Implementation of Computed Tomography Angiography (CTA) and Computed Tomography Perfusion (CTP) in Polish Guidelines for Determination of Cerebral Circulatory Arrest (CCA) during Brain Death/Death by Neurological Criteria (BD/DNC) Diagnosis Procedure
Abstract
:1. Background
- Catheter digital subtraction angiography (DSA), selective or from the aortic arch;
- Transcranial Doppler ultrasonography (TCD);
- Cerebral perfusion scintigraphy.
2. Early Research into Using CTA/CTP for Diagnosing BD/DNC in Poland
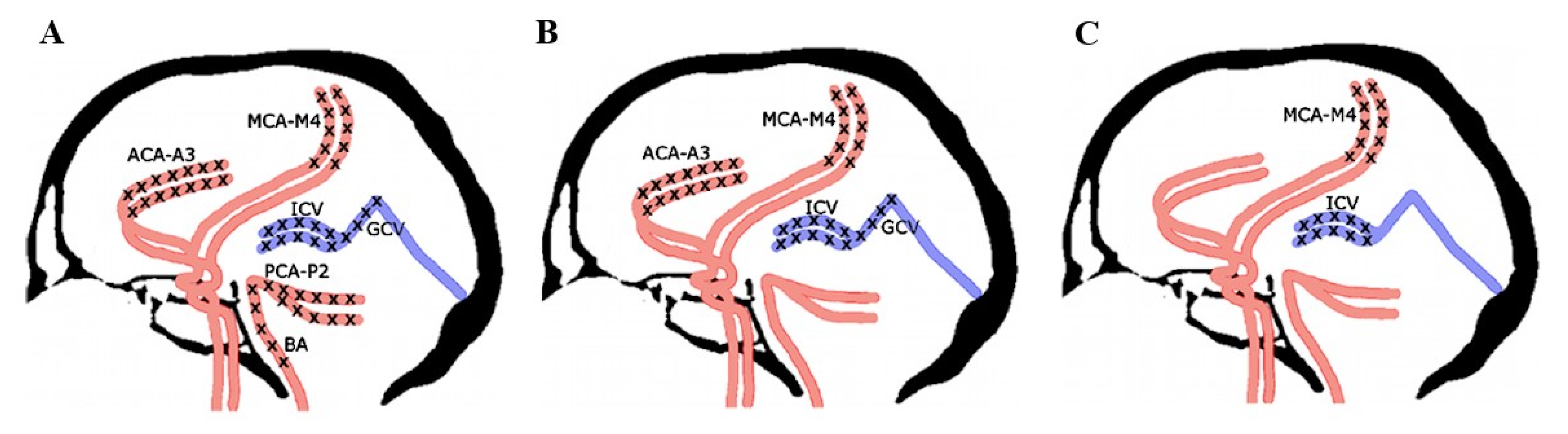
- Pericallosal segments of the right and left anterior cerebral artery (ACA-A3);
- Cortical segments of the right and left middle cerebral artery (MCA-M4);
- Cortical segments of the right and left posterior cerebral artery (PCA-P2);
- Basilar artery (BA);
- Right and left internal cerebral vein (ICV);
- Great cerebral vein (GCV)—the vein of Galen.
