Subjective and Electroencephalographic Sleep Parameters in Children and Adolescents with Autism Spectrum Disorder: A Systematic Review
Abstract
:1. Introduction
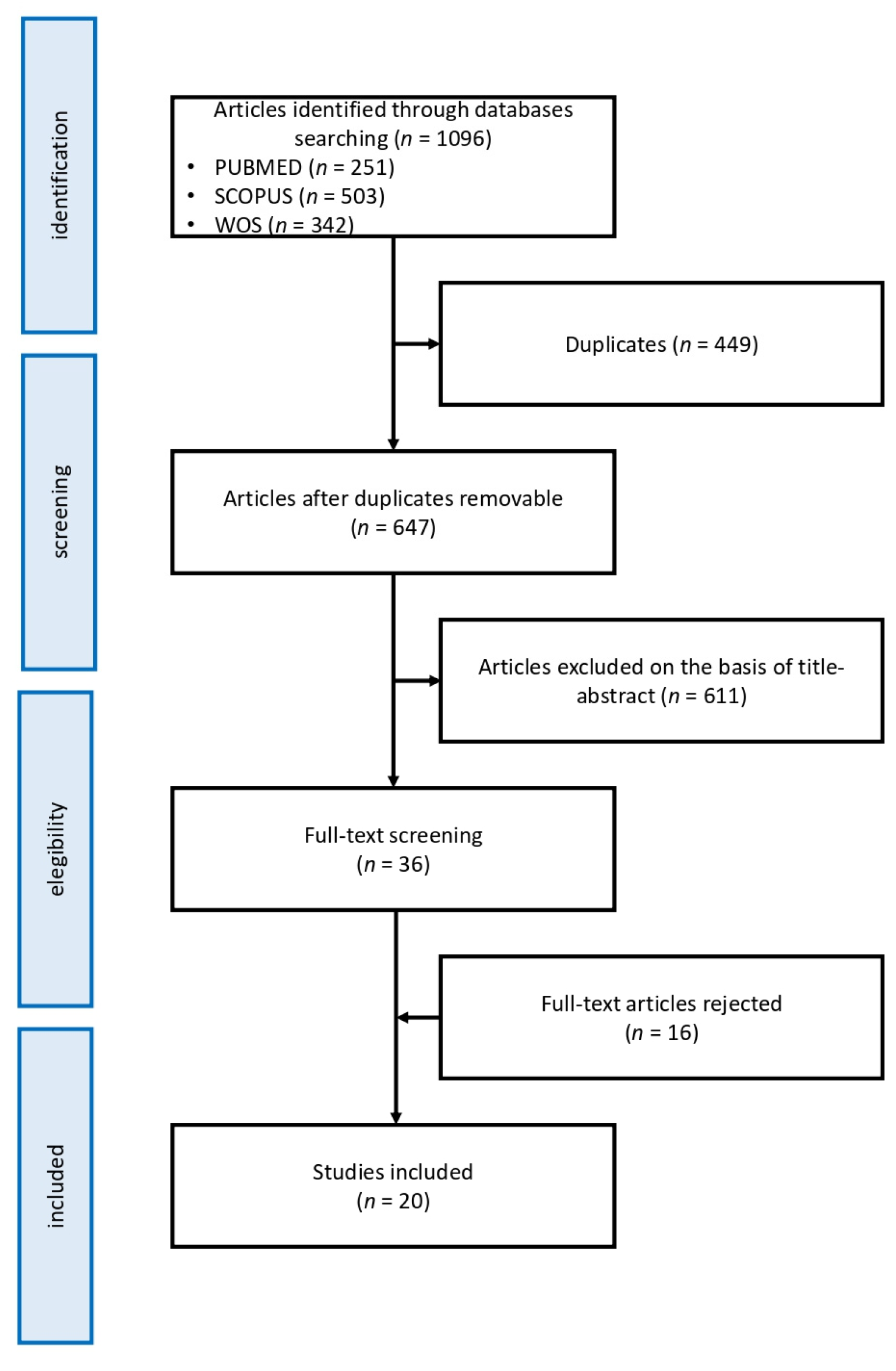
2. Methods
2.1. Study Selection Criteria
- (a)
- original observational studies published between January 2000 and December 2020;
- (b)
- studies conducted on children and/or adolescents aged between 0 and 18 years old;
- (c)
- clear indication of the procedure followed to arrive at the formal diagnosis of ASD, conducted through clinical judgment alone or with the support of standardized diagnostic tools in accordance with the criteria of the DSM-IV or DSM-5;
- (d)
- studies reporting objective sleep parameters measured using sleep EEG or polysomnography and/or subjective sleep parameters from any sleep questionnaire;
- (e)
- English language was mandatory.
- (a)
- reviews, meta-analysis articles, book chapters, meeting abstracts or case report/case series articles;
- (b)
- studies on subjects with autistic traits or in which the diagnostic process and/or tools were not clearly specified;
- (c)
- clinical or human research studies (i.e., not animal models);
- (d)
- papers referring to ASD related to specific genetic mutations or syndromes.
