Targeting Oxidative Stress in Septic Acute Kidney Injury: From Theory to Practice
Abstract
1. Introduction
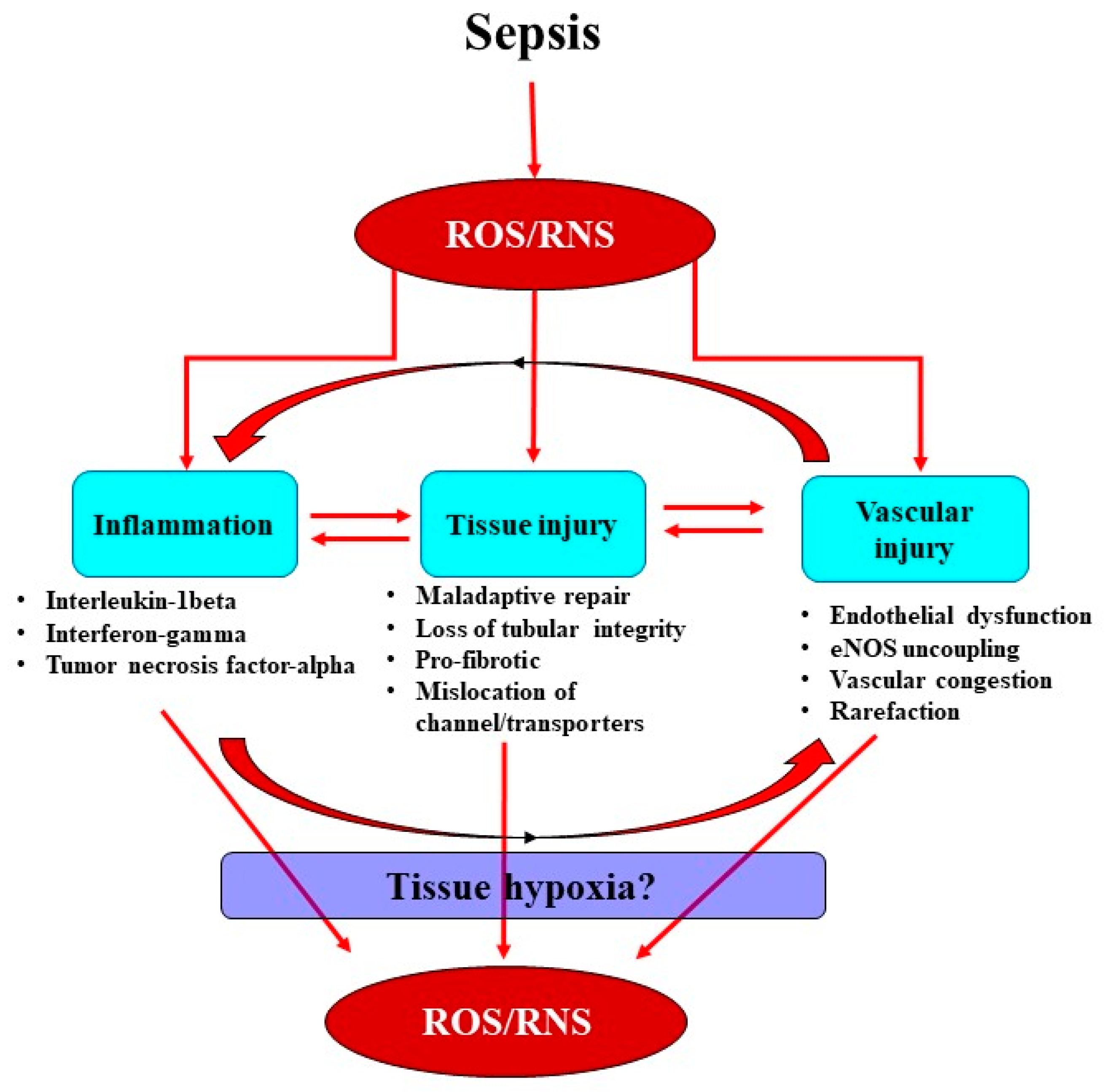
2. Interactions between the Septic Inflammatory Cascade and Oxidative Stress
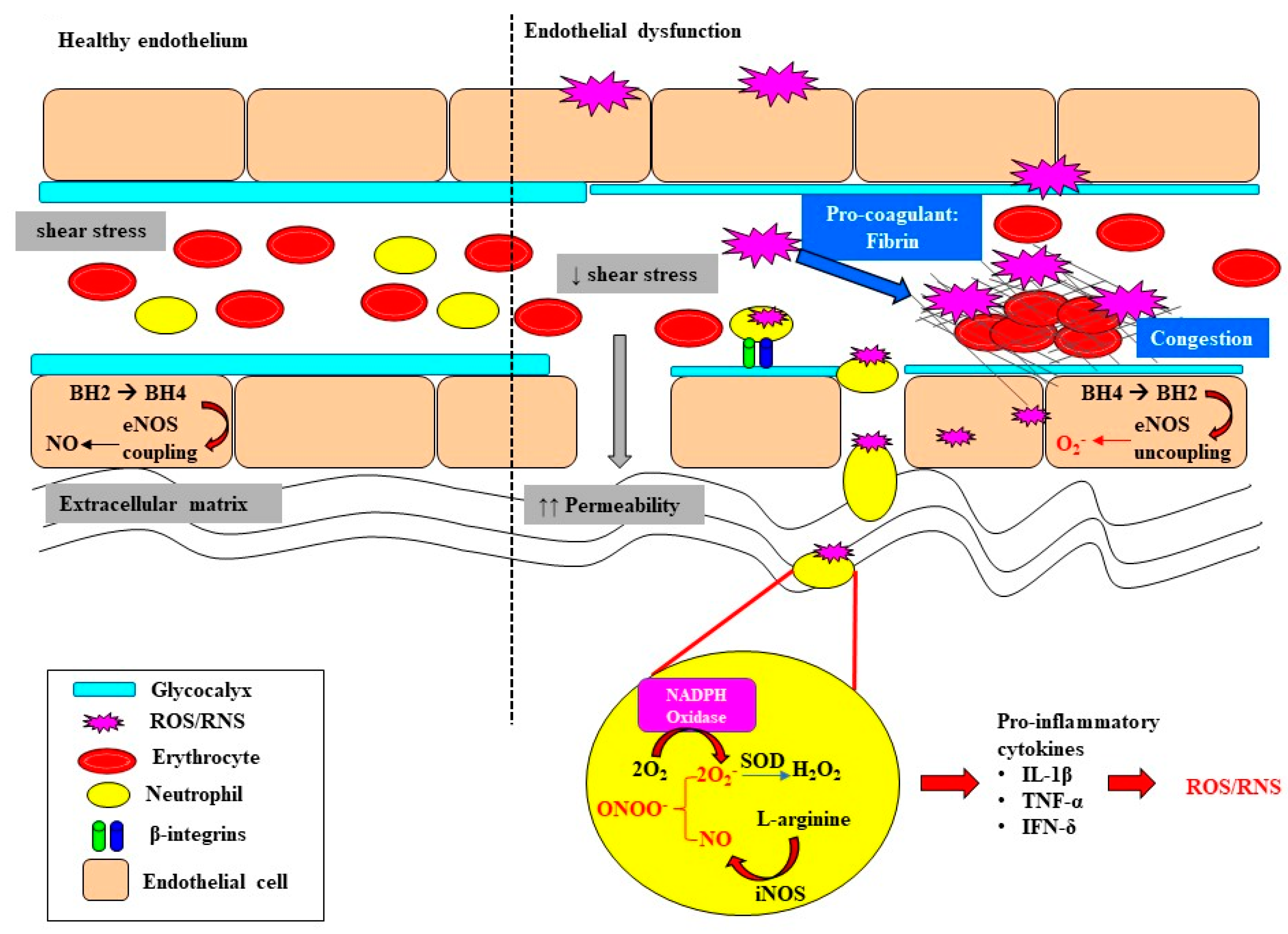
3. Oxidative Stress Exacerbates Microcirculatory Abnormalities and Vascular Rarefaction
4. Renal Medullary Tissue Hypoxia: A Critical Event in Acute Kidney Injury?
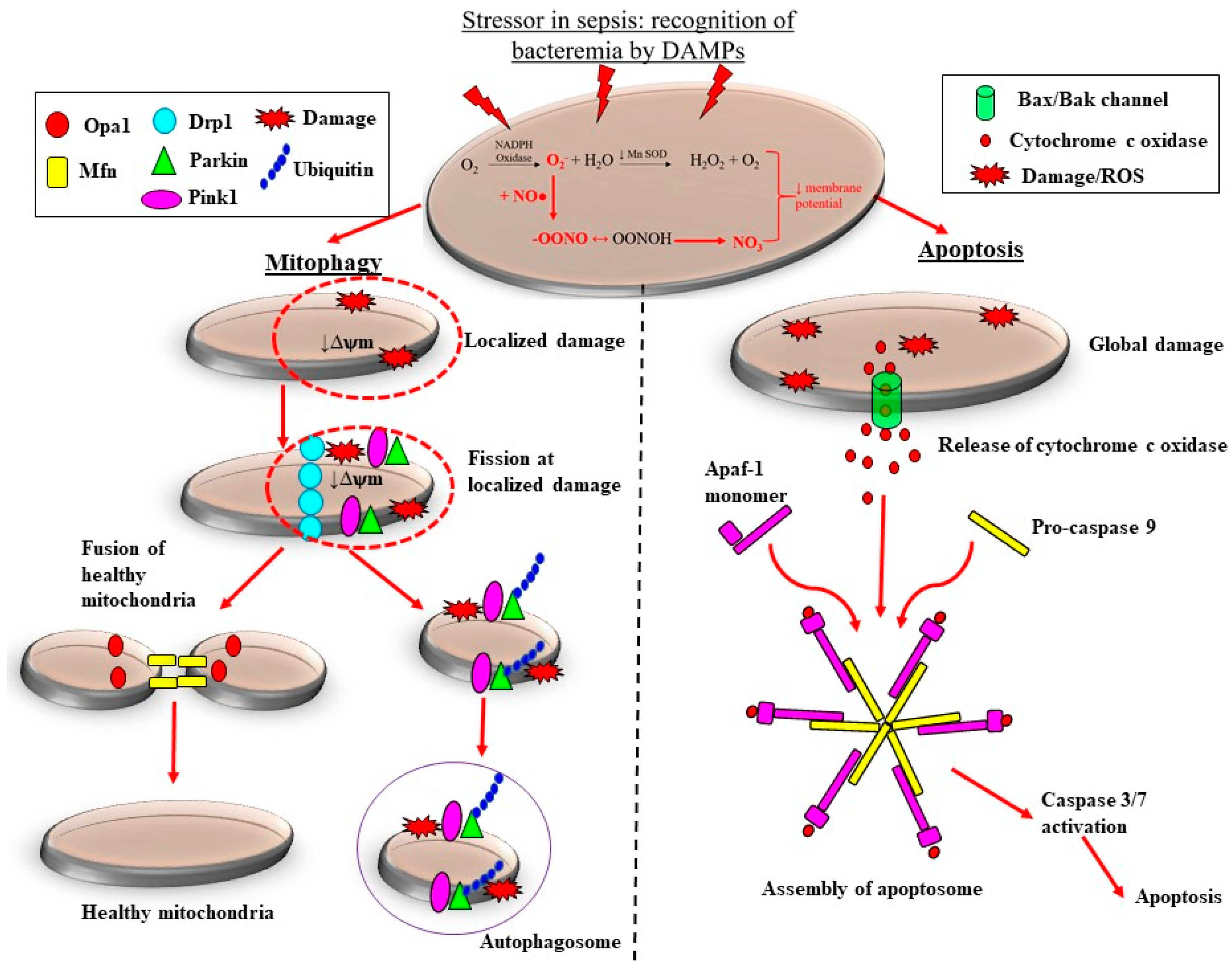
5. Sepsis-Induced Mitochondrial Dysfunction Activates Production of ROS
6. N-Acetylcysteine
7. Vitamin C
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bagshaw, S.M.; George, C.; Dinu, I.; Bellomo, R. A multi-centre evaluation of the RIFLE criteria for early acute kidney injury in critically ill patients. Nephrol. Dial. Transplant. 2007, 23, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, R.; Kellum, J.A.; Ronco, C.; Wald, R.; Martensson, J.; Maiden, M.; Bagshaw, S.M.; Glassford, N.J.; Lankadeva, Y.R.; Vaara, S.T.; et al. Acute kidney injury in sepsis. Intensive Care Med. 2017, 43, 816–828. [Google Scholar] [CrossRef] [PubMed]
- Odutayo, A.; Wong, C.X.; Farkouh, M.; Altman, D.G.; Hopewell, S.; Emdin, C.A.; Hunn, B.H. AKI and long-rerm risk for cardiovascular events and mortality. J. Am. Soc. Nephrol. 2017, 28, 377–387. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef]
- Schrier, R.W.; Wang, W. Acute Renal Failure and Sepsis. N. Engl. J. Med. 2004, 351, 159–169. [Google Scholar] [CrossRef]
- Takasu, O.; Gaut, J.P.; Watanabe, E.; To, K.; Fagley, R.E.; Sato, B.; Jarman, S.; Efimov, I.R.; Janks, D.L.; Srivastava, A.; et al. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am. J. Respir. Crit. Care Med. 2013, 187, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Lerolle, N.; Nochy, D.; Guérot, E.; Bruneval, P.; Fagon, J.-Y.; Diehl, J.-L.; Hill, G. Histopathology of septic shock induced acute kidney injury: Apoptosis and leukocytic infiltration. Intensive Care Med. 2009, 36, 471–478. [Google Scholar] [CrossRef]
- Corrêa, T.D.; Jeger, V.; Pereira, A.J.; Takala, J.; Djafarzadeh, S.; Jakob, S.M. Angiotensin II in septic shock: Effects on tissue perfusion, organ function, and mitochondrial respiration in a porcine model of fecal peritonitis. Crit. Care Med. 2014, 42, e550–e559. [Google Scholar] [CrossRef] [PubMed]
- Maiden, M.J.; Otto, S.; Brealey, J.K.; Finnis, M.E.; Chapman, M.J.; Kuchel, T.R.; Nash, C.H.; Edwards, J.; Bellomo, R. Structure and Function of the Kidney in Septic Shock. A Prospective Controlled Experimental Study. Am. J. Respir. Crit. Care Med. 2016, 194, 692–700. [Google Scholar] [CrossRef]
- Di Giantomasso, D.; May, C.N.; Bellomo, R. Vital Organ Blood Flow During Hyperdynamic Sepsis. Chest 2003, 124, 1053–1059. [Google Scholar] [CrossRef]
- Langenberg, C.; Gobe, G.; Hood, S.; May, C.N.; Bellomo, R. Renal Histopathology During Experimental Septic Acute Kidney Injury and Recovery. Crit. Care Med. 2014, 42, e58–e67. [Google Scholar] [CrossRef]
- Ma, S.; Evans, R.; Iguchi, N.; Tare, M.; Parkington, H.C.; Bellomo, R.; May, C.N.; Lankadeva, Y.R. Sepsis-induced acute kidney injury: A disease of the microcirculation. Microcirculation 2018, 26, e12483. [Google Scholar] [CrossRef] [PubMed]
- Calzavacca, P.; Evans, R.G.; Bailey, M.; Bellomo, R.; May, C.N. Cortical and Medullary Tissue Perfusion and Oxygenation in Experimental Septic Acute Kidney Injury. Crit. Care Med. 2015, 43, e431–e439. [Google Scholar] [CrossRef]
- Lankadeva, Y.R.; Kosaka, J.; Evans, R.G.; Bellomo, R.; May, C.N. Urinary oxygenation as a surrogate marker of medullary oxy-genation during angiotensin II therapy in septic acute kidney injury. Crit. Care Med. 2018, 46, e41–e48. [Google Scholar] [CrossRef]