3. Comparison with the Other Instructions for CCA Confirmation by CTA Imaging
4. Sensitivity and Specificity of CTA in CCA Determination during BD/DNC Diagnosis Procedures
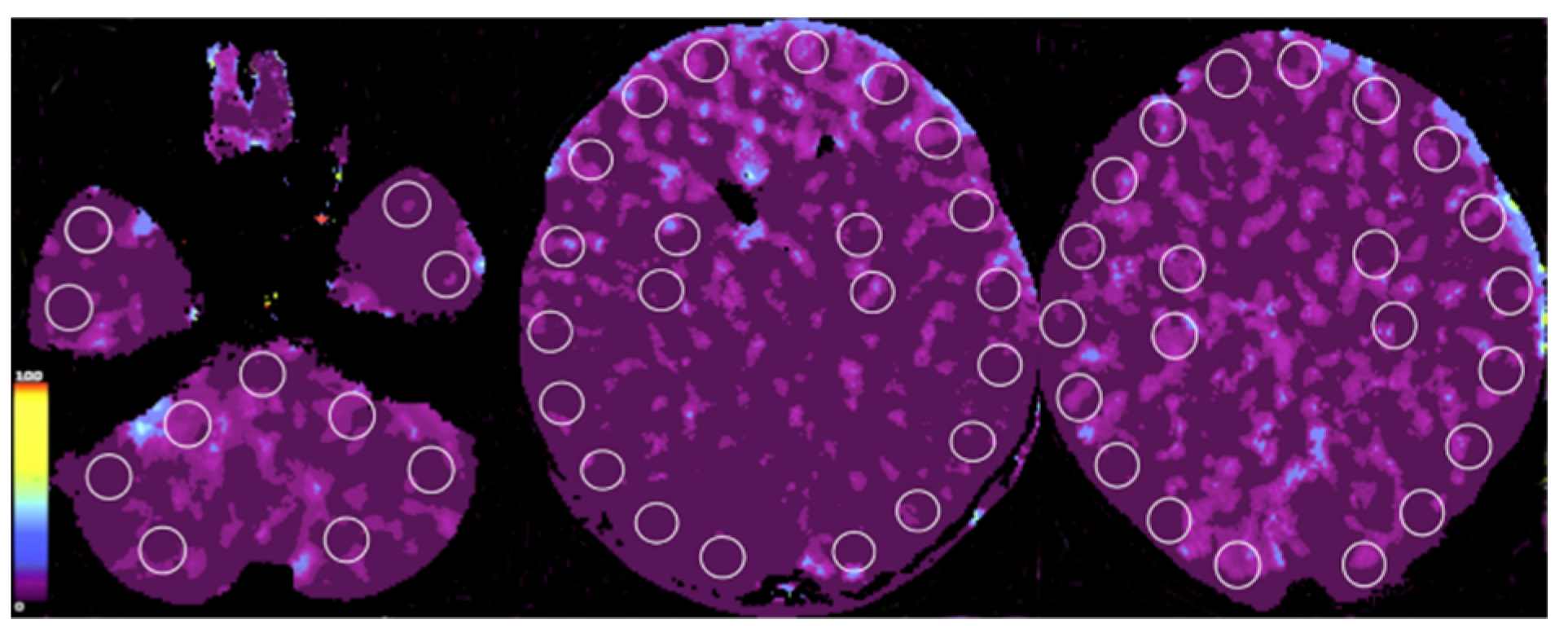
5. Research Advancement with CTP in Poland
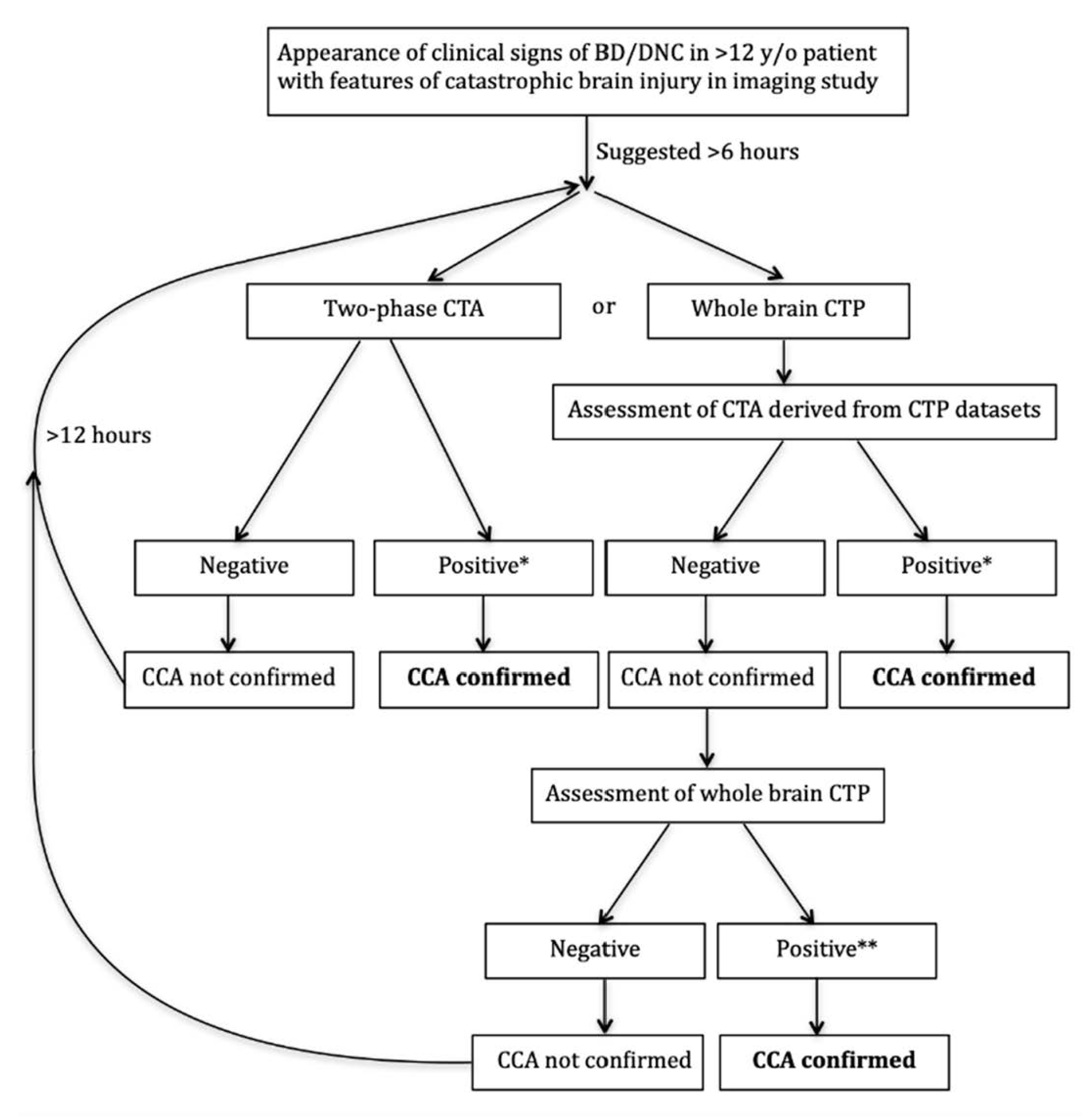
6. Implementation of CTA/CTP Examination into the Polish National Guidelines for BD/DNC
7. Comparison with Recommendations Made in the World Brain Death Project
- Recently Greer at al. published in JAMA great work elaborated by international group of experts, entitled “Determination of Brain Death/Death by Neurologic Criteria: The World Brain Death Project” consisting of introduction part and 17 supplements [4]. It summarizes current knowledge about various aspects of pathophysiology of brain injury leading, finally, to BD/DNC, all aspects of diagnostic procedures, possible organ procurement and, finally, future research agenda. The following is stated in it: “It is recommended that when ancillary testing is performed and demonstrates the presence of brain blood flow, BD/DNC cannot be declared at that time”. This indirectly points out the necessity for the elaboration of precise diagnostic criteria for CTA/CTP after implementation of these new technologies for investigation of CCA, both in infratentorial and supratentorial spaces. Our research results and their interpretations are consistent with this point of view.
- The following is stated in the “Determination of Brain Death/Death by Neurologic Criteria. The World Brain Death Project” publication: “It is recommended that when ancillary testing is performed and demonstrates the presence of brain blood flow, BD/DNC cannot be declared at that time” [4]. This indirectly points out the necessity for the elaboration of precise criteria for CTA/CTP after implementation of these new technologies for investigation of CCA, both in infratentorial and supratentorial spaces. Our research results and their interpretations are consistent with this point of view.
