrossRef]
- Rasouli, M.; Kalantari, K.R. Comparison of methods for calculating serum osmolality: Multivariate linear regression analysis. Clin. Chem. Lab. Med. 2005, 43, 635–640. [Google Scholar] [CrossRef]
- Sweeney, T.E.; Beuchat, C.A. Limitation of methods of osmometry: Measuring the osmolality of biological FLUIDS. Am. J. Physiol. 1993, 264, R469–R480. [Google Scholar] [CrossRef] [PubMed]
- Kruse, J.A.; Cadnapaphornachai, P. The serum osmole gap. J. Crit. Care 1994, 9, 185–197. [Google Scholar] [CrossRef]
- Skaaland, H.; Larstorp, A.C.K.; Lindberg, M.; Jacobsen, D. Reference values for osmolal gap in healthy subject and in medical inpatients. Scand. J. Clin. Lab. Investig. 2020, 80, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Liams, G.; Fillppatos, T.D.; Liontos, A.; Elisaf, M.S. Serum osmolal gap in clinical practice: Usefulness and limitations. Postgrad. Med. 2017, 129, 456–459. [Google Scholar] [CrossRef]
- Dabrowski, W.; Siwicka-Gieroba, D.; Robba, C.; Badenes, R.; Bialy, M.; Iwaniuk, P.; Schlegel, T.T.; Jaroszynski, A. Plasma hyperosmolality prolongs QTc interval and increases rosk for atrial fibrillation in traumatic brain injury patients. J. Clin. Med. 2020, 9, 1293. [Google Scholar] [CrossRef]
- Bealer, S.L. Peripheral hyperosmolality reduces cardiac baroreflex sensitivity. Auton. Neurosci. 2003, 104, 24–31. [Google Scholar] [CrossRef]
- Kultz, D. Hyperosmolality triggers oxidative damage in kidney cells. Proc. Nat. Acad. Sci. USA 2004, 101, 9177–9178. [Google Scholar] [CrossRef]
- Steinberg, C. Diagnosis and clinical management of long-QT syndrome. Curr. Opin. Cardiol. 2018, 33, 31–41. [Google Scholar] [CrossRef]
- Tatlisu, M.A.; Kaya, A.; Keskin, M.; Uzman, O.; Borklu, E.B.; Cinier, G.; Hayiroglu, M.I.; Tatlisu, K.; Eren, M. Can we use plasma hyperosmolality as a predictor of mortality for ST-segment elevation myocardial infarction? Coron. Artery Dis. 2017, 28, 70–76. [Google Scholar] [CrossRef]
- Kaya, H.; Yücel, O.; Ege, M.R.; Zorlu, A.; Yücel, H.; Günes, H.; Ekmekci, A.; Yilmaz, M.B. Plasma osmolality predicts mortality in patients with heart failure with reduced ejection fraction. Kardiol. Pol. 2017, 75, 316–322. [Google Scholar] [CrossRef]
- Rohla, M.; Freynhofer, M.K.; Tentzeris, I.; Farhan, S.; Wojte, J.; Huber, K.; Weiss, T.W. Plasma osmolality predicts clinical outcome in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Eur. Heart J. Acuta Cardiovasc. Care 2014, 3, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, R.A.; Bassani, R.A.; Bassani, J.W.M. Osmolality- and Na+-dependent effect of hyperosmotic NaCl solution on contractile activity and Ca2+ cycling in rat ventricular myocytes. Pflugers Arch. 2008, 455, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Burgos, J.I.; Morell, M.; Mariangelo, J.I.E.; Petroff, M.V. Hyperosmotic stress promotes endoplasmic reticulum stress-dependent apoptosis in adult rat cardiac myocytes. Apoptosis 2019, 24, 785–797. [Google Scholar] [CrossRef]
- Galvez, A.; Morales, M.P.; Eltit, J.M.; Ocaranza, P.; Carrasco, L.; Campos, X.; Sapag-Hagar, M.; Díaz-Araya, G.; Lavandero, S. A rapid and strong apoptotic process is triggering by hyperosmotic stress in cultured rat cardiac myocytes. Cell Tissue Res. 2001, 304, 279–285. [Google Scholar] [CrossRef]
- Page, E.; Winterfield, J.; Goings, G.; Bastawrous, A.; Upshaw-Early, J. Water channel proteins in rat cardiac myocyte caveolae: Osmolarity-dependent reversible internalization. Am. J. Physiol. 1998, 274, H1988–H2000. [Google Scholar] [CrossRef]