- Lankadeva, Y.R.; Kosaka, J.; Evans, R.G.; Bailey, M.; Bellomo, R.; May, C.N. Intra-renal and urinary oxygenation during norepinephrine resuscitation in ovine septic acute kidney injury. Kidney Int. 2016, 90, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Kellum, J.A.; Prowle, J. Paradigms of acute kidney injury in the intensive care setting. Nat. Rev. Nephrol. 2018, 14, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Gomez, H.; Ince, C.; De Backer, D.; Pickkers, P.; Payen, D.; Hotchkiss, J.; Kellum, J.A. A unified theory of sepsis-induced acute kidney injury: Inflammation, microcirculatory dysfunction, bioenergetics and the tubular cell adaptation to injury. Shock 2014, 41, 3–11. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Graham, D.B.; Robertson, C.M.; Bautista, J.; Mascarenhas, F.; Diacovo, M.J.; Montgrain, V.; Lam, S.K.; Cremasco, V.; Dunne, W.M.; Faccio, R.; et al. Neutrophil-mediated oxidative burst and host defense are controlled by a Vav-PLCgamma2 signaling axis in mice. J. Clin. Investig. 2007, 117, 3445–3452. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.T.; Green, E.R.; Mecsas, J. Neutrophils to the ROScue: Mechanisms of NADPH Oxidase Activation and Bacterial Resistance. Front. Cell. Infect. Microbiol. 2017, 7, 373. [Google Scholar] [CrossRef]
- Herter, J.M.; Rossaint, J.; Spieker, T.; Zarbock, A. Adhesion Molecules Involved in Neutrophil Recruitment during Sepsis-Induced Acute Kidney Injury. J. Innate Immun. 2014, 6, 597–606. [Google Scholar] [CrossRef]
- Fujimi, S.; Ogura, H.; Tanaka, H.; Koh, T.; Hosotsubo, H.; Nakamori, Y.; Kuwagata, Y.; Shimazu, T.; Sugimoto, H. Activated Polymorphonuclear Leukocytes Enhance Production of Leukocyte Microparticles with Increased Adhesion Molecules in Patients with Sepsis. J. Trauma Acute Care Surg. 2002, 52, 443–448. [Google Scholar] [CrossRef]
- Ware, L.B.; Fessel, J.P.; May, A.K.; Roberts, L.J. Plasma Biomarkers of Oxidant Stress and Development of Organ Failure in Severe Sepsis. Shock 2011, 36, 12–17. [Google Scholar] [CrossRef]
- Satriano, J.A.; Banas, B.; Luckow, B.; Nelson, P.; Schlondorff, D.O. Regulation of RANTES and ICAM-1 expression in murine mesangial cells. J. Am. Soc. Nephrol. 1997, 8, 596–603. [Google Scholar] [CrossRef]
- Ley, K.; Laudanna, C.; Cybulsky, M.; Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef]
- Michie, H.R.; Manogue, K.R.; Spriggs, D.R.; Revhaug, A.; O’Dwyer, S.; Dinarello, C.A.; Cerami, A.; Wolff, S.M.; Wilmore, D.W. Detection of Circulating Tumor Necrosis Factor after Endotoxin Administration. N. Engl. J. Med. 1988, 318, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.G.; Tompkins, R.G.; Gelfand, J.A.; Michie, H.R.; Stanford, G.G.; van der Meer, J.W.; Endres, S.; Lonnemann, G.; Corsetti, J.; Chernow, B.; et al. Circulating interleukin-1 and tumor necrosis factor in septic shock and experimental endotoxin fever. J. Infect. Dis. 1990, 161, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jin, S.; Teng, X.; Hu, Z.; Zhang, Z.; Qiu, X.; Tian, D.; Wu, Y. Hydrogen Sulfide Attenuates LPS-Induced Acute Kidney Injury by Inhibiting Inflammation and Oxidative Stress. Oxidative Med. Cell. Longev. 2018, 2018, 6717212. [Google Scholar] [CrossRef]
- Ehling, J.L.A.; Babickova, J.; Gremse, F.; Klinkhammer, B.M.; Baetke, S.C.; Knuechel, R.; Kiessling, F.; Floege, J.; Lammers, T.; Boor, P. Quantitative Micro-Computed Tomography Imaging of Vascular Dysfunction in Progressive Kidney Diseases. J. Am. Soc. Nephrol. 2016, 27, 520–532. [Google Scholar] [CrossRef]
- Bábíčková, J.; Klinkhammer, B.M.; Buhl, E.M.; Djudjaj, S.; Hoss, M.; Heymann, F.; Tacke, F.; Floege, J.; Becker, J.U.; Boor, P. Regardless of etiology, progressive renal disease causes ultrastructural and functional alterations of peritubular capillaries. Kidney Int. 2017, 91, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Trzeciak, S.; Dellinger, R.P.; Parrillo, J.E.; Guglielmi, M.; Bajaj, J.; Abate, N.L.; Arnold, R.C.; Colilla, S.; Zanotti, S.; Hollenberg, S.M. Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock: Relationship to hemody-namics, oxygen transport, and survival. Ann. Emerg. Med. 2007, 49, 88–98. [Google Scholar] [CrossRef]
- McNeill, E.; Channon, K.M. The role of tetrahydrobiopterin in inflammation and cardiovascular disease. Thromb. Haemost. 2012, 108, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Bendall, J.K.; Alp, N.J.; Warrick, N.; Cai, S.; Adlam, D.; Rockett, K.; Yokoyama, M.; Kawashima, S.; Channon, K.M. Stoichi-ometric relationships between endothelial tetrahydrobiopterin, endothelial NO synthase (eNOS) activity, and eNOS coupling In Vivo: Insights from transgenic mice with endothelial-targeted GTP cyclohydrolase 1 and eNOS overexpression. Circ. Res. 2005, 97, 864–871. [Google Scholar] [CrossRef]