2.2. Results Analysis
3. Results
3.1. Macrostructural EEG Parameters
3.1.1. ASD Children vs. TD Children
3.1.2. ASD Children vs. Developmentally Delayed Children
3.1.3. Differences in ASD Subgroups
3.2. Microstructural EEG Parameters
3.3. Subjective Parameter
4. Discussion
Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 5th Edition, DSM-5; American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Lai, M.C.; Kassee, C.; Besney, R.; Bonato, S.; Hull, L.; Mandy, W.; Szatmari, P.; Ameis, S.H. Prevalence of co-occurring mental health diagnoses in the autism population: A systematic review and meta-analysis. Lancet Psychiatry 2019, 6, 819–829. [Google Scholar] [CrossRef]
- Malorgio, E. I Disturbi Del Sonno in Età Pediatrica; Guida Pratica; Editeam: Ferrara, Italy, 2021. [Google Scholar]
- Rana, M.; Kothare, S.; DeBassio, W.J. The Assessment and Treatment of Sleep Abnormalities in Children and Adolescents with Autism Spectrum Disorder: A Review. J. Can. Acad. Child Adolesc. Psychiatry 2021, 30, 25–35. [Google Scholar]
- Polimeni, A.L.; Richdale, A.J. A survey of sleep problems in autism, Asperger’s disorder and typically developing children. J. Intellect. Disabil. Res. 2005, 49, 260–268. [Google Scholar] [CrossRef]
- Kotagal, S.; Broomall, E. Sleep in children with autism spectrum disorder. Pediatric Neurol. 2012, 47, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Souders, M.C.; Zavodny, S.; Eriksen, W.; Sinko, R.; Connell, J.; Kerns, C.; Schaaf, R.; Pinto-Martin, J. Sleep in Children with Autism Spectrum Disorder. Curr. Psychiatry Rep. 2017, 19, 34. [Google Scholar] [CrossRef] [PubMed]
- Carmassi, C.; Palagini, L.; Caruso, D.; Masci, I.; Nobili, L.; Vita, A.; Dell’Osso, L. Systematic Review of Sleep Disturbances and Circadian Sleep Desynchronization in Autism Spectrum Disorder: Toward an Integrative Model of a Self-Reinforcing Loop. Front. Psychiatry 2019, 10, 366. [Google Scholar] [CrossRef] [PubMed]
- Cortese, S.; Wang, F.; Angriman, M.; Masi, G.; Bruni, O. Sleep Disorders in Children and Adolescents with Autism Spectrum Disorder: Diagnosis, Epidemiology, and Management. CNS Drugs 2020, 34, 415–423. [Google Scholar] [CrossRef]
- Devnani, P.A.; Hegde, A.U. Autism and sleep disorders. J. Pediatric Neurosci. 2015, 10, 304–307. [Google Scholar] [CrossRef] [Green Version]
- Veatch, O.J.; Maxwell-Horn, A.C.; Malow, B.A. Sleep in Autism Spectrum Disorders. Curr. Sleep Med. Rep. 2015, 1, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Román, A.; Zhang, J.; Delorme, R.; Beggiato, A.; Cortese, S. Sleep in youth with autism spectrum disorders: Systematic review and meta-analysis of subjective and objective studies. Evid. Based Ment. Health 2018, 21, 146–154. [Google Scholar] [CrossRef]
- Johnson, C.R.; Smith, T.; DeMand, A.; Lecavalier, L.; Evans, V.; Gurka, M.; Swiezy, N.; Bearss, K.; Scahill, L. Exploring sleep quality of young children with autism spectrum disorder and disruptive behaviors. Sleep Med. 2018, 44, 61–66. [Google Scholar] [CrossRef]
- Köse, S.; Yılmaz, H.; Ocakoğlu, F.T.; Özbaran, N.B. Sleep problems in children with autism spectrum disorder and intellectual disability without autism spectrum disorder. Sleep Med. 2017, 40, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Romeo, D.M.; Brogna, C.; Belli, A.; Lucibello, S.; Cutrona, C.; Apicella, M.; Mercuri, E.; Mariotti, P. Sleep Disorders in Autism Spectrum Disorder Pre-School Children: An Evaluation Using the Sleep Disturbance Scale for Children. Medicina 2021, 57, 95. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, V.; Juneja, M.; Jain, R. Sleep Problems and Their Correlates in Children with Autism Spectrum Disorder: An Indian Study. J. Autism Dev. Disord. 2019, 49, 1169–1181. [Google Scholar] [CrossRef] [PubMed]
- Schreck, K.A.; Richdale, A.L. Sleep problems, behavior, and psychopathology in autism: Inter-relationships across the lifespan. Curr. Opin. Psychol. 2020, 34, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, H.; Wu, Y.; Xuan, K.; Zhao, T.; Sun, Y. Characteristics of sleep architecture in autism spectrum disorders: A meta-analysis based on polysomnographic research. Psychiatry Res. 2021, 296, 113677. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Cardinali, D.P.; Shakunthala, V.; Spence, D.W.; Brown, G.M.; Pandi-Perumal, S.R. Understanding the role of sleep and its disturbances in Autism spectrum disorder. Int. J. Neurosci. 2020, 130, 1033–1046. [Google Scholar] [CrossRef]
- Tye, C.; Runicles, A.K.; Whitehouse, A.; Alvares, G.A. Characterizing the Interplay Between Autism Spectrum Disorder and Comorbid Medical Conditions: An Integrative Review. Front. Psychiatry 2019, 9, 751. [Google Scholar] [CrossRef] [Green Version]
- Gorgoni, M.; Scarpelli, S.; Reda, F.; De Gennaro, L. Sleep EEG oscillations in neurodevelopmental disorders without intellectual disabilities. Sleep Med. Rev. 2020, 49, 101224. [Google Scholar] [CrossRef]
- Sun, L.; Zhou, H.; Cichon, J.; Yang, G. Experience and sleep-dependent synaptic plasticity: From structure to activity. Philos. Trans. R. Soc. B 2020, 375, 20190234. [Google Scholar] [CrossRef]
