Evaluation of Biomarkers of Severity in Patients with COVID-19 Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Measurement of Laboratory Biomarkers
2.2. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Jiang, F.; Deng, L.; Zhang, L.; Cai, Y.; Cheung, C.W.; Xia, Z.J. Review of the Clinical Characteristics of Coronavirus Disease 2019 (COVID-19). Gen Intern Med. 2020, 35, 1545–1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gavriatopoulou, M.; Ntanasis-Stathopoulos, I.; Korompoki, E.; Fotiou, D.; Migkou, M.; Tzanninis, I.-G.; Psaltopoulou, T.; Kastritis, E.; Terpos, E.; Dimopoulos, M.A. Emerging treatment strategies for COVID-19 infection. Clin. Exp. Med. 2020, 21, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Machhi, J.; Herskovitz, J.; Senan, A.M.; Dutta, D.; Nath, B.; Oleynikov, M.D.; Blomberg, W.R.; Meigs, D.D.; Hasan, M.; Patel, M.; et al. The Natural History, Pathobiology, and Clinical Manifestations of SARS-CoV-2 Infections. J. Neuroimmune Pharmacol. 2020, 21, 1–28. [Google Scholar]
- Hertanto, D.M.; Wiratama, B.S.; Sutanto, H.; Wungu, C.D.K. Immunomodulation as a Potent COVID-19 Pharmacotherapy: Past, Present and Future. J. Inflamm. Res. 2021, 14, 3419–3428. [Google Scholar] [CrossRef]
- Berlin, D.A.; Gulick, R.M.; Martinez, F.J. Severe Covid-19. N. Engl. J. Med. 2020, 383, 2451–2460. [Google Scholar] [CrossRef]
- Gandhi, R.T.; Lynch, J.B.; Del Rio, C. Mild or Moderate Covid-19. N. Engl. J. Med. 2020, 383, 1757–1766. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, N.; Hayakawa, K.; Terada, M.; Ohtsu, H.; Asai, Y.; Tsuzuki, S.; Suzuki, S.; Toyoda, A.; Suzuki, K.; Endo, M.; et al. Clinical epidemiology of hospitalized patients with COVID-19 in Japan: Report of the COVID-19 Registry Japan. Clin. Infect. Dis. 2020, 28, ciaa1470. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Guan, W.; Chen, R.; Wang, W.; Li, J.; Xu, K.; Li, C.; Ai, Q.; Lu, W.; Liang, H.; et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020, 21, 335–337. [Google Scholar] [CrossRef]
- Lippi, G.; Henry, B.M. Chronic obstructive pulmonary disease is associated with severe coronavirus disease 2019 (COVID-19). Respir. Med. 2020, 167, 105941. [Google Scholar] [CrossRef]
- Myers, L.C.; Parodi, S.M.; Escobar, G.J.; Liu, V.X. Characteristics of Hospitalized Adults With COVID-19 in an Integrated Health Care System in California. JAMA 2020, 323, 2195–2198. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Morieri, M.L.; Longato, E.; Avogaro, A. Prevalence and impact of diabetes among people infected with SARS-CoV-2. J. Endocrinol. Investig. 2020, 43, 867–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Z.; Peng, F.; Xu, B.; Zhao, J.; Liu, H.; Peng, J.; Li, Q.; Jiang, C.; Zhou, Y.; Liu, S.; et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J. Infect. 2020, 81, e16–e25. [Google Scholar] [CrossRef]
- Popkin, B.M.; Du, S.; Green, W.D.; Beck, M.A.; Algaith, T.; Herbst, C.H.; Alsukait, R.; Alluhidan, M.; Alazemi, N.; Shekar, M. Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes. Rev. 2020, 21, e13128. [Google Scholar] [CrossRef] [PubMed]
- Latif, F.; Farr, M.A.; Clerkin, K.J.; Habal, M.V.; Takeda, K.; Naka, Y.; Restaino, S.; Sayer, G.; Uriel, N. Characteristics and outcomes of recipients of heart transplant with coronavirus isease 2019. JAMA Cardiol. 2020, 5, 1165–1169. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.mhlw.go.jp/content/000785119.pdf (accessed on 25 July 2021).