- Kuzkaya, N.; Weissmann, N.; Harrison, D.G.; Dikalov, S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: Implications for uncoupling endothelial nitric-oxide synthase. J. Biol. Chem. 2003, 278, 22546–22554. [Google Scholar] [CrossRef] [PubMed]
- Alkaitis, M.S.; Crabtree, M.J. Recoupling the Cardiac Nitric Oxide Synthases: Tetrahydrobiopterin Synthesis and Recycling. Curr. Heart Fail. Rep. 2012, 9, 200–210. [Google Scholar] [CrossRef]
- Kietadisorn, R.; Juni, R.P.; Moens, A.L. Tackling endothelial dysfunction by modulating NOS uncoupling: New insights into its pathogenesis and therapeutic possibilities. Am. J. Physiol. Metab. 2012, 302, E481–E495. [Google Scholar] [CrossRef]
- Lankadeva, Y.R.; Singh, R.R.; Moritz, K.M.; Parkington, H.C.; Denton, K.M.; Tare, M. Renal Dysfunction Is Associated with a Reduced Contribution of Nitric Oxide and Enhanced Vasoconstriction After a Congenital Renal Mass Reduction in Sheep. Circulation 2015, 131, 280–288. [Google Scholar] [CrossRef]
- He, X.; Su, F.; Velissaris, D.; Salgado, D.R.; de Souza Barros, D.; Lorent, S.; Taccone, F.S.; Vincent, J.L.; De Backer, D. Admin-istration of tetrahydrobiopterin improves the microcirculation and outcome in an ovine model of septic shock. Crit. Care Med. 2012, 40, 2833–2840. [Google Scholar] [CrossRef]
- Chelazzi, C.; Villa, G.; Mancinelli, P.; De Gaudio, A.R.; Adembri, C. Glycocalyx and sepsis-induced alterations in vascular permeability. Crit. Care 2015, 19, 1–7. [Google Scholar] [CrossRef]
- Butler, M.J.; Down, C.J.; Foster, R.; Satchell, S.C. The Pathological Relevance of Increased Endothelial Glycocalyx Permeability. Am. J. Pathol. 2020, 190, 742–751. [Google Scholar] [CrossRef]
- Dane, M.J.C.; Berg, B.M.V.D.; Lee, D.H.; Boels, M.G.S.; Tiemeier, G.L.; Avramut, M.C.; van Zonneveld, A.J.; Van der Vlag, J.; Vink, H.; Rabelink, T.J. A microscopic view on the renal endothelial glycocalyx. Am. J. Physiol. Physiol. 2015, 308, F956–F966. [Google Scholar] [CrossRef]
- Wiesinger, A.; Peters, W.; Chappell, D.; Kentrup, D.; Reuter, S.; Pavenstädt, H.; Oberleithner, H.; Kümpers, P. Nanomechanics of the endothelial glycocalyx in experimental sepsis. PLoS ONE 2013, 8, e80905. [Google Scholar]
- Marechal, X.; Favory, R.; Joulin, O.; Montaigne, D.; Hassoun, S.; Decoster, B.; Zerimech, F.; Neviere, R. Endothelial Glycocalyx Damage During Endotoxemia Coincides with Microcirculatory Dysfunction and Vascular Oxidative Stress. Shock 2008, 29, 572–576. [Google Scholar] [CrossRef]
- Ince, C.; Mayeux, P.R.; Nguyen, T.; Gomez, H.; Kellum, J.A.; Ospina-Tascón, G.A.; Hernandez, G.; Murray, P.; De Backer, D. The Endothelium in Sepsis. Shock 2016, 45, 259–270. [Google Scholar] [CrossRef]
- Nelson, A.; Berkestedt, I.; Schmidtchen, A.; Ljunggren, L.; Bodelsson, M. Increased levels of glycosaminoglycans during septic shock: Relation to mortality and the antibacterial actions of plasma. Shock 2008, 30, 623–627. [Google Scholar] [CrossRef]
- Yu, W.-K.; McNeil, J.B.; Wickersham, N.E.; Shaver, C.M.; Bastarache, J.A.; Ware, L.B. Vascular endothelial cadherin shedding is more severe in sepsis patients with severe acute kidney injury. Crit. Care 2019, 23, 18. [Google Scholar] [CrossRef] [PubMed]
- Clapp, B.R.; Hingorani, A.D.; Kharbanda, R.K.; Mohamed-Ali, V.; Stephens, J.W.; Vallance, P.; MacAllister, R.J. Inflamma-tion-induced endothelial dysfunction involves reduced nitric oxide bioavailability and increased oxidant stress. Cardiovasc. Res. 2004, 64, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.C.P.; Yuen, P.S.; Star, R.A. Microparticles: Markers and mediators of sepsis-induced microvascular dysfunction, immunosuppression, and AKI. Kidney Int. 2015, 87, 1100–1108. [Google Scholar] [CrossRef]
- Lankadeva, Y.R.; Okazaki, N.; Evans, R.G.; Bellomo, R.; May, C.N. Renal Medullary Hypoxia: A New Therapeutic Target for Septic Acute Kidney Injury? Semin. Nephrol. 2019, 39, 543–553. [Google Scholar] [CrossRef]
- Evans, R.G.; Lankadeva, Y.R.; Cochrane, A.D.; Marino, B.; Iguchi, N.; Zhu, M.Z.L.; Hood, S.G.; Smith, J.A.; Bellomo, R.; Gardiner, B.S.; et al. Renal haemodynamics and oxygenation during and after cardiac surgery and cardiopulmonary bypass. Acta Physiol. 2018, 222, e12995. [Google Scholar] [CrossRef]
- Lankadeva, Y.R.; Cochrane, A.D.; Marino, B.; Iguchi, N.; Hood, S.G.; Bellomo, R.; May, C.N.; Evans, R.G. Strategies that improve renal medullary oxygenation during experimental cardiopulmonary bypass may mitigate postoperative acute kidney injury. Kidney Int. 2019, 95, 1338–1346. [Google Scholar] [CrossRef]