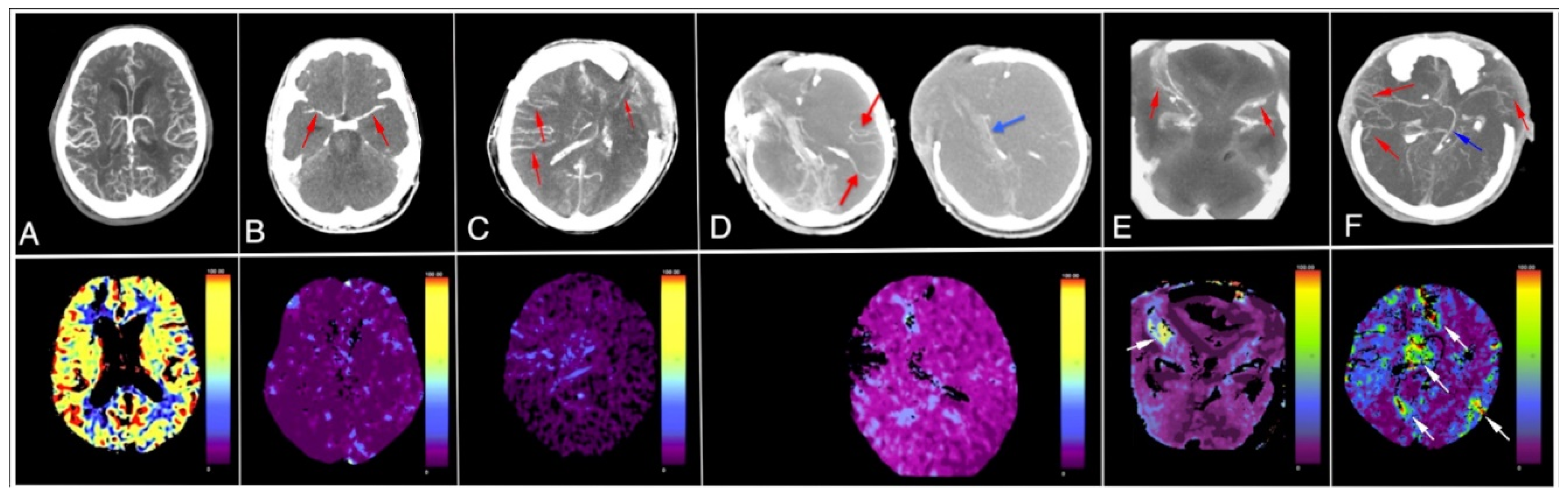
- Non contrast CT (Figure 1) in our opinion confirms severe edema in course of devastating brain injury indirectly indicating presence of severe intracranial hypertension,
- CTA imaging pattern is typical for CCA (Figure 2); however, the authors declare it doubtful because of possible hypotension during the procedure which is an obvious diagnostic protocol violation and makes the examination not interpretative,
- In TCD imaging (Figure 3) intracranial arteries might be not properly identified and flow spectra incorrectly interpreted:
- The typical flow spectrum in OA (ophthalmic artery) is usually different from the one showed in Figure 3. In transorbital window in TCD (transcranial Doppler) it should be higher resistive than presented on the depth 50–60 mm as OA is an artery of predominantly elastic type. Furthermore, presented flow was inconsistent with intracranial hypertension. Therefore, the flow described as the right OA perhaps does not represent true flow in OA. Regardless of these doubts the flow in OA is not a TCD criterion for CCA diagnosis. Therefore, the reason for demonstration of flow in a vessel identified as OA by default in order to support supposition of preserved cerebral perfusion remains doubtful.
- In patients with high intracranial pressure the flow spectra in cerebral arteries change in a very characteristic manner. The systolic phase of spectrum become very short (velocities are normal or diminished) and all diastolic velocities decline to baseline or near it. Such type of flow can persist for some time and usually leads to CCA, while in Figure 3, the flow spectrum in artery recognized by the authors as left middle cerebral artery (LMCA) is low resistant with gradual reduction of velocity during systole and diastole. This does not represent residual flow consistent with severe intracranial hypertension.