- Puentes-Mestril, C.; Aton, S.J. Linking Network Activity to Synaptic Plasticity during Sleep: Hypotheses and Recent Data. Front. Neural Circuits 2017, 11, 61. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Elia, M.; Ferri, R.; Musumeci, S.A.; Del Gracco, S.; Bottitta, M.; Scuderi, C.; Miano, G.; Panerai, S.; Bertrand, T.; Grubar, J.C. Sleep in subjects with autistic disorder: A neurophysiological and psychological study. Brain Dev. 2000, 22, 88–92. [Google Scholar] [CrossRef]
- Malow, B.A.; Marzec, M.L.; McGrew, S.G.; Wang, L.; Henderson, L.M.; Stone, W.L. Characterizing sleep in children with autism spectrum disorders: A multidimensional approach. Sleep 2006, 29, 1563–1571. [Google Scholar] [CrossRef] [Green Version]
- Miano, S.; Bruni, O.; Elia, M.; Trovato, A.; Smerieri, A.; Verrillo, E.; Roccella, M.; Terzano, M.G.; Ferri, R. Sleep in children with autistic spectrum disorder: A questionnaire and polysomnographic study. Sleep Med. 2007, 9, 64–70. [Google Scholar] [CrossRef]
- Bruni, O.; Ferri, R.; Vittori, E.; Novelli, L.; Vignati, M.; Porfirio, M.C.; Aricò, D.; Bernabei, P.; Curatolo, P. Sleep architecture and NREM alterations in children and adolescents with Asperger syndrome. Sleep 2007, 30, 1577–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldman, S.E.; Surdyka, K.; Cuevas, R.; Adkins, K.; Wang, L.; Malow, B.A. Defining the sleep phenotype in children with autism. Dev. Neuropsychol. 2009, 34, 560–573. [Google Scholar] [CrossRef] [Green Version]
- Ming, X.; Sun, Y.M.; Nachajon, R.V.; Brimacombe, M.; Walters, A.S. Prevalence of parasomnia in autistic children with sleep disorders. Clin. Med. Pediatrics 2009, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Giannotti, F.; Cortesi, F.; Cerquiglini, A.; Vagnoni, C.; Valente, D. Sleep in children with autism with and without autistic regression. J. Sleep Res. 2011, 20, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Buckley, A.W.; Rodriguez, A.J.; Jennison, K.; Buckley, J.; Thurm, A.; Sato, S.; Swedo, S. Rapid eye movement sleep percentage in children with autism compared with children with developmental delay and typical development. Arch. Pediatrics Adolesc. Med. 2010, 164, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Tessier, S.; Lambert, A.; Chicoine, M.; Scherzer, P.; Soulières, I.; Godbout, R. Intelligence measures and stage 2 sleep in typically-developing and autistic children. Int. J. Psychophysiol. 2015, 97, 58–65. [Google Scholar] [CrossRef]
- Tessier, S.; Lambert, A.; Scherzer, P.; Jemel, B.; Godbout, R. REM sleep and emotional face memory in typically-developing children and children with autism. Biol. Psychol. 2015, 110, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.; Tessier, S.; Rochette, A.C.; Scherzer, P.; Mottron, L.; Godbout, R. Poor sleep affects daytime functioning in typically developing and autistic children not complaining of sleep problems: A questionnaire-based and polysomnographic study. Res. Autism Spectrum Disord. 2016, 23, 94–106. [Google Scholar] [CrossRef]
- Sahroni, A.; Igasaki, T.; Murayama, N. Band powers analysis of spontaneous EEG with uncooperative autism children during short sleep condition. In Proceedings of the 2015 8th International Conference on Biomedical Engineering and Informatics (BMEI), Shenyang, China, 14–16 October 2015; pp. 163–168. [Google Scholar]
- Maski, K.; Holbrook, H.; Manoach, D.; Hanson, E.; Kapur, K.; Stickgold, R. Sleep Dependent Memory Consolidation in Children with Autism Spectrum Disorder. Sleep 2015, 38, 1955–1963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehoux, T.; Carrier, J.; Godbout, R. NREM sleep EEG slow waves in autistic and typically developing children: Morphological characteristics and scalp distribution. J. Sleep Res. 2019, 28, e12775. [Google Scholar] [CrossRef]
- Aathira, R.; Gulati, S.; Tripathi, M.; Shukla, G.; Chakrabarty, B.; Sapra, S.; Dang, N.; Gupta, A.; Kabra, M.; Pandey, R.M. Prevalence of Sleep Abnormalities in Indian Children with Autism Spectrum Disorder: A Cross-Sectional Study. Pediatric Neurol. 2017, 74, 62–67. [Google Scholar] [CrossRef]
- Vite, T.K.G.; Guerrero, F.A.; Salgado, E.L.M.; Paniagua, R.C. Characterization of the Mu rhythm during the sleep of children with autism spectrum disorder level 1. Salud Ment. 2018, 41, 109–116. [Google Scholar] [CrossRef]
- Farmer, C.A.; Chilakamarri, P.; Thurm, A.E.; Swedo, S.E.; Holmes, G.L.; Buckley, A.W. Spindle activity in young children with autism, developmental delay, or typical development. Neurology 2018, 91, e112–e122. [Google Scholar] [CrossRef]
- Page, J.; Lustenberger, C.; Fröhlich, F. Nonrapid eye movement sleep and risk for autism spectrum disorder in early development: A topographical electroencephalogram pilot study. Brain Behav. 2020, 10, e01557. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, F.E.; Knowland, V.; Walker, S.; Gaskell, M.G.; Norbury, C.; Henderson, L.M. Atypicalities in sleep and semantic consolidation in autism. Dev. Sci. 2020, 23, e12906. [Google Scholar] [CrossRef]
- Arazi, A.; Meiri, G.; Danan, D.; Michaelovski, A.; Flusser, H.; Menashe, I.; Tarasiuk, A.; Dinstein, I. Reduced sleep pressure in young children with autism. Sleep 2020, 43, zsz309. [Google Scholar] [CrossRef] [PubMed]
- Rechtschaffen, A.; Kales, A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stage of Human Subjects; U.S. Government Printing Office: Washington, DC, USA, 1968.