- Heyman, S.N.; Reichman, J.; Brezis, M. Pathophysiology of radiocontrast nephropathy: A role for medullary hypoxia. Investig. Radiol. 1999, 34, 685–691. [Google Scholar] [CrossRef]
- Tanaka, S.; Tanaka, T.; Nangaku, M. Hypoxia as a key player in the AKI-to-CKD transition. Am. J. Physiol. Physiol. 2014, 307, F1187–F1195. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.; Basile, D.P. Role of Renal Hypoxia in the Progression From Acute Kidney Injury to Chronic Kidney Disease. Semin. Nephrol. 2019, 39, 567–580. [Google Scholar] [CrossRef]
- Evans, R.G.; Smith, D.W.; Lee, C.J.; Ngo, J.P.; Gardiner, B.S. What makes the kidney susceptible to hypoxia? Anat. Rec. 2020, 303, 2544–2552. [Google Scholar] [CrossRef]
- Evans, R.G.; Ince, C.; Joles, J.A.; Smith, D.W.; May, C.N.; O’Connor, P.M.; Gardiner, B. Haemodynamic influences on kidney oxygenation: Clinical implications of integrative physiology. Clin. Exp. Pharmacol. Physiol. 2013, 40, 106–122. [Google Scholar] [CrossRef]
- Calzavacca, P.; Evans, R.; Bailey, M.; Lankadeva, Y.R.; Bellomo, R.; May, C.N. Long-term measurement of renal cortical and medullary tissue oxygenation and perfusion in unanesthetized sheep. Am. J. Physiol. Integr. Comp. Physiol. 2015, 308, R832–R839. [Google Scholar] [CrossRef]
- Bonventre, J.V.; Weinberg, J.M. Recent Advances in the Pathophysiology of Ischemic Acute Renal Failure. J. Am. Soc. Nephrol. 2003, 14, 2199–2210. [Google Scholar] [CrossRef]
- Evans, R.G.; Gardiner, B.S.; Smith, D.W.; O’Connor, P.M. Intrarenal oxygenation: Unique challenges and the biophysical basis of homeostasis. Am. J. Physiol. Physiol. 2008, 295, F1259–F1270. [Google Scholar] [CrossRef]
- Evans, R.G.; Fitzgerald, S. Nitric oxide and superoxide in the renal medulla: A delicate balancing act. Curr. Opin. Nephrol. Hypertens. 2005, 14, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Lankadeva, Y.R.; Kosaka, J.; Iguchi, N.; Evans, R.; Booth, L.C.; Bellomo, R.; May, C.N. Effects of Fluid Bolus Therapy on Renal Perfusion, Oxygenation, and Function in Early Experimental Septic Kidney Injury. Crit. Care Med. 2019, 47, e36–e43. [Google Scholar] [CrossRef]
- Iguchi, N.; Lankadeva, Y.R.; Mori, T.A.; Osawa, E.A.; Cutuli, S.L.; Evans, R.G.; Bellomo, R.; May, C.N. Furosemide reverses medullary tissue hypoxia in ovine septic acute kidney injury. Am. J. Physiol. Integr. Comp. Physiol. 2019, 317, R232–R239. [Google Scholar] [CrossRef]
- Osawa, E.A.; Cutuli, S.L.; Bitker, L.; Canet, E.; Cioccari, L.; Iguchi, N.; Lankadeva, Y.R.; Eastwood, G.M.; Evans, R.G.; May, C.N.; et al. Effect of furosemide on urinary oxygenation in patients with septic shock. Blood Purif. 2019, 23, 1–10. [Google Scholar] [CrossRef]
- O’Connor, P.M.; Kett, M.M.; Anderson, W.P.; Evans, R. Renal medullary tissue oxygenation is dependent on both cortical and medullary blood flow. Am. J. Physiol. Physiol. 2006, 290, F688–F694. [Google Scholar] [CrossRef]
- Haase, V.H. Hypoxia-inducible factors in the kidney. Am. J. Physiol. Physiol. 2006, 291, F271–F281. [Google Scholar] [CrossRef]
- Ke, Q.; Costa, M. Hypoxia-Inducible Factor-1 (HIF-1). Mol. Pharmacol. 2006, 70, 1469–1480. [Google Scholar] [CrossRef]
- Beck, I.; Weinmann, R.; Caro, J. Characterization of hypoxia-responsive enhancer in the human erythropoietin gene shows presence of hypoxia-inducible 120-Kd nuclear DNA-binding protein in erythropoietin-producing and nonproducing cells. Blood 1993, 82, 704–711. [Google Scholar] [CrossRef]
- Lee, F.S.; Percy, M.J. The HIF Pathway and Erythrocytosis. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 165–192. [Google Scholar] [CrossRef]
- Melillo, G.; Musso, T.; Sica, A.; Taylor, L.S.; Cox, G.W.; Varesio, L. A hypoxia-responsive element mediates a novel pathway of activation of the inducible nitric oxide synthase promoter. J. Exp. Med. 1995, 182, 1683–1693. [Google Scholar] [CrossRef]
- Higgins, D.F.; Kimura, K.; Bernhardt, W.M.; Shrimanker, N.; Akai, Y.; Hohenstein, B.; Saito, Y.; Johnson, R.S.; Kretzler, M.; Cohen, C.D.; et al. Hypoxia promotes fibrogenesis In Vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J. Clin. Investig. 2007, 117, 3810–3820. [Google Scholar] [CrossRef]