8. Conclusions
- CTA and CTP-derived CTA might be in future the tests of choice for CCA diagnosis due to increasing availability and relatively easy interpretation.
- Proper timing based on time elapse after the appearance of brain stem areflexia and/or Doppler pretest might significantly reduce preterm examinations and significantly increase sensitivity of CTA in CCA diagnosis procedures.
- Whole brain CTP might be decisive in some cases of inconclusive CTA.
- The monitoring of the implementation of CTA/CTP according to recently amended Polish BD/DNC diagnosis guidelines does not show any major obstacles, occasionally appearing teaching troubles and excessively large sticking to traditional diagnostic schemes are gradually eliminated.
- We strongly believe that in next edition of “The World Brain Death Project”, CTA and CTP will be recommended as ancillary tests of choice for CCA diagnosis during BD/DNC diagnosis procedures. We strongly believe that in next edition of “The World Brain Death Project” CTA and CTP will be recommended as ancillary tests of choice for CCA confirmation during BD/DNC diagnosis procedures.
Author Contributions
Funding
Conflicts of Interest
References
- Ad Hoc Committee of the Harvard Medical School to Examine the Definition of Brain Death. A definition of irreversible coma. Report of the ad hoc committee of the harvard medical school to examine the definition of brain death. J. Am. Med. Assoc. 1968, 205, 5694976. [Google Scholar]
- Bohatyrewicz, R.; Zukowski, M.; Marzec-Lewenstein, E.; Biernawska, J.; Solek-Pastuszka, J.; Sienko, J.; Sulikowski, T.; Bohatyrewicz, A. Reversal to whole-brain death criteria after 15-year experience with brain stem death criteria in Poland. Transplant. Proc. 2009, 41, 2959–2960. [Google Scholar] [CrossRef]
- Announcement of the Minister of Health from the 4th of December 2019 on the Method and Criteria for Establishing Permanent Irreversible Cessation of Brain Function. 2020. Available online: https://monitorpolski.gov.pl/M2020000007301.pdf (accessed on 11 June 2021).
- Greer, D.M.; Shemie, S.D.; Lewis, A.; Torrance, S.; Varelas, P.; Goldenberg, F.D.; Bernat, J.L.; Souter, M.; Topcuoglu, M.A.; Alexandrov, A.W.; et al. Determination of brain death/death by neurologic criteria. J. Am. Med. Assoc. 2020, 324, 1078–1097. [Google Scholar] [CrossRef] [PubMed]
- Bohatyrewicz, R.; Sawicki, M.; Walecka, A.; Walecki, J.; Rowinski, O.; Czajkowski, Z.; Krzysztalowski, A.; Solek-Pastuszka, J.; Zukowski, M.; Marzec-Lewenstein, E.; et al. Computed tomographic angiography and perfusion in the diagnosis of brain death. Transplant. Proc. 2010, 42, 3941–3946. [Google Scholar] [CrossRef] [PubMed]
- Dupas, B.; Gayet-Delacroix, M.; Villers, D.; Antonioli, D.; Veccherini, M.F.; Soulillou, J.P. Diagnosis of brain death using two-phase spiral CT. Am. J. Neuroradiol. 1998, 19, 641–647. [Google Scholar] [PubMed]
- Leclerc, X.; Taschner, C.A.; Vidal, A.; Strecker, G.; Savage, J.; Gauvrit, J.Y.; Pruvo, J.P. The role of spiral CT for the assessment of the intracranial circulation in suspected brain-death. J. Neuroradiol. 2006, 33, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Combes, J.-C.; Chomel, A.; Ricolfi, F.; D’Athis, P.; Freysz, M. Reliability of computed tomographic angiography in the diagnosis of brain death. Transplant. Proc. 2007, 39, 16–20. [Google Scholar] [CrossRef]