- Owens, J.A.; Spirito, A.; McGuinn, M. The Children’s Sleep Habits Questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep 2000, 23, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Drake, C.; Nickel, C.; Burduvali, E.; Roth, T.; Jefferson, C.; Pietro, B. The pediatric daytime sleepiness scale (PDSS): Sleep habits and school outcomes in middle-school children. Sleep 2003, 26, 455–458. [Google Scholar] [PubMed]
- Elrod, M.G.; Hood, B.S. Sleep differences among children with autism spectrum disorders and typically developing peers: A Meta-analysis. J. Dev. Behav. Pediatrics 2015, 36, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Hollway, J.A.; Aman, M.G.; Butter, E. Correlates and risk markers for sleep disturbance in participants of the Autism Treatment Network. J. Autism Dev. Disord. 2013, 43, 2830–2843. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.; Evans, V.; Hanvey, G.; Johnson, C. Assessment of Sleep in Children with Autism Spectrum Disorder. Children 2017, 4, 72. [Google Scholar] [CrossRef]
- Wintler, T.; Schoch, H.; Frank, M.G.; Peixoto, L. Sleep, brain development, and autism spectrum disorders: Insights from animal models. J. Neurosci. Res. 2020, 98, 1137–1149. [Google Scholar] [CrossRef]
- Bourgeron, T. The possible interplay of synaptic and clock genes in autism spectrum disorders. Cold Spring Harb. Symp. Quant. Biol. 2007, 72, 645–654. [Google Scholar] [CrossRef] [Green Version]
- Ingiosi, A.M.; Schoch, H.; Wintler, T.; Singletary, K.G.; Righelli, D.; Roser, L.G.; Medina, E.; Risso, D.; Frank, M.G.; Peixoto, L. Shank3 modulates sleep and expression of circadian transcription factors. eLife 2019, 8, e42819. [Google Scholar] [CrossRef]
- Wong, K.; Leonard, H.; Jacoby, P.; Ellaway, C.; Downs, J. The trajectories of sleep disturbances in Rett syndrome. J. Sleep Res. 2015, 24, 223–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, L.M.J.; Lüthi, A. Sleep spindles: Mechanisms and functions. Physiol. Rev. 2020, 100, 805–868. [Google Scholar] [CrossRef] [PubMed]
- Novelli, L.; D’atri, A.; Marzano, C.; Finotti, E.; Ferrara, M.; Bruni, O.; De Gennaro, L. Mapping changes in cortical activity during sleep in the first 4 years of life. J. Sleep Res. 2016, 25, 381–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hornung, T.; Chan, W.H.; Müller, R.A.; Townsend, J.; Keehn, B. Dopaminergic hypo-activity and reduced theta-band power in autism spectrum disorder: A resting-state EEG study. Int. J. Psychophysiol. 2019, 146, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Rizzolatti, G.; Craighero, L. The mirror-neuron system. Ann. Rev. Neurosci. 2004, 27, 169–192. [Google Scholar] [CrossRef] [Green Version]
- Oberman, L.M.; Hubbard, E.M.; McCleery, J.P.; Altschuler, E.L.; Ramachandran, V.S.; Pineda, J.A. EEG evidence for mirror neuron dysfunction in autism spectrum disorders. Cogn. Brain Res. 2005, 24, 190–198. [Google Scholar] [CrossRef]
- Hamilton, A.F.D.C. Reflecting on the mirror neuron system in autism: A systematic review of current theories. Dev. Cogn. Neurosci. 2013, 3, 91–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palau-Baduell, M.; Valls-Santasusana, A.; Salvadó-Salvadó, B. Trastornos del espectro autista y ritmo mu. Una nueva perspectiva neurofisiologica [Autism spectrum disorders and mu rhythm. A new neurophysiological view]. Rev. Neurol. 2011, 52 (Suppl. 1), S141–S146. [Google Scholar]
- Gorgoni, M.; D’Atri, A.; Scarpelli, S.; Reda, F.; De Gennaro, L. Sleep electroencephalography and brain maturation: Developmental trajectories and the relation with cognitive functioning. Sleep Med. 2020, 66, 33–50. [Google Scholar] [CrossRef]
| Author, Year, Country | ASD Group | Control Group | PSG/EEG Features | Subjective Measures | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n (% Male) | Mean Age (Age Range) | Diagnosis | Diagnostic Tools | Inclusion/Exclusion Criteria | Type | n (% Male) | Mean Age (Age Range) | |||
| Elia, 2000, Italy [25] | 17 (100%) | 10.36 years (5.7–16.8 years) | Autistic disorder (DSM-IV) | CARS | Inclusion criteria in AD patients:
| fragile XTD | 7 (100%) 5 (100%) | 9.92 years (8.25–12 years) 9.22 years (7.17–11.58 years) | 2 PSG with one adaptation night | - |
| Malow, 2006, USA [26] | 21(85.7%) | - (4–10 years) | Autism, pervasive developmental disorder, Asperger’s disorder (DSM-IV) | ADOS | Inclusion criteria in ASD patients:
| TD | 10 (80%) | (4–10 years) | 2 consecutive nights of video monitoring combined with EEG and PSG with 21 EEG channels | Sleep diaries CSHQ |
| Miano, 2007, Italy [27] | 31 (90.3%) | 9.53 years (3.7–19 years) | Autistic disorder (DSM-IV) | CARS | Inclusion criteria in ASD patients:
| TD | 18 (50%) | age-matched | Overnight PSG recording after one adaptation night. The PSG montage includes at least 3 EEG channels (including C3 and C4 in particular) | Sleep Questionnaire |
| Bruni, 2007, Italy [28] | 10 autism (90%) 8 Asperger syndrome (87.5%) | autism: 11.9 ± 2.5 years (7–15 years) Asperger syndrome: 12.7 ± 2.6 years (7–15 years) | Autism (DSM-IV) Asperger syndrome (DSM-IV) | CARS | Inclusion criteria in patients with AS:
| TD | 12 (41.6%) | 12.6 ± 3.7 years (7–15 years) | PSG overnight The PSG montage included at least 8 EEG channels (Fp1, Fp2, C3, C4, T3, T4, O1, O2) All recordings started at the patients’ usual bedtime and continued until spontaneous awakening. | Sleep Questionnaire PDSS |
| Goldman, 2009, USA [29] | 27 psASD (88.9%) 15 gsASD: (93.3%) | PS: 5.8 years GS: 5.9 years | ASD (DSM-IV) | ADOS | Inclusion criteria in ASD children:
| TD | 16 (75%) | 6.9 years | Two consecutive nights of PSG with 21-channel EEG and actigraphy monitoring | PCQ CSHQ |
| Ming, 2009, USA [30] | 23 (82.6%) | 6 years (3–15 years) | autistic disorder, PDD-NOS, Asperger’s disorder (DSM-IV) | ASD ADI-R, ADOS-G | Inclusion criteria in ASD patients:
| TD | 23 (65.2%) | 5 years (3–12 years) | PSG for 2 consecutive nights with standard four channel EEG for PSG | Sleep Questionnaires |
| Giannotti, 2010, Italy [31] | 22 NRegASD (75%) 18 RegASD (80%) | 5.5 years 5.10 years | autistic disorder (DSM-IV) | ADI-R, ADOS-G | Inclusion criteria in autistic groups:
| TD | 12 (75%) | 5.8 years | Overnight PSG including at least 11 EEG channels (Fp1, Fp2, C3, C4, Cz, P3, P4, T3, T4, O1, O2) for 2 consecutive nights | CSHQ |
| Buckley, 2010, USA [32] | 60 (82%) | 4.81 years (2.24–13.11 years) | Autism (DSM-IV) | ADOS, ADI-R | Inclusion criteria in patients with autism:
| developmental delay TD | 13 (54%) 15 (73%) | 4.29 years (2.69–7.11 years) 3.69 years (1.35–5.84 years) | Overnight PSG with 21 lead electroencephalogram montage | CSHQ |
| Tessier, 2015, Canada [33] | 13 (100%) | 10.23 years (6–13 years) | hfASD(DSM-IV-TR) | ADI, ADOS | Inclusion criteria in patients with autism:
| TD | 13 (100%) | 10.23 years (7–12 years) | Recordings took place on 2 consecutive nights in individual bedrooms using bilateral central, frontal and occipital EEG leads (C3, C4, F3, F4, O1, O2) | Sleep diary |
| Tessier, 2015 Canada [34] | 13 (100%) | 10.23 years (6–13 years) | hfASD(DSM-IV-TR) | ADI-R, ADOS | Inclusion criteria in patients with autism:
| TD | 13 (100%) | 10.23 years (7–12 years) | Recordings took place on 2 consecutive nights in individual bedrooms using bilateral central, occipital and parietal EEG leads (C3, C4, O1, O2, P7, P8) | Sleep diary |
| Lambert, 2015 Canada [35] | 11 (100%) | 10.27 years (6–13 years) | hfASD(DSM-IV) | ADI-R, ADOS | Inclusion criteria in patients with autism:
| TD | 13 (100%) | 10.23 years (7–12 years) | Participants spent two consecutive nights in the sleep laboratory, the first night served for adaptation to recording conditions. All PSG data reported were recorded during the second night. The electrodes that have been used are not specified | CSHQ |
| Sahroni, 2015, Japan [36] | 8 (87.5%) | 10.23 years (7–12 years) | Autistic disorder (DSM-IV) | Inclusion criteria in patients with autism:
| TD | 8 (62.5%) | 6.14 ± 2.19 years | Both groups were given some sedative before EEG recordings to make subjects sleep in a short time of period (10–15 min) 19 electrodes were placed according to the 10–20 international system | ___ | |