- Parikh, S.M.; Yang, Y.; He, L.; Tang, C.; Zhan, M.; Dong, Z. Mitochondrial Function and Disturbances in the Septic Kidney. Semin. Nephrol. 2015, 35, 108–119. [Google Scholar] [CrossRef]
- Ince, C.; Mik, E.G. Microcirculatory and mitochondrial hypoxia in sepsis, shock, and resuscitation. J. Appl. Physiol. 2016, 120, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Chandel, N.S.; Maltepe, E.; Goldwasser, E.; Mathieu, C.E.; Simon, M.C.; Schumacker, P.T. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc. Natl. Acad. Sci. USA 1998, 95, 11715–11720. [Google Scholar] [CrossRef] [PubMed]
- Scherz-Shouval, R.; Shvets, E.; Fass, E.; Shorer, H.; Gil, L.; Elazar, Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007, 26, 1749–1760. [Google Scholar] [CrossRef]
- Cruz, C.M.; Rinna, A.; Forman, H.J.; Ventura, A.L.; Persechini, P.M.; Ojcius, D.M. ATP activates a reactive oxygen spe-cies-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J. Biol. Chem. 2007, 282, 2871–2879. [Google Scholar] [CrossRef] [PubMed]
- Bar-Or, D.; Carrick, M.M.; Mains, C.W.; Rael, L.T.; Slone, D.; Brody, E.N. Sepsis, oxidative stress, and hypoxia: Are there clues to better treatment? Redox Rep. 2015, 20, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Nagar, H.; Piao, S.; Kim, C.-S. Role of Mitochondrial Oxidative Stress in Sepsis. Acute Crit. Care 2018, 33, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Guzy, R.D.; Schumacker, P.T. Oxygen sensing by mitochondria at complex III: The paradox of increased reactive oxygen species during hypoxia. Exp. Physiol. 2006, 91, 807–819. [Google Scholar] [CrossRef]
- Chandel, N.S.; McClintock, D.S.; Feliciano, C.E.; Wood, T.M.; Melendez, J.A.; Rodriguez, A.M.; Schumacker, P.T. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: A mechanism of O2 sensing. J. Biol. Chem. 2000, 275, 25130–25138. [Google Scholar] [CrossRef]
- Hernansanz-Agustín, P.; Ramos, E.; Navarro, E.; Parada, E.; Sánchez-López, N.; Peláez-Aguado, L.; Cabrera-García, J.D.; Tello, D.; Buendia, I.; Marina, A.; et al. Mitochondrial complex I deactivation is related to superoxide production in acute hypoxia. Redox Biol. 2017, 12, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Sureshbabu, A.; Patino, E.; Ma, K.C.; Laursen, K.; Finkelsztein, E.J.; Akchurin, O.; Muthukumar, T.; Ryter, S.W.; Gudas, L.; Choi, A.M.K.; et al. RIPK3 promotes sepsis-induced acute kidney injury via mitochondrial dysfunction. JCI Insight. 2018, 3, e98411. [Google Scholar] [CrossRef]
- Kitur, K.; Wachtel, S.; Brown, A.; Wickersham, M.; Paulino, F.; Peñaloza, H.F.; Soong, G.; Bueno, S.; Parker, D.; Prince, A. Necroptosis Promotes Staphylococcus aureus Clearance by Inhibiting Excessive Inflammatory Signaling. Cell Rep. 2016, 16, 2219–2230. [Google Scholar] [CrossRef]
- Duprez, L.; Takahashi, N.; Van Hauwermeiren, F.; Vandendriessche, B.; Goossens, V.; Berghe, T.V.; Declercq, W.; Libert, C.; Cauwels, A.; Vandenabeele, P. RIP Kinase-Dependent Necrosis Drives Lethal Systemic Inflammatory Response Syndrome. Immunity 2011, 35, 908–918. [Google Scholar] [CrossRef]
- Brealey, D.; Brand, M.; Hargreaves, I.; Heales, S.; Land, J.; Smolenski, R.; Davies, N.A.; Cooper, C.; Singer, M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 2002, 360, 219–223. [Google Scholar] [CrossRef]
- Plotnikov, E.Y.; Pevzner, I.B.; Zorova, L.D.; Chernikov, V.P.; Prusov, A.N.; Kireev, I.I.; Silachev, D.N.; Skulachev, V.P.; Zorov, D.B. Mitochondrial damage and mitochondria-Targeted antioxidant protection in LPS-induced acute kidney injury. Antioxidants 2019, 8, 176. [Google Scholar] [CrossRef]
- Pathak, E.; MacMillan-Crow, L.A.; Mayeux, P.R. Role of Mitochondrial Oxidants in an In Vitro Model of Sepsis-Induced Renal Injury. J. Pharmacol. Exp. Ther. 2012, 340, 192–201. [Google Scholar] [CrossRef]
- Ding, Y.; Zheng, Y.; Huang, J.; Peng, W.; Chen, X.; Kang, X.; Zeng, Q. UCP2 ameliorates mitochondrial dysfunction, inflammation, and oxidative stress in lipopolysaccharide-induced acute kidney injury. Int. Immunopharmacol. 2019, 71, 336–349. [Google Scholar] [CrossRef]
- Divakaruni, A.S.; Brand, M.D. The Regulation and Physiology of Mitochondrial Proton Leak. Physiology 2011, 26, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-X.; Yang, C.; Zhang, W.-H.; Su, H.-Y.; Liu, Z.-J.; Pan, Q.; Liu, H.-F. Disturbance of mitochondrial dynamics and mitophagy in sepsis-induced acute kidney injury. Life Sci. 2019, 235, 116828. [Google Scholar] [CrossRef] [PubMed]