- Escudero, D.; Otero, J.; Marqués, L.; Parra, D.; Gonzalo, J.A.; Albaiceta, G.M.; Cofiño, L.; Blanco, A.; Vega, P.; Murias, E.; et al. Diagnosing brain death by CT perfusion and multislice CT angiography. Neurocrit. Care 2009, 11, 261–271. [Google Scholar] [CrossRef]
- Frampas, E.; Videcoq, M.; De Kerviler, E.; Ricolfi, F.; Kuoch, V.; Mourey, F.; Tenaillon, A.; Dupas, B. CT Angiography for brain death diagnosis. Am. J. Neuroradiol. 2009, 30, 1566–1570. [Google Scholar] [CrossRef]
- Welschehold, S.; Boor, S.; Reuland, K.; Thömke, F.; Kerz, T.; Reuland, A.; Beyer, C.; Gartenschläger, M.; Wagner, W.; Giese, A.; et al. Technical aids in the diagnosis of brain death: A comparison of SEP, AEP, EEG, TCD and CT angiography. Dtsch. Arztebl. Int. 2012, 109, 624–630. [Google Scholar] [CrossRef]
- Welschehold, S.; Kerz, T.; Boor, S.; Reuland, K.; Thömke, F.; Beyer, C.; Wagner, W.; Müller-Forell, W.; Giese, A. Detection of intracranial circulatory arrest in brain death using cranial CT-angiography. Eur. J. Neurol. 2013, 20, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Welschehold, S.; Kerz, T.; Boor, S.; Reuland, K.; Thömke, F.; Reuland, A.; Beyer, C.; Tschan, C.; Wagner, W.; Müller-Forell, W.; et al. Computed tomographic angiography as a useful adjunct in the diagnosis of brain death. J. Trauma Acute Care Surg. 2013, 74, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, M.; Bohatyrewicz, R.; Safranow, K.; Walecka, A.; Walecki, J.; Rowinski, O.; Solek-Pastuszka, J.; Czajkowski, Z.; Guzinski, M.; Burzynska, M.; et al. Computed tomographic angiography criteria in the diagnosis of brain death—Comparison of sensitivity and interobserver reliability of different evaluation scales. Neuroradiology 2014, 56, 609–620. [Google Scholar] [CrossRef] [PubMed]
- De Radiologie, S.F.; De Neuroradiologie, S.F.; De La Biomédecine, A. Recommandations sur les critères diagnostiques de la mort encéphalique par la technique d’angioscanner cérébral. J. Neuroradiol. 2011, 38, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Kerhuel, L.; Srairi, M.; Georget, G.; Bonneville, F.; Mrozek, S.; Mayeur, N.; Lonjaret, L.; Sacrista, S.; Hermant, N.; Marhar, F.; et al. The optimal time between clinical brain death diagnosis and confirmation using CT angiography: A retrospective study. Minerva Anestesiol. 2016, 82, 1180–1188. [Google Scholar] [PubMed]
- Lewis, A.; Liebman, J.; Kreiger-Benson, E.; Kumpfbeck, A.; Bakkar, A.; Shemie, S.D.; Sung, G.; Torrance, S.; Greer, D. Ancillary Testing for Determination of Death by Neurologic Criteria Around the World. Neurocrit. Care 2020, 34, 473–484. [Google Scholar] [CrossRef]
- Beschluss der Bundesärztekammer über die Richtlinie gemäß §16 Abs. 1 S. 1 Nr. 1 TPG für die Regeln zur Feststellung des Todes nach § 3 Abs. 1 S. 1 Nr. 2 TPG und die Verfahrensregeln zur Feststellung des endgültigen, nicht behebbaren Ausfalls der Gesamtfunktion des Großhirns, des Kleinhirns und des Hirnstamms nach § 3 Abs. 2 Nr. 2 TPG, Vierte Fortschreibung. Deutsches Ärzteblatt, 30 March 2015; 112(A-1256/B-1052/C-1024).