- Rutkovskiy, A.; Mariero, L.H.; Nygard, S.; Stenslokken, K.O.; Valen, G.; Vaage, J. Transient hyperosmolality modulates expression of cardiac aquaporins. Biochem. Biophys. Res. Commun. 2012, 425, 70–75. [Google Scholar] [CrossRef]
- Aggeli, I.K.; Kapogiannatou, A.; Paraskevopoulou, F.; Gaitanaki, C. Differential response of cardiac aquaporins of hyperosmotic stress; salutary role of AQ1 against the induced apoptosis. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 313–325. [Google Scholar]
- Farquhar, W.B.; Wenner, M.M.; Delaney, E.P.; Prettyman, A.V.; Stillabower, M.E. Sympathetic neural response to increased osmolality in humans. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H2181–H2186. [Google Scholar] [CrossRef] [PubMed]
- Kinsman, B.J.; Browning, K.N.; Stocker, S.D. NaCl and osmolarity produce different responses in organum vasculosum if the lamina terminalis neurons, sympathetic nerve activity and blood pressure. J. Physiol. 2017, 595, 6187–6201. [Google Scholar] [CrossRef]
- Brooks, V.L.; Freeman, K.L.; O’Donaughy, T.L. Acute and chronic increase in osmolality increase excitatory amino acid drive of the rostral ventrolateral medulla in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R1359–R1368. [Google Scholar] [CrossRef] [PubMed]
- Kamijo, Y.I.; Okazaki, K.; Ikegawa, S.; Okada, Y.; Nose, H. Rapid saline infusion and/or drinking enhance skin sympathetic nerve activity components reduced by hypovolaemia and hyperosmolality in hyperthermia. J. Physiol. 2018, 596, 544–5459. [Google Scholar] [CrossRef]
- Andreas-Villarreal, M.; Barba, I.; Poncelas, M.; Inserte, J.; Rodriguea-Palomares, J.; Pineda, V.; Gracia-Dorado, D. Measuring water distribution in the heart: Preventing edema reducas ischemia-reperfusion injury. J. Am. Hert. Assoc. 2016, 5, e003843. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, L.; Chen, H.; Lv, Z. Beneficial effect of hyperosmotic perfusion in the myocardium after ischemia/reperfusion injury in isolated rat hearts. Rev. Bras. Cir. Cardiovasc. 2013, 28, 545–560. [Google Scholar] [CrossRef][Green Version]
- Falck, G.; Schjott, J.; Jynge, P. Hyperosmotic pretreatment reduces infarct size in the rat heart. Physiol. Rev. 1999, 48, 331–340. [Google Scholar]
- Feige, K.; Rubbert, J.; Raupach, A.; Stroethoff, M.; Heinen, A.; Hollmann, M.W.; Huhn, R.; Torregroza, C. Cardioprotective properties of mannitol-involvement of mitochondrial potassium channels. Int. J. Mol. Sci. 2021, 22, 2395. [Google Scholar] [CrossRef]
- Bie, P.; Damkjaer, M. Renin secretion and total body sodium: Pathways of integrative control. Clin. Exp. Pharmacol. Physiol. 2010, 37, e34–e42. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Patel, R.N. Pathophysiology of contrast-induced acute kidney injury. Interv. Cardiol. Clin. 2020, 9, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Berl, T. How do kidney cells adopt to survive in hypertonic inner medulla? Trans. Am. Clin. Climatol. Assoc. 2009, 120, 389–401. [Google Scholar]
- Blasum, B.S.; Schröter, R.; Neugebauer, U.; Hofchsröer, V.; Pavenstädt, H.; Ciarimboli, G.; Schlatter, E.; Edemir, B. The kidney-sepcific expression of genes can be modulated by the extracellular osmolality. FASEB J. 2016, 30, 3588–3597. [Google Scholar] [CrossRef]