| Maski, 2015, USA [37] | 22 (86%) | 11.3 years (9–16 years) | ASD (DSM-IV) | ADOS, ADI R | Inclusion criteria in ASD group:
| TD | 20 (90%) | 12.3 years (9–16 years) | Home PSG recordings with seven channels of EEG (F1, F2, C3, Cz, C4, O1, O2) | CSHQ |
| Lehoux, 2017, Canada [38] | 13 (100%) | 10.23 years (6–13 years) | hfASD(DSM-IV) | ADI R | Inclusion criteria in ASD patients:
| TD | 13 | 10.23 years (7–12 years) | PSG montage included 7 EEG channels (F3, F4, C3, P3, P4, O1, O2). | Sleep diary |
| Aathira, 2017, India [39] | 71(90.3%) | 5.3 ± 1.8 years (3–10 years) | ASD (DSM-IV) | Inclusion criteria in ASD group:
| TD | 65 (61.5%) | 5.7 ± 1.6 years (3–10 years) | Single overnight PSG | CSHQ | |
| Vite, 2018, Messico [40] | 10 (100%) | 8.2 years (6–10 years) | ASD, level 1 (DSM-IV and DSM-V) | Inclusion criteria in ASD group:
| TD | 7 (100%) | 8.3 years (6–10 years) | 2 eight-hour PSG were performed for 2 consecutive nights. The referrals for night 1 were: C3, C4, O2, O1 The referrals for night 2 were: F3, F4, C3, C4, T3, T4, P3, P4, O1, O2 | - | |
| Farmer, 2018 USA [41] | 85 (84%) | 2–6 years | Autistic disorder (DSM-IV-TR) | ADI, ADOS | Inclusion criteria in patients with autism:
| TD DD | 29 (72%) 21 (62%) | 2–6 years 2–6 years | Overnight video-EEG | - |
| Page, 2019, USA [42] | 7 (71.4%) | 21.8 months (13–30 months) | ASD (DSM-5) | FYI, M-CHAT-R/F, ADOS-2 | Inclusion criteria in ASD group:
| TD | 13 (38.4%) | 21.8 months (13–30 months) | EEG was recorded during a daytime nap (average duration of 78 min) with a 124- or 128-channel high density EEG electrode net | Sleep diary |
| Fletcher, 2019, UK [43] | 20 (80%) | 125.55 months | autism | GARS | Inclusion criteria in ASD group:
| TD | 34 (50%) | 118.94 months | Home PSG with a montage of six EEG (F3, F4, C3, C4, O1, O2) | CSHQ |
| Arazi, 2019, Israel [44] | 29 (72.4%) | 4.6 years (1.9–7.8 years) | autism (DSM-5) | ADOS | Inclusion criteria in ASD group:
| TD | 23 (65.2%) | 5.3 ± 1.5 years | PSG with 6 EEG electrodes (C3, C4, O1, O2, A1, A2) | CSHQ |
| First Author, Year, Country | Macrostructural EEG Features | Microstructural EEG Features | Sleep Subjective Features | Other Findings |
|---|---|---|---|---|
| Elia, 2000, Italy [25] | ASD vs. TD ↓ TIB (p < 0.01), TST (p < 0.02), SPT (p < 0.01) ASD vs. X-fragile ↓ SPT (p < 0.03), RL (p < 0.01), N1 (p < 0.05) | |||
| Malow, 2006, USA [26] | psASD vs. gsASD 1st night ↓ SE (p = 0.0091), REM% (p = 0.0226) ↑ SL (p < 0.0079), N3,N4 (p = 0.446) 2nd night ↓ TST (p= 0.3800) gsASD vs. TD 1st night ↓ TST (p= 0.5507) 2nd night ↓ TST (p= 0. 5483) | |||
| Miano, 2007, Italy [27] | ASD vs. TD ↓ TIB (p < 0.044), SPT (p < 0.007), RL (p < 0.02) | ASD vs. TD ↓ CAP rate during N3,4 (p < 0.02) A1% (p < 0.0004) ↑ A2% (p < 0.006) A3% (p < 0.02) | ASD vs. TD Sleep Questionnaire: ↑ Sleep less than 8 hours (p < 0.02) Latency to sleep > 30 min (p < 0.000001) Difficulty falling asleep at night (p < 0.002) Fluids or drugs to facilitate sleep (p < 0.00001) Hypnic jerks (p < 0.00001) Rhythmic movements while falling asleep (p < 0.00001) Poor sleep quality (p < 0.00001) More than two awakenings per night (p < 0.05) Waking up to drink or to eat at night (p < 0.015) Difficulty to fall asleep after awakenings (p < 0.00001) Parasomnias – bedwetting (p < 0.00001) Daytime somnolence (p < 0.03) Falling asleep at school (p < 0.02) ↓ Drinks stimulant beverages in the evening (p < 0.00001) | |
| Bruni, 2007, Italy [28] | No significant results | AS vs. TD ↑ A1% (η2 = 1.43; p < 0.009) ↓ A2% (η2 = −1.88; p < 0.003) AS vs. ASD ↑ CAP rate during N3,4 (η2 = 1.41; p < 0.02) A1% (η2 = 2.05; p < 0.001) | AS Sleep Questionnaires: reluctant to go to bed (50%) need for light or TV in the bedroom (75%) difficulty getting to sleep at night (87%) falling asleep sweating (75%) nocturnal hyperkinesia(50%) feeling unrefreshed upon morning awakening (50%) difficulty in waking up in the morning (87%) daytime somnolence 87%) PDSS mean score 16.5 ± 3.4 | AS Positive correlation between verbal IQ and: total CAP rate (r = 0.99) CAP rate in SWS (r = 0.95) global A1 index (r = 0.94) SWS A1 index (r = 0.76) Negative correlation between A2% and: FSIQ (r = −0.086) VIQ (r = −0.86) PIQ (r = −0.81) Positive correlation between CBCL total score and: cap rate (r = 0.76) A1 index (r = 0.88) Negative correlation between externalizing score and A3% (r= −0.81) |