- Wujtewicz, M.A.; Szarmach, A.; Chwojnicki, K.; Sawicki, M.; Owczuk, R. Subtotal Cerebral Circulatory Arrest with Preserved Breathing Activity: A Case Report. Transplant. Proc. 2016, 48, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Bohatyrewicz, R.; Walecka, A.; Bohatyrewicz, A.; Zukowski, M.; Kepinski, S.; Marzec-Lewenstein, E.; Sawicki, M.; Kordowski, J. Unusual movements, “spontaneous” breathing, and unclear cerebral vessels sonography in a brain-dead patient: A case report. Transplant. Proc. 2007, 39, 2707–2708. [Google Scholar] [CrossRef]
- Sawicki, M.; Solek-Pastuszka, J.; Chamier-Cieminska, K.; Walecka, A.; Bohatyrewicz, R. Accuracy of Computed Tomographic Perfusion in Published: Diagnosis of Brain Death: A Prospective Cohort Study. Med. Sci. Monit. 2018, 24, 2777–2785. [Google Scholar] [CrossRef] [Green Version]
- Sawicki, M.; Bohatyrewicz, R.; Safranow, K.; Walecka, A.; Walecki, J.; Rowinski, O.; Solek-Pastuszka, J.; Czajkowski, Z.; Marzec-Lewenstein, E.; Motyl, K.; et al. Dynamic evaluation of stasis filling phenomenon with computed tomography in diagnosis of brain death. Neuroradiology 2013, 55, 1061–1069. [Google Scholar] [CrossRef] [Green Version]
- Astrup, J.; Siesjö, B.K.; Symon, L. Thresholds in cerebral ischemia—The ischemic penumbra. Stroke 1981, 12, 723–725. [Google Scholar] [CrossRef] [Green Version]
- Shankar, J.; Vandorpe, R. CT Perfusion for Confirmation of Brain Death. Am. J. Neuroradiol. 2012, 34, 1175–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawicki, M.; Sołek-Pastuszka, J.; Chamier-Ciemińska, K.; Walecka, A.; Walecki, J.; Bohatyrewicz, R. Computed tomography perfusion is a useful adjunct to computed tomography angiography in the diagnosis of brain death. Clin. Neuroradiol. 2017, 29, 101–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smit, E.J.; Vonken, E.-J.; Van Der Schaaf, I.C.; Mendrik, A.M.; Dankbaar, J.W.; Horsch, A.D.; Van Seeters, T.; Van Ginneken, B.; Prokop, M. Timing-Invariant Reconstruction for Deriving High-Quality CT Angiographic Data from Cerebral CT Perfusion Data. Radiology 2012, 263, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Shankar, J.J.S.; Stewart-Perrin, B.; Quraishi, A.-U.-R.; Bata, I.; Vandorpe, R. Computed Tomography Perfusion Aids in the Prognostication of Comatose Postcardiac Arrest Patients. Am. J. Cardiol. 2018, 121, 874–878. [Google Scholar] [CrossRef] [PubMed]
- Bohatyrewicz, R.; Solek-Pastuszka, J.; Sawicki, M. Invitation to participate in multi-center study for validation of cerebral computed tomography angiography (CTA) and computed tomography perfusion (CTP) in determination of cerebral circulatory arrest (CCA) during brain death/death by neurological criteria (BD/DNC) diagnosis procedure in pediatric population below 12 years of age. Anaesthesiol. Intensiv. Ther. 2021, 53, 97–102. [Google Scholar]
- Sawicki, M.; Solek-Pastuszka, J.; Jurczyk, K.; Skrzywanek, P.; Guzinski, M.; Czajkowski, Z.; Manko, W.; Burzynska, M.; Safranow, K.; Poncyljusz, W.; et al. Original Protocol Using Computed Tomographic Angiography for Diagnosis of Brain Death: A Better Alternative to Standard Two-Phase Technique? Ann. Transplant. 2015, 20, 449–460. [Google Scholar]
- Greer, D.M.; Strozyk, D.; Schwamm, L.H. False positive CT angiography in brain death. Neurocrit. Care 2009, 11, 272–275. [Google Scholar] [CrossRef] [PubMed]