- Orbach, H.; Tishler, M.; Shoenfeld, Y. Intravenous immunoglobulin and the kidney—A two-edged sword. Semin. Arthritis Rheum. 2004, 34, 593–601. [Google Scholar] [CrossRef]
- Kwan, T.H.; Tong, M.K.; Siu, Y.P.; Leung, K.T.; Lee, H.K.; Young, C.Y.; Au, T.C. Acute renal failure related to intravenous immunoglobulin infusion in an elderly woman. Hong Kong Med. J. 2005, 11, 45–49. [Google Scholar] [PubMed]
- Gaut, J.P.; Liapis, H. Acute kidney injury pathology and pathophysiology: A retrospective review. Clin. Kidney J. 2020, 14, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Qian, J.; Li, H.; Luo, H.; Luo, W.; Lin, Z. Renal tubular epithelial cells injury induced by mannitol and its potential mechanism. Ren. Fail. 2018, 40, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Dickenmann, M.; Oettl, T.; Mihatsch, M.J. Osmotic nephrosis: Acute kidney injury with accumulation of proximal tubular lysosomes due to administration of exogenous solutes. Am. J. Kidney Dis. 2008, 51, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Visweswaran, P.; Massin, E.K.; Dubose, T.D., Jr. Mannitol-induced acute renal failure. J. Am. Soc. Nephrol. 1997, 8, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Tang, S.C.; Tsai, L.K.; Yeh, S.J.; Shen, L.J.; Wu, F.L.; Jeng, J.S. Incidence and risk factors for acute kidney injury following mannitol infusion in patients with acute stroke: A retrospective cohort study. Medicine 2015, 94, e2032. [Google Scholar] [CrossRef] [PubMed]
- Asif, A.; Preston, R.A.; Roth, D. Radiocontrast-induced nephropathy. Am. J. Ther. 2003, 10, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Seeliger, E.; Lenhard, D.C.; Persson, P.B. Contrast media velocity versus osmolality in kidney injury: Lesson from animal studies. BioMed Res. Int. 2014, 2014, 358136. [Google Scholar] [CrossRef]
- Lancelot, E.; Idee, J.P.; Lacledere, C.; Santus, R.; Corot, C. Effects of two dimetric iodinated contrast media on renal medullary blood perfusion and oxygenation in dogs. Invest. Radiol. 2002, 37, 368–375. [Google Scholar] [CrossRef]
- Skrifvars, M.B.; Bailey, M.; Moore, E.; Martensson, J.; French, C.; Presneill, J.; Nichol, A.; Little, L.; Duranteau, J.; Huet, O.; et al. A post hoc analysis of osmotherapy use in the erythropoietin in traumatic brain injury study—Associations with acute kidney injury and mortality. Crit. Care Med. 2021, 49, e394–e403. [Google Scholar] [CrossRef]
- Dorman, H.R.; Sondheimer, J.H.; Cadnapaphornchai, P. Mannitol-induced acute renal failure. Medicine 1990, 69, 153–159. [Google Scholar] [CrossRef]
- Moustafa, H.; Schoene, D.; Altarsha, E.; Rahmig, J.; Schneider, H.; Pallesen, L.P.; Prakapenia, A.; Siepmann, T.; Barlinn, J.; Paussauer, J.; et al. Acute kidney injury in patients with malignant middle cerebral artery infarction undergoing hyperosmolar therapy with mannitol. J. Crit. Care 2021, 64, 22–28. [Google Scholar] [CrossRef]
- Schmitt, J.; Rahman, A.F.; Ashraf, A. Concurrent diabetic ketoacidosis with hyperosmolality and/or severe hyperglycemia in youth with type 2 diabetes. Endocrinol. Diab. Metab. 2020, 3, e00160. [Google Scholar] [CrossRef] [PubMed]
- Royal Australian College of General Practioners. General Practice Management of Type 2 Diabetes 2016–2018. Available online: www.racgp.org.au/clinical-resources/clinical-guidelines/key-racgp-guidelines/view-all-racgp-guidelines/management-of-type-2-diabetes (accessed on 5 May 2021).