| Goldman, 2009, USA [29] | psASD vs. TD ↑ SL (p < 0.05) psASD vs. gsASD ↑ SL (p < 0.05) | PCQ: poor sleepers rate among ASD: 64% psASD vs. gsASD CSHQ: ↑ sleep onset delay (p < 0.01), sleep duration (p < 0.01), night wakings and total (p < 0.01) psASD vs. TD ↑ for all dimensions except sleep disordered breathing | ||
| Ming, 2009, USA [30] | ASD vs. TD ↓ REM% (p = 0.002) | ASD Sleep Questionnaires: Parasomnias (60.8%), Disorder of Partial Arousal (55.6%) | ||
| Giannotti, 2010, Italy [31] | NregASD vs. RegASD ↑ TST (p < 0.001), SE (p < 0.001) ↓ WASO (p < 0.001), SL (p < 0.001) RegASD vs. TD ↓ TST (p < 0.001), SE (p < 0.001), REM% (p < 0.01), N3,4 (p < 0.001) ↑ WASO (p < 0.001), SL (p < 0.001), RL (p < 0.01), N2 (p < 0.001) NRegASD vs. TD ↓ TST (p < 0.001), SE (p < 0.001) ↑ WASO (p < 0.001), SL (p < 0.001), RL (p < 0.01) | ASD vs. TD ↓A1% (p < 0.001) ↑A2% (p < 0.01) A3% (p < 0.001) RegASD vs. TD ↓CAP rate during N1,2 (p < 0.01) | NregASD vs. RegASD CSHQ: ↓ Bedtime, Bedtime resistance, Sleep onset delay, Sleep duration, Night-wakings (p < 0.001); Sleep latency (p < 0.05) ↑ Sleep length (p < 0.001) RegASD/NRegASD vs. TD ↓ Sleep length, (p < 0.001) ↑ Bedtime, Sleep latency, Bedtime resistance, Sleep onset delay, Sleep duration, Night-wakings (p < 0.001) | |
| Buckley, 2010, USA [32] | ASD vs. TD ↓TST (p = 0.004), REM% (p < 0.001) ↑RL (p = 0.016), N3,4 (p = 0.001) ASD vs. DD ↓TST (p = 0.001), REM% (p < 0.001) ↑RL (p = 0.012), N3,4 (p < 0.001) | CSHQ: Median wake time: ASD 06.17 DD 06.45 TD 06.46 | ||
| Tessier, 2015, Canada [33] | hfASD vs. TD Fp1 ↓Sleep Spindles duration (p < 0.05) Fp2 ↓Sleep Spindles density (p < 0.05) ↓Fast sigma EEG activity at C3, C4 (p < 0.05) | Sleep diary: No sleep disturbances complained in the previous 14 days. | TD negative correlation between VIQ and Fp2 spindle density for the last quarter of the night (r= −0.6, p < 0.04) positive correlation: between VIQ and C4 spindle duration for the total night (r = 0.72, p = 0.01) between PIQ and fast sigma activity in the end of the night at the C4 electrode (r = 0.59, p = 0.04) ASD negative correlation: between VIQ and C3 spindle density for the total night (r= −0.62, p = 0.02) between FSIQ and C3 spindle density for the total night (r= −0.55, p = 0.05) | |
| Tessier, 2015 Canada [34] | No significant results | Sleep diary: No sleep disturbances complained in the previous 14 days. | ASD vs. TD ↑ neutral emotion reaction times on the delayed recognition task ((η2 = 0.16, p = 0.04) | |
| Lambert, 2015 Canada [35] | ASD vs. TD ↑SL (p = 0.02) ↓N3,4 (p = 0.026) | ASD vs. TD Fp1 ↓K-complex (p = 0.006) Fp2 ↓Sleep Spindles density (p = 0.03), K-complex (p = 0.013) C3 ↓K-complex (p = 0.002) C4 ↓K-complex (p = 0.006) | ASD vs. TD CSHQ: No significant results. Agendas: ↓Sleep onset latency (p < 0.05) Sleep quality (p < 0.02) | ASD Negative correlation between N1% and FSIQ (r = −0.53, p = 0.009) and PIQ (r = −0.65, p = 0.001) Negative correlation between N3,4% and CBCL internalized behaviors (r = −0.41, p = 0.046). Positive correlation between SL reported in daily sleep agendas and in PSG in both groups (r = 0.75, p < 0.001). |
| Sahroni, 2015, Japan [36] | ASD vs. TD ↑ absolute theta band power in T6 (p = 0.0379) ↑ absolute alpha band power in F7, Fz, F4, T3, Cz, C4, P3 (p < 0.03) ↑ relative delta band power in Fz, T6 (p = 0.0379) ↓ relative beta band power in T6 (p < 0.04) ↓ absolute and relative gamma band power in Fp1, T5, P3, T6, O1, O2 (p < 0.04) | |||