- Sun, H.-B.; Zhang, Y.-M.; Huang, L.-G.; Lai, Q.-N.; Mo, Q.; Ye, X.-Z.; Wang, T.; Zhu, Z.-Z.; Lv, X.-L.; Luo, Y.-J.; et al. The changes of the peripheral CD4+ lymphocytes and inflammatory cytokines in Patients with COVID-19. PLoS ONE 2020, 15, e0239532. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Li, H.; Li, L.; Liu, C.; Yan, S.; Chen, H.; Li, Y. Ferritin in the coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. J. Clin. Lab. Anal. 2020, 34, e23618. [Google Scholar] [CrossRef]
- Soy, M.; Keser, G.; Atagündüz, P.; Tabak, F.; Atagündüz, I.; Kayhan, S. Cytokine storm in COVID-19: Pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020, 39, 2085–2094. [Google Scholar] [CrossRef]
- Hamade, B.; Huang, D.T. Procalcitonin: Where Are We Now? Crit. Care Clin. 2020, 36, 23–40. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, H.; Mu, S.; Wei, W.; Jin, C.; Tong, C.; Song, Z.; Zha, Y.; Xue, Y.; Gu, G. Lactate dehydrogenase, an independent risk factor of severe COVID-19 patients: A retrospective and observational study. Aging 2020, 12, 11245–11258. [Google Scholar] [CrossRef]
- Cui, S.; Chen, S.; Li, X.; Liu, S.; Wang, F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 1421–1424. [Google Scholar] [CrossRef]
- Wada, H.; Matsumoto, T.; Yamashita, Y. Natural History of Thrombotic Thrombocytopenic Purpura and Hemolytic Uremic Syndrome. Semin. Thromb. Hemost. 2014, 40, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Taylor, F.B., Jr.; Toh, C.H.; Hoots, K.; Wada, H.; Levi, M. Towards a definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb. Haemost. 2001, 86, 1327–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wada, H.; Matsumoto, T.; Suzuki, K.; Imai, H.; Katayama, N.; Iba, T.; Matsumoto, M. Differences and similarities between disseminated intravascular coagulation and thrombotic microangiopathy. Thromb. J. 2018, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Salah, H.M.; Mehta, J.L. Meta-Analysis of the Effect of Aspirin on Mortality in COVID-19. Am. J. Cardiol. 2021, 142, 158–159. [Google Scholar] [CrossRef]
| Stage | Mild | Moderate I | Moderate II | Severe |
|---|---|---|---|---|
| Number | 63 | 48 | 32 | 9 |
| Age, years (25–75th percentile) | 41.0 (29.3–57.8) | 50.5 * (38.0–64.0) | 66.5 ***, ### (58.5–80.5) | 70.0 **, ## (63.8–74.0) |
| Male (percent in stage) | 25 (39.7%) | 22 (45.8%) | 19 (59.4%) | 8 * (88.8%) |
| Death (mortality) | 0 | 0 | 4 (12.5%) | 1 (11.1%) |
| Died of old age | 0 | 0 | 2 (6.3%) | 0 |
| Pregnant patient, n (%) | 3 (4.8%) | 0 | 0 | 0 |
| Comorbidity | Patient number (percent) in each disease stage | |||
| No comorbidity | 28 (45.0%) | 15 (31.2%) | 3 **, # (9.4%) | 0 * |
| Hypertension | 12 (19.4%) | 10 (20.8%) | 9 (28.1%) | 5 * (55.6%) |
| Hyperlipidemia | 6 (9.7%) | 11 (22.9%) | 6 (18.8%) | 2 (22.2%) |
| Diabetes mellitus | 6 (9.5%) | 4 (8.3%) | 11 **, ## (34.4%) | 5 **, ## (55.6%) |