- Nomani, A.Z.; Nabi, Z.; Rashid, H.; Janjua, J.; Nomani, H.; Majeed, A.; Chaudry, S.R.; Mazhar, A.S. Osmotic nephrosis with mannitol: A review article. Ren. Fail. 2014, 36, 1169–1176. [Google Scholar] [CrossRef]
- Kim, M.Y.; Park, J.H.; Kang, N.R.; Jang, H.R.; Lee, J.E.; Huh, W.; Kim, Y.G.; Kim, D.J.; Hong, S.C.; Kim, J.S.; et al. Intreased risk of acute kidney injury associated with higher infusion rate of mannitol in patients with intracranial haemorrhage. J. Neurosurg. 2014, 120, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Narayan, S.W.; Castelino, R.; Hammond, N.; Patanwala, A.E. Effect of mannitol plus hypertonic saline combination versus hypertonic saline monotherapy on acute kidney injury after traumatic brain injury. J. Crit. Care 2020, 57, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Huang, H.; Huang, Y.; Sun, H.; Xu, H. Hypertonic saline or mannitol for treating elevated intracranial pressure in traumatic brain injury: A meta-analysis of randomized controlled trials. Neurosurg. Rev. 2019, 42, 499–509. [Google Scholar] [CrossRef]
- Huang, X.; Yang, L.; Ye, J.; He, S.; Wang, B. Equimolar doses of hypertonic agents (saline or mannitol) in the treatment of intracranial hypertension after severe traumatic brain injury. Medicine 2020, 99, e22004. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Tan, L.; Ye, J.; Hu, L. Hypertonic saline and mannitol in patients with traumatic brain injury: A systematic and meta-analysis. Medicine 2020, 99, e21655. [Google Scholar] [CrossRef]
- Patil, H.; Gupta, R. A comparative study of bolus dose of hypertonic saline, mannitol, and mannitol plus glycerol combination in patients with severe traumatic brain injury. World Neurosurg. 2019, 125, e221–e228. [Google Scholar] [CrossRef]
- DeNett, T.; Feltner, C. Hypertonic saline versus mannitol for the treatment of increased intracranial pressure in traumatic brain injury. J. Am. Assoc. Nurse Pract. 2019, 33, 283–293. [Google Scholar] [CrossRef]
- Wiorek, A.; Jaworski, T.; Krzych, Ł.J. Hyperosmolar threatment for patients at risk for increased intracranial pressure: A single-center cohort study. Int. J. Environ. Res. Public Health 2020, 17, 4573. [Google Scholar] [CrossRef]
- Popovic, Z.V.; Embgenbroich, M.; Chessa, F.; Nordström, V.; Bonrouhy, M.; Hielscher, T.; Gretz, N.; Wang, S.; Mathow, D.; Quast, T.; et al. Hyperosmolarity impedes the cross-priming competence of dendric cells in a TRIF-dependent manner. Sci. Rep. 2017, 7, 311. [Google Scholar] [CrossRef] [PubMed]
- Junger, W.T.; Hoyt, D.B.; Davis, R.E.; Herdon-Remelius, C.; Namiki, S.; Junger, H.; Loomis, W.; Altman, A. Hypertonicity regulates the function of human neutrophils by modulating chemoattractant receptor signalling and activating mitogen-activated protein kinase p38. J. Clin. Investig. 1998, 101, 2768–2779. [Google Scholar] [CrossRef]
- Cvetkovic, L.; Perisic, S.; Titze, J.; Jäck, H.M.; Schuh, W. The impact of hyperosmolality on activation and differentiation of B lymphoid cells. Front. Immunol. 2019, 10, 808. [Google Scholar] [CrossRef] [PubMed]