- Van der Slikke, E.C.; Star, B.S.; van Meurs, M.; Henning, R.H.; Moser, J.; Bouma, H.R. Sepsis is associated with mitochondrial DNA damage and a reduced mitochondrial mass in the kidney of patients with sepsis-AKI. Crit. Care 2021, 25, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cain, K.; Bratton, S.B.; Cohen, G.M. The Apaf-1 apoptosome: A large caspase-activating complex. Biochimie 2002, 84, 203–214. [Google Scholar] [CrossRef]
- Yuan, S.; Akey, C.W. Apoptosome structure, assembly, and procaspase activation. Structure 2013, 21, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I.; Halliwell, B.; Hoey, B.M.; Butler, J. The antioxidant action of N-acetylcysteine: Its reaction with hydrogen per-oxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic. Biol. Med. 1989, 6, 593–597. [Google Scholar] [CrossRef]
- Kharazmi, A.; Nielsen, H.; Schiøtz, P.O. N-acetylcysteine inhibits human neutrophil and monocyte chemotaxis and oxidative metabolism. Int. J. Immunopharmacol. 1988, 10, 39–46. [Google Scholar] [CrossRef]
- Schmidt, H.; Schmidt, W.; Muller, T.; Bohrer, H.; Gebhard, M.M.; Martin, E. N-acetylcysteine attenuates endotoxin-induced leukocyte-endothelial cell adhesion and macromolecular leakage In Vivo. Crit. Care Med. 1997, 25, 858–863. [Google Scholar] [CrossRef]
- Paterson, R.L.; Galley, H.F.; Webster, N.R. The effect of N-acetylcysteine on nuclear factor-κB activation, interleukin-6, interleukin-8, and intercellular adhesion molecule-1 expression in patients with sepsis. Crit. Care Med. 2003, 31, 2574–2578. [Google Scholar] [CrossRef]
- Ritter, C.; Andrades, M.E.; Reinke, A.; Menna-Barreto, S.; Moreira, J.C.F.; Dal-Pizzol, F. Treatment with N-acetylcysteine plus deferoxamine protects rats against oxidative stress and improves survival in sepsis. Crit. Care Med. 2004, 32, 342–349. [Google Scholar] [CrossRef]
- Hsu, B.-G.; Lee, R.-P.; Yang, F.-L.; Harn, H.-J.; Chen, H.I. Post-treatment with N-acetylcysteine ameliorates endotoxin shock-induced organ damage in conscious rats. Life Sci. 2006, 79, 2010–2016. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.C.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.C.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Vassilev, D.; Hauser, B.; Bracht, H.; Iványi, Z.; Schoaff, M.; Asfar, P.; Vogt, J.; Wachter, U.; Schelzig, H.; Georgieff, M.; et al. Systemic, pulmonary, and hepatosplanchnic effects of N-acetylcysteine during long-term porcine endotoxemia. Crit. Care Med. 2004, 32, 525–532. [Google Scholar] [CrossRef]
- Szakmany, T.; Hauser, B.; Matejovic, M.; Radermacher, P. N-acetylcysteine for sepsis and systemic inflammatory response in adults. Cochrane Database Syst. Rev. 2007, CD006616. [Google Scholar] [CrossRef]
- Spapen, H.D.; Diltoer, M.W.; Nguyen, D.N.; Hendrickx, I.; Huyghens, L.P. Effects of N-acetylcysteine on microalbuminuria and organ failure in acute severe sepsis: Results of a pilot study. Chest 2005, 127, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- Peake, S.L.; Moran, J.L.; Leppard, P.I. N-acetyl-L-cysteine depresses cardiac performance in patients with septic shock. Crit. Care Med. 1996, 24, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Molnár, Z.; Shearer, E.; Lowe, D. N-Acetylcysteine treatment to prevent the progression of multisystem organ failure: A pro-spective, randomized, placebo-controlled study. Crit. Care Med. 1999, 27, 1100–1104. [Google Scholar] [CrossRef]
- Najafi, A.; Mojtahedzadeh, M.; Ahmadi, K.H.; Abdollahi, M.; Mousavi, M.; Chelkeba, L.; Najmeddin, F.; Ahmadi, A. The immunological benefit of higher dose N-acetyl cysteine following mechanical ventilation in critically ill patients. DARU J. Pharm. Sci. 2014, 22, 57. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Levine, M. Vitamin C: The known and the unknown and Goldilocks. Oral Dis. 2016, 22, 463–493. [Google Scholar] [CrossRef]
- Buettner, G.R.; Jurkiewicz, B.A. Catalytic Metals, Ascorbate and Free Radicals: Combinations to Avoid. Radiat. Res. 1996, 145, 532. [Google Scholar] [CrossRef]