| Polish (2020 *) | French (2011 *) | German (2015 *) | |
|---|---|---|---|
| Recommended delay after appearance of clinical signs of BD/DNC (h) | 6 | 6 ** | not specified |
| 1. Non-contrast scanning used as a reference | |||
| 2. Early post-contrast scanning | |||
| Contrast volume (mL) | 80 | 2 mL/kg (max 120) | 65 |
| Scanning time | triggered by bolus-tracking in extracranial carotid arteries | 20 s after start of contrast injection | not performed |
| Assessed vessel | |||
| STA (bilaterally) *** | 2 | 2 | not performed |
| 3. Late post-contrast scanning | |||
| Scanning time | 40 s after start of early post-contrast scanning | 60 s after start of contrast injection | 15 s after filling of extracranial carotid arteries detected with bolus-tracking |
| Evaluation scale | 4-point | 4-point | 7-point late arterial |
| Assessed vessel | |||
| STA (bilaterally) *** | 2 | ||
| MCA-M1 (bilaterally) | 2 | ||
| ACA-A1 (bilaterally) | 2 | ||
| BA | 1 | ||
| PCA-P1 (bilaterally) | 2 | ||
| MCA-M4 (bilaterally) | 2 | 2 | |
| ICV (bilaterally) | 2 | 2 | |
| Delay to next exam if the previous was inconclusive (h) | 12 | not specified | not specified |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bohatyrewicz, R.; Pastuszka, J.; Walas, W.; Chamier-Cieminska, K.; Poncyljusz, W.; Dabrowski, W.; Wojczal, J.; Luchowski, P.; Guzinski, M.; Jurkiewicz, E.; et al. Implementation of Computed Tomography Angiography (CTA) and Computed Tomography Perfusion (CTP) in Polish Guidelines for Determination of Cerebral Circulatory Arrest (CCA) during Brain Death/Death by Neurological Criteria (BD/DNC) Diagnosis Procedure. J. Clin. Med. 2021, 10, 4237. https://doi.org/10.3390/jcm10184237
Bohatyrewicz R, Pastuszka J, Walas W, Chamier-Cieminska K, Poncyljusz W, Dabrowski W, Wojczal J, Luchowski P, Guzinski M, Jurkiewicz E, et al. Implementation of Computed Tomography Angiography (CTA) and Computed Tomography Perfusion (CTP) in Polish Guidelines for Determination of Cerebral Circulatory Arrest (CCA) during Brain Death/Death by Neurological Criteria (BD/DNC) Diagnosis Procedure. Journal of Clinical Medicine. 2021; 10(18):4237. https://doi.org/10.3390/jcm10184237
Chicago/Turabian StyleBohatyrewicz, Romuald, Joanna Pastuszka, Wojciech Walas, Katarzyna Chamier-Cieminska, Wojciech Poncyljusz, Wojciech Dabrowski, Joanna Wojczal, Piotr Luchowski, Maciej Guzinski, Elzbieta Jurkiewicz, and et al. 2021. "Implementation of Computed Tomography Angiography (CTA) and Computed Tomography Perfusion (CTP) in Polish Guidelines for Determination of Cerebral Circulatory Arrest (CCA) during Brain Death/Death by Neurological Criteria (BD/DNC) Diagnosis Procedure" Journal of Clinical Medicine 10, no. 18: 4237. https://doi.org/10.3390/jcm10184237
APA StyleBohatyrewicz, R., Pastuszka, J., Walas, W., Chamier-Cieminska, K., Poncyljusz, W., Dabrowski, W., Wojczal, J., Luchowski, P., Guzinski, M., Jurkiewicz, E., Bekiesinska-Figatowska, M., Owczuk, R., Walecki, J., Rowinski, O., Zukowski, M., Kusza, K., Piechota, M., Piotrowski, A., Migdal, M., ... Sawicki, M. (2021). Implementation of Computed Tomography Angiography (CTA) and Computed Tomography Perfusion (CTP) in Polish Guidelines for Determination of Cerebral Circulatory Arrest (CCA) during Brain Death/Death by Neurological Criteria (BD/DNC) Diagnosis Procedure. Journal of Clinical Medicine, 10(18), 4237. https://doi.org/10.3390/jcm10184237