- Powers, K.A.; Zurawska, J.; Szaszi, K.; Khadaroo, R.G.; Kapus, A.; Rotstein, O.D. Hypertonic resuscitation of hemorrhagic shock prevents alveolar macrophage activation by preventing systemic oxidative stress due to gut ischemia/reperfusion. Surgery 2005, 137, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Wright, F.L.; Gamboni, F.; Moore, E.E.; Nydam, T.L.; Mitra, S.; Silliman, C.C.; Banerjee, A. Hyperosmolarity invokes distinct anti-inflammatory mechanisms in pulmonary epithelial cells: Evidence from signalling and transcription layers. PLoS ONE 2014, 9, e114129. [Google Scholar] [CrossRef]
- Lopez-Rodríguez, C.; Aramburu, J.; Jin, L.; Rakeman, A.S.; Michino, M.; Rao, A. Bridging the NFAT and NF-κB families: NFAT5 dimerization regulates cytokine gene transcription in response to osmotic stress. Immunity 2001, 15, 47–58. [Google Scholar] [CrossRef]
- Dong, Z.H.; Wang, D.C.; Liu, T.T.; Li, F.H.; Liu, R.L.; Wei, J.W.; Zhou, C.L. The roles of MAPKs in rabbit nucleus pulposus cell apoptosis induced by high osmolality. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2835–2845. [Google Scholar]
- Malek, A.M.; Goss, G.G.; Jiang, L.; Izumo, S.; Alper, S.L. Mannitol at clinical concentrations activated multiple signaling pathways and induces apoptosis in endothelial cells. Stroke 1998, 29, 2631–2640. [Google Scholar] [CrossRef]
- Schwartz, L.; Guais, A.; Pooya, M.; Abolhassani, M. Is inflammation a concequence of extracellular hyperosmolarity? J. Inflamm. 2009, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, N.C.; Gilman, T.L.; Daws, L.C.; Toney, G.M. High salt intake enhances swim stress-induced PVN vasopressin cell activation and active stress coping. Psychoneuroendocrinology 2018, 93, 29–38. [Google Scholar] [CrossRef]
- Sailer, C.O.; Wiedemann, S.J.; Strauss, K.; Schnyder, I.; Fenske, W.K.; Christ-Crain, M. Markers of systemic inflammation in response to osmotic stimuli in healthy volunteers. Endocr. Connect. 2019, 8, 1282–1287. [Google Scholar] [CrossRef]
- Ziegeler, S.; Raddatz, A.; Schneider, S.O.; Sandman, I.; Sasse, H.; Bauer, I.; Kubulus, D.; Mathes, A.; Lausberg, H.F.; Rensing, H. Effect of haemofiltration and mannitol treatment on cardiopulmonary-bypass induced immunosuppression. Scand. J. Immunol. 2009, 69, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Al-Sarraf, H.; Ghaaedi, F.; Redic, Z. Time course of hyperosmolar opening of the blood-brain and blood-CSF barriers in spontaneously hypertensive rats. J. Vasc. Res. 2007, 44, 99–109. [Google Scholar] [CrossRef]
- Huang, K.; Zhou, L.; Alanis, K.; Hou, J.; Baker, L.A. Imaging effect of hyperosmolality on individual tricellular junctions. Chem. Sci. 2020, 11, 1307–1315. [Google Scholar] [CrossRef]
- Burks, S.R.; Kersch, C.N.; Witko, J.A.; Pagel, M.A.; Sundby, M.; Muldoon, L.L.; Neuwelt, E.A.; Frank, J.A. Blood-brain barrier opening by intracarotid artery hyperosmolar mannitol induces sterile inflammatory and innate immune responses. Proc. Natl. Acad. Sci. USA 2021, 118, e2021915118. [Google Scholar] [CrossRef] [PubMed]