- Cárcamo, J.M.; Pedraza, A.; Bórquez-Ojeda, O.; Golde, D.W. Vitamin C suppresses TNF alpha-induced NF kappa B activation by inhibiting I kappa B alpha phosphorylation. Biochemistry 2002, 41, 12995–13002. [Google Scholar] [CrossRef]
- Victor, V.V.; Guayerbas, N.; Puerto, M.; Medina, S.; De la Fuente, M. Ascorbic acid modulates In Vitro the function of mac-rophages from mice with endotoxic shock. Immunopharmacology 2000, 46, 89–101. [Google Scholar] [CrossRef]
- Armour, J.; Tyml, K.; Lidington, D.; Wilson, J.X. Ascorbate prevents microvascular dysfunction in the skeletal muscle of the septic rat. J. Appl. Physiol. 2001, 90, 795–803. [Google Scholar] [CrossRef]
- Mo, S.-J.; Son, E.-W.; Rhee, D.-K.; Pyo, S. Modulation of tnf-α-induced icam-1 expression, NO and H2O2 production by alginate, allicin and ascorbic acid in human endothelial cells. Arch. Pharmacal Res. 2003, 26, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, S.I.; Lee, D.-H.; Smith, D.; Jo, H.; Schellhorn, H.E.; Boo, Y.C. Ascorbic acid synthesis due to l-gulono-1,4-lactone oxidase expression enhances NO production in endothelial cells. Biochem. Biophys. Res. Commun. 2006, 345, 1657–1662. [Google Scholar] [CrossRef] [PubMed]
- Ladurner, A.; Schmitt, C.A.; Schachner, D.; Atanasov, A.; Werner, E.R.; Dirsch, V.M.; Heiss, E.H. Ascorbate stimulates endothelial nitric oxide synthase enzyme activity by rapid modulation of its phosphorylation status. Free Radic. Biol. Med. 2012, 52, 2082–2090. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.P.; Delles, C.; Schmidt, B.M.; Oehmer, S.; Schwarz, T.K.; Schmieder, R.E.; John, S. Superoxide scavenging effects of N-acetylcysteine and vitamin C in subjects with essential hypertension. Am. J. Hypertens. 2005, 18, 1111–1117. [Google Scholar] [CrossRef]
- Carr, A.C.; Shaw, G.M.; Fowler, A.A.; Natarajan, R. Ascorbate-dependent vasopressor synthesis: A rationale for vitamin C administration in severe sepsis and septic shock? Crit. Care 2015, 19, 418. [Google Scholar] [CrossRef]
- Hudson, E.P.; Collie, J.T.; Fujii, T.; Luethi, N.; Udy, A.A.; Doherty, S.; Eastwood, G.; Yanase, F.; Naorungroj, T.; Bitker, L.; et al. Pharmacokinetic data support 6-hourly dosing of intravenous vitamin C to critically ill patients with septic shock. Crit. Care Resusc 2019, 21, 236–242. [Google Scholar]
- Schorah, C.J.; Downing, C.; Piripitsi, A.; Gallivan, L.; Al-Hazaa, A.H.; Sanderson, M.J.; Bodenham, A. Total vitamin C, ascorbic acid, and dehydroascorbic acid concentrations in plasma of critically ill patients. Am. J. Clin. Nutr. 1996, 63, 760–765. [Google Scholar] [CrossRef]
- Carr, A.C.; Rosengrave, P.C.; Bayer, S.; Chambers, S.; Mehrtens, J.; Shaw, G.M. Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit. Care 2017, 21, 1–10. [Google Scholar] [CrossRef]
- May, C.N.; Bellomo, R.; Lankadeva, Y.R. Therapeutic potential of mega-dose vitamin C to reverse organ dysfunction in sepsis and COVID-19. Br. J. Pharmacol. 2021. [Google Scholar] [CrossRef]
- Wilson, J.X. Evaluation of Vitamin C for Adjuvant Sepsis Therapy. Antioxid. Redox Signal. 2013, 19, 2129–2140. [Google Scholar] [CrossRef]
- Zhou, G.; Kamenos, G.; Pendem, S.; Wilson, J.X.; Wu, F. Ascorbate protects against vascular leakage in cecal ligation and puncture-induced septic peritonitis. Am. J. Physiol. Integr. Comp. Physiol. 2012, 302, R409–R416. [Google Scholar] [CrossRef]
- Secor, D.; Li, F.; Ellis, C.; Sharpe, M.D.; Gross, P.L.; Wilson, J.X.; Tyml, K. Impaired microvascular perfusion in sepsis requires activated coagulation and P-selectin-mediated platelet adhesion in capillaries. Intensive Care Med. 2010, 36, 1928–1934. [Google Scholar] [CrossRef] [PubMed]
- Tyml, K.; Li, F.; Wilson, J.X. Septic impairment of capillary blood flow requires nicotinamide adenine dinucleotide phosphate oxidase but not nitric oxide synthase and is rapidly reversed by ascorbate through an endothelial nitric oxide syn-thase-dependent mechanism. Crit. Care Med. 2008, 36, 2355–2362. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wilson, J.X.; Tyml, K. Ascorbate inhibits iNOS expression and preserves vasoconstrictor responsiveness in skeletal muscle of septic mice. Am. J. Physiol. Integr. Comp. Physiol. 2003, 285, R50–R56. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wilson, J.X.; Tyml, K. Ascorbate protects against impaired arteriolar constriction in sepsis by inhibiting inducible nitric oxide synthase expression. Free Radic. Biol. Med. 2004, 37, 1282–1289. [Google Scholar] [CrossRef]