| Maski, 2015, USA [37] | ASD vs. TD ↑ TIB (p = 0.01), WASO (p = 0.02), SL (p = 0.01) ↓ SE (p < 0.001), REM% (p = 0.007) | ASD vs. TD CSHQ: ↑ Bedtime resistance (p = 0.03), Sleep onset delay (p = 0.02), Sleep duration (p = 0.04), Sleep anxiety (p = 0.001), Daytime sleepiness (p < 0.02), Parasomnias (p = 0.02) | ASD vs. TD No significant differences in benefiting from sleep in memory consolidation tasks | |
| Lehoux, 2017, Canada [38] | ASD vs. TD ↓N3,4 (p = 0.007) | Sleep diary: No sleep disturbances complained in the previous 14 days. | ||
| Aathira, 2017, India [39] | poor sleepers rate among ASD: 77.5% ASD vs. TD CSHQ: ↑ Daytime sleepiness (p < 0.001), Parasomnias (p < 0.001), Sleep anxiety (p = 0.002), Bedtime resistance (p < 0.001) | psASD vs. gsASD ↑ higher CBCL mean score (p = 0.004), CBCL “withdrawn” score in the borderline or clinical range (p = 0.03) Not significant results about IQ and CARS | ||
| Vite, 2018, Messico [40] | ASD vs. TD ↑ Mu rhythm peak in C3 (p = 0.003) ↓ Mu rhythm peak in C4 (p = 0.003) | |||
| Farmer, 2018 USA [41] | ASD vs. TD ↓Sleep Spindles density (p < 0.0001) ↓Sleep Spindles duration (p = 0.006) ASD vs. DD ↓Sleep Spindles density (p = 0.017) | For the full sample, significant correlation between: spindle density and IQ (r = 0.26, p < 0.002) spindle density and Vineland subscales: socialization (r = 0.33, p = 0.0001) communication (r = 0.32, p = 0.0002) living skills (r = 0.25, p = 0.003) | ||
| Page, 2019, USA [42] | ASD vs. TD ↓ theta band power in temporo-central regions (p < 0.05) ↑ beta band power in right temporo-occipital region (p < 0.05) ↑ slower sigma power over occipital and central regions (p < 0.05) ↓ higher frequency sigma power over frontal, central, and parietal regions (p < 0.05) | ASD vs. TD Sleep diary: No significant differences in the naptime nor in the duration of wakefulness before the nap. | No significant correlation between ADOS-2 score and NREM spectral power | |
| Fletcher, 2019, UK [43] | ASD vs. TD ↓ TST (p≤0.05), NREM (p < 0.05) | ASD vs. TD ↓ sigma power(p ≤ 0.001) | ASD vs. TD CSHQ: ↑Total sleep problems (p < 0.001) | ASD vs. TD ↓performances in specific memory tasks with memory recalling after a month |
| Arazi, 2019, Israel [44] | ASD vs. TD ↓ TIB (p = 0.02), TST (p = 0.03), REM% - second half of the night (p = 0.007) | ASD vs. TD CSHQ: 50% of ASD children had scores that were above the mean score from previously published CSHQ scores from a large population of typically developing children in all domains, excluding sleep duration and sleep disordered breathing | Negative correlation between SWA power and Bedtime resistance (r= −0.49, p = 0.01), Total sleep disturbances (r= −0.38, p = 0.05) and time to fall asleep (r = 0.42, p = 0.02). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petruzzelli, M.G.; Matera, E.; Giambersio, D.; Marzulli, L.; Gabellone, A.; Legrottaglie, A.R.; Margari, A.; Margari, L. Subjective and Electroencephalographic Sleep Parameters in Children and Adolescents with Autism Spectrum Disorder: A Systematic Review. J. Clin. Med. 2021, 10, 3893. https://doi.org/10.3390/jcm10173893
Petruzzelli MG, Matera E, Giambersio D, Marzulli L, Gabellone A, Legrottaglie AR, Margari A, Margari L. Subjective and Electroencephalographic Sleep Parameters in Children and Adolescents with Autism Spectrum Disorder: A Systematic Review. Journal of Clinical Medicine. 2021; 10(17):3893. https://doi.org/10.3390/jcm10173893
Chicago/Turabian StylePetruzzelli, Maria Giuseppina, Emilia Matera, Donatella Giambersio, Lucia Marzulli, Alessandra Gabellone, Anna Rosi Legrottaglie, Anna Margari, and Lucia Margari. 2021. "Subjective and Electroencephalographic Sleep Parameters in Children and Adolescents with Autism Spectrum Disorder: A Systematic Review" Journal of Clinical Medicine 10, no. 17: 3893. https://doi.org/10.3390/jcm10173893
APA StylePetruzzelli, M. G., Matera, E., Giambersio, D., Marzulli, L., Gabellone, A., Legrottaglie, A. R., Margari, A., & Margari, L. (2021). Subjective and Electroencephalographic Sleep Parameters in Children and Adolescents with Autism Spectrum Disorder: A Systematic Review. Journal of Clinical Medicine, 10(17), 3893. https://doi.org/10.3390/jcm10173893