- Stonestreet, B.S.; Sadowska, G.B.; Leeman, J.; Hanumara, R.C.; Petersson, K.H.; Patlak, C.S. Effects of acute hyperosmolality on blood-brain barrier function in ovine foetuses and lambs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R1031–R1039. [Google Scholar] [CrossRef][Green Version]
- Joshi, S.; Ellis, J.A.; Ornstein, E.; Bruce, J.N. Intraarterial drug delivery for glioblastoma multiforme: Will the phoenix rise again? J. Neurooncol. 2015, 124, 333–343. [Google Scholar] [CrossRef]
- Fortin, D.; Desjardins, A.; Benko, A.; Niyonsega, T.; Boudrias, M. Enhanced chemotherapy delivery by intraarterial infusion and blood-brain barrier disruption in malignant brain tumors: The Sherbrooke experience. Cancer 2005, 103, 2606–2615. [Google Scholar] [CrossRef]
- Marchi, N.; Betto, G.; Fazio, V.; Fan, Q.; Ghosh, C.; Machado, A.; Janigro, D. Blood-brain barrier damage and brain penetration of antiepileptic drugs: Role of serum proteins and brain edema. Epilepsia 2009, 50, 664–677. [Google Scholar] [CrossRef] [PubMed]
- Godinho, B.M.D.C.; Henninger, N.; Bouley, J.; Alterman, J.F.; Haraszti, R.A.; Gilbert, J.W.; Sapp, E.; Coles, A.H.; Biscans, A.; Nikan, M.; et al. Transvascular delivery of hydrophobically modified siRNAs: Gene silencing in the rat brain upon disruption of the blood-brain barrier. Mol. Ther. 2018, 26, 2580–2591. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.H.; Chen, N.Y.; Tu, P.H.; Wu, C.T.; Chiu, S.C.; Huang, Y.C.; Lim, S.N.; Yip, P.K. DHA attenuates cerebral edema following traumatic brain injury via the reduction in blood-brain barrier permeability. Int. J. Mol. Sci. 2020, 21, 6291. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dabrowski, W.; Siwicka-Gieroba, D.; Robba, C.; Bielacz, M.; Sołek-Pastuszka, J.; Kotfis, K.; Bohatyrewicz, R.; Jaroszyński, A.; Malbrain, M.L.N.G.; Badenes, R. Potentially Detrimental Effects of Hyperosmolality in Patients Treated for Traumatic Brain Injury. J. Clin. Med. 2021, 10, 4141. https://doi.org/10.3390/jcm10184141
Dabrowski W, Siwicka-Gieroba D, Robba C, Bielacz M, Sołek-Pastuszka J, Kotfis K, Bohatyrewicz R, Jaroszyński A, Malbrain MLNG, Badenes R. Potentially Detrimental Effects of Hyperosmolality in Patients Treated for Traumatic Brain Injury. Journal of Clinical Medicine. 2021; 10(18):4141. https://doi.org/10.3390/jcm10184141
Chicago/Turabian StyleDabrowski, Wojciech, Dorota Siwicka-Gieroba, Chiara Robba, Magdalena Bielacz, Joanna Sołek-Pastuszka, Katarzyna Kotfis, Romuald Bohatyrewicz, Andrzej Jaroszyński, Manu L. N. G. Malbrain, and Rafael Badenes. 2021. "Potentially Detrimental Effects of Hyperosmolality in Patients Treated for Traumatic Brain Injury" Journal of Clinical Medicine 10, no. 18: 4141. https://doi.org/10.3390/jcm10184141
APA StyleDabrowski, W., Siwicka-Gieroba, D., Robba, C., Bielacz, M., Sołek-Pastuszka, J., Kotfis, K., Bohatyrewicz, R., Jaroszyński, A., Malbrain, M. L. N. G., & Badenes, R. (2021). Potentially Detrimental Effects of Hyperosmolality in Patients Treated for Traumatic Brain Injury. Journal of Clinical Medicine, 10(18), 4141. https://doi.org/10.3390/jcm10184141