- Fowler, A.A., III; Syed, A.A.; Knowlson, S.; Sculthorpe, R.; Farthing, D.; De Wilde, C.; Farthing, C.A.; Larus, T.L.; Martin, E.; Brophy, D.F.; et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J. Transl. Med. 2014, 12, 32. [Google Scholar] [CrossRef]
- Khalili, H.; Zabet, M.H.; Mohammadi, M.; Ramezani, M. Effect of high-dose Ascorbic acid on vasopressor′s requirement in septic shock. J. Res. Pharm. Pract. 2016, 5, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.E.; Khangoora, V.; Rivera, R.; Hooper, M.H.; Catravas, J. Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock: A retrospective before-after study. Chest 2017, 151, 1229–1238. [Google Scholar] [CrossRef]
- Fujii, T.; Luethi, N.; Young, P.J.; Frei, D.R.; Eastwood, G.M.; French, C.J.; Deane, A.M.; Shehabi, Y.; Hajjar, L.A.; Oliveira, G.; et al. Effect of vitamin C, hydrocortisone, and thiamine vs hydrocortisone alone on time alive and free of vasopressor support among patients with septic shock: The VITAMINS randomized clinical trial. JAMA 2020, 323, 423–431. [Google Scholar] [CrossRef]
- Moskowitz, A.; Huang, D.T.; Hou, P.C.; Gong, J.; Doshi, P.B.; Grossestreuer, A.V.; Andersen, L.W.; Ngo, L.; Sherwin, R.L.; Berg, K.M.; et al. Effect of ascorbic acid, corticosteroids, and thiamine on organ injury in septic shock: The ACTS randomized clinical trial. JAMA 2020, 324, 642–650. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Ryoo, S.M.; Park, J.E.; Jo, Y.H.; Jang, D.H.; Suh, G.J.; Kim, T.; Kim, Y.J.; Kim, S.; Cho, H.; et al. Combination therapy of vitamin C and thiamine for septic shock: A multi-centre, double-blinded randomized, controlled study. Intensive Care Med. 2020, 46, 2015–2025. [Google Scholar] [CrossRef] [PubMed]
- Fowler, A.A., III; Truwit, J.D.; Hite, R.D.; Morris, P.E.; De Wilde, C.; Priday, A.; Fisher, B.; Thacker, L.R., II; Natarajan, R.; Brophy, D.F.; et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: The CITRIS-ALI randomized clinical trial. JAMA 2019, 322, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, V.S.; Sabui, S.; Moradi, H.; Marchant, J.S.; Said, H.M. Inhibition of intestinal ascorbic acid uptake by lipopoly-saccharide is mediated via transcriptional mechanisms. Biochim. Biophys. Acta Biomembr. 2018, 1860, 556–565. [Google Scholar] [CrossRef]
- Subramanian, V.S.; Sabui, S.; Subramenium, G.A.; Marchant, J.S.; Said, H.M. Tumor necrosis factor alpha reduces intestinal vitamin C uptake: A role for NF-κB-mediated signaling. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G241–G248. [Google Scholar] [CrossRef] [PubMed]
- Yanase, F.; Fujii, T.; Naorungroj, T.; Belletti, A.; Luethi, N.; Carr, A.C.; Young, P.J.; Bellomo, R. Harm of IV high-dose vitamin C therapy in adult patients: A scoping review. Crit. Care Med. 2020, 48, e620–e628. [Google Scholar] [CrossRef]
- Lankadeva, Y.R.; Peiris, R.M.; Okazaki, N.; Birchall, I.E.; Trask-Marino, A.; Dornom, A.; Vale, T.A.M.; Evans, R.G.; Yanase, F.; Bellomo, R.; et al. Reversal of the pathophysiological responses to gram-negative sepsis by megadose vitamin C. Crit. Care Med. 2021, 49, e179–e190. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ow, C.P.C.; Trask-Marino, A.; Betrie, A.H.; Evans, R.G.; May, C.N.; Lankadeva, Y.R. Targeting Oxidative Stress in Septic Acute Kidney Injury: From Theory to Practice. J. Clin. Med. 2021, 10, 3798. https://doi.org/10.3390/jcm10173798
Ow CPC, Trask-Marino A, Betrie AH, Evans RG, May CN, Lankadeva YR. Targeting Oxidative Stress in Septic Acute Kidney Injury: From Theory to Practice. Journal of Clinical Medicine. 2021; 10(17):3798. https://doi.org/10.3390/jcm10173798
Chicago/Turabian StyleOw, Connie P. C., Anton Trask-Marino, Ashenafi H. Betrie, Roger G. Evans, Clive N. May, and Yugeesh R. Lankadeva. 2021. "Targeting Oxidative Stress in Septic Acute Kidney Injury: From Theory to Practice" Journal of Clinical Medicine 10, no. 17: 3798. https://doi.org/10.3390/jcm10173798
APA StyleOw, C. P. C., Trask-Marino, A., Betrie, A. H., Evans, R. G., May, C. N., & Lankadeva, Y. R. (2021). Targeting Oxidative Stress in Septic Acute Kidney Injury: From Theory to Practice. Journal of Clinical Medicine, 10(17), 3798. https://doi.org/10.3390/jcm10173798