| Heart failure | 2 (3.2%) | 1 (2.1%) | 3 (9.4%) | 1 (11.1%) |
| Renal failure | 2 (3.3%) | 3 (6.3%) | 5 (15.6%) | 2 (22.2%) |
| Other pneumonia * | 1 (1.6%) | 1 (2.1%) | 5 * (15.6%) | 0 (0.0%) |
| Cerebrovascular accident | 0 | 1 (2.1%) | 5 ** (15.6%) | 1 (11.1%) |
| Asthma | 4 (6.3%) | 5 (10.4%) | 2 (6.3%) | 0 |
| Neurodegenerative diseases | 5 (7.9%) | 2 (4.2%) | 4 (12.5%) | 0 |
| Digestive diseases | 3 (4.8%) | 2 (4.2%) | 4 (12.5%) | 1 (11.1%) |
| HIV infection | 2 (3.2%) | 1 (2.1%) | 0 | 0 |
| Autoimmune diseases | 3 (4.8%) | 2 (4.2%) | 2 (6.2%) | 0 |
| Solid cancer | 3 (4.8%) | 0 | 0 | 0 |
| Endocrine diseases | 2 (3.2%) | 2 (4.2%) | 1 (3.1%) | 0 |
| Other diseases | 11 (17.5%) | 11 (22.9%) | 13 (40.6%) | 3 (33.3%) |
| Mild | Moderate I | Moderate II | Severe | |
|---|---|---|---|---|
| WBC (×109/L) | 5.2 (4.2–6.1) | 5.5 (4.0–6.7) | 5.7 (4.0–7.6) | 6.0 (5.4–8.0) |
| Neutrophil (×109/L) | 2.7 (1.7–3.3) | 3.2 (1.9–4.1) | 4.4 **, ## (2.2–5.9) | 4.8 ***, ### (4.4–7.1) |
| Lymphocyte (×109/L) | 1.5 (1.1–1.7) | 1.3 (1.0–1.7) | 9.8 **, # (0.6–1.3) | 0.7 **, ## (0.5–1.1) |
| Hemoglobin (g/dL) | 14.4 (12.7–15.4) | 14.3 (13.5–15.0) | 13.4 (12.4–14.9) | 14.6 (13.4–14.7) |
| Platelet (×1010/L) | 21.7 (18.7–25.2) | 22.0 (17.7–26.6) | 19.5 * (14.5–23.2) | 13.8 **, ## (10.6–19.0) |
| PT-INR | 0.96 (0.90–1.01) | 0.98 (0.94–1.02) | 1.02 ** (0.96–1.07) | 1.02 **, # (0.99–1.10) |
| APTT (sec) | 32.0 (29.0–33.0) | 32.0 (30.0–35.0) | 32.0 (30.0–35.0) | 33.0 (28.5–34.8) |
| D-dimer (μg/mL) | 0.5 (0.5–0.7) | 0.5 (0.5–0.9) | 0.8 ***, # (0.5–2.0) | 1.2 ***, ## (0.7–1.8) |
| CRP (mg/mL) | 0.20 (0.05–0.65) | 0.58 *** (0.31–2.67) | 5.59 ***, ### (2.69–8.11) | 9.21 ***, ### (7.53–13.0) |
| Procalcitonin (ng/mL) | 0.05 (0.04–0.06) | 0.06 * (0.05–0.08) | 0.08 ***, ## (0.07–0.10) | 0.95 **, ## |
| Ferritin (ng/mL) | 150 (83–342) | 258 (115–369) | 541 ***, ### (378–1288) | 1530 **, ## |
| Total protein (g/dL) | 7.10 (6.88–7.50) | 7.10 (6.80–7.45) | 6.65 **, # (6.30–7.25) | 6.55 **, # (6.30–6.95) |
| Albumin (g/dL) | 4.20 (3.90–4.50) | 4.00 * (3.80–4.30) | 3.20 ***, ### (3.00–3.70) | 3.45 ***, ### (2.80–3.55) |
| A/G ratio | 1.40 (1.30–1.60) | 1.30 ** (1.20–1.40) | 1.00 ***, ### (0.80–1.10) | 1.05 ***, ## (0.90–1.10) |
| T-Bil (mg/dL) | 0.53 (0.37–0.68) | 0.60 (0.46–0.77) | 0.71 ***, ## (0.65–1.09) | 0.69 (0.50–0.98) |
| AST (U/L) | 20.0 (16.3–24.0) | 22.0 * (19.0–31.5) | 35.0 ***, ## (25.5–47.0) | 43.0 ***, ### (34.0–140) |
| LDH (U/L) | 176 (155–195) | 202 ** (175–249) | 254 ***, ## (216–333) | 428 ***, ### (318–731) |
| Creatinine (mg/dL) | 0.67 (0.55–0.82) | 0.69 (0.58–0.89) | 0.76 (0.65–1.01) | 1.24 * (0.68–1.51) |
| eGFR | 84.5 (73.5–104) | 79.5 (68.5–92.5) | 65.0 ***, # (54.3–84.3) | 45.0 * (37.0–84.0) |
| Hemoglobin A1c (%) | 5.60 (5.40–6.08) | 5.80 (5.50–6.10) | 6.45 ***, ### (5.90–7.45) | 6.70 ***, ### (6.55–8.43) |
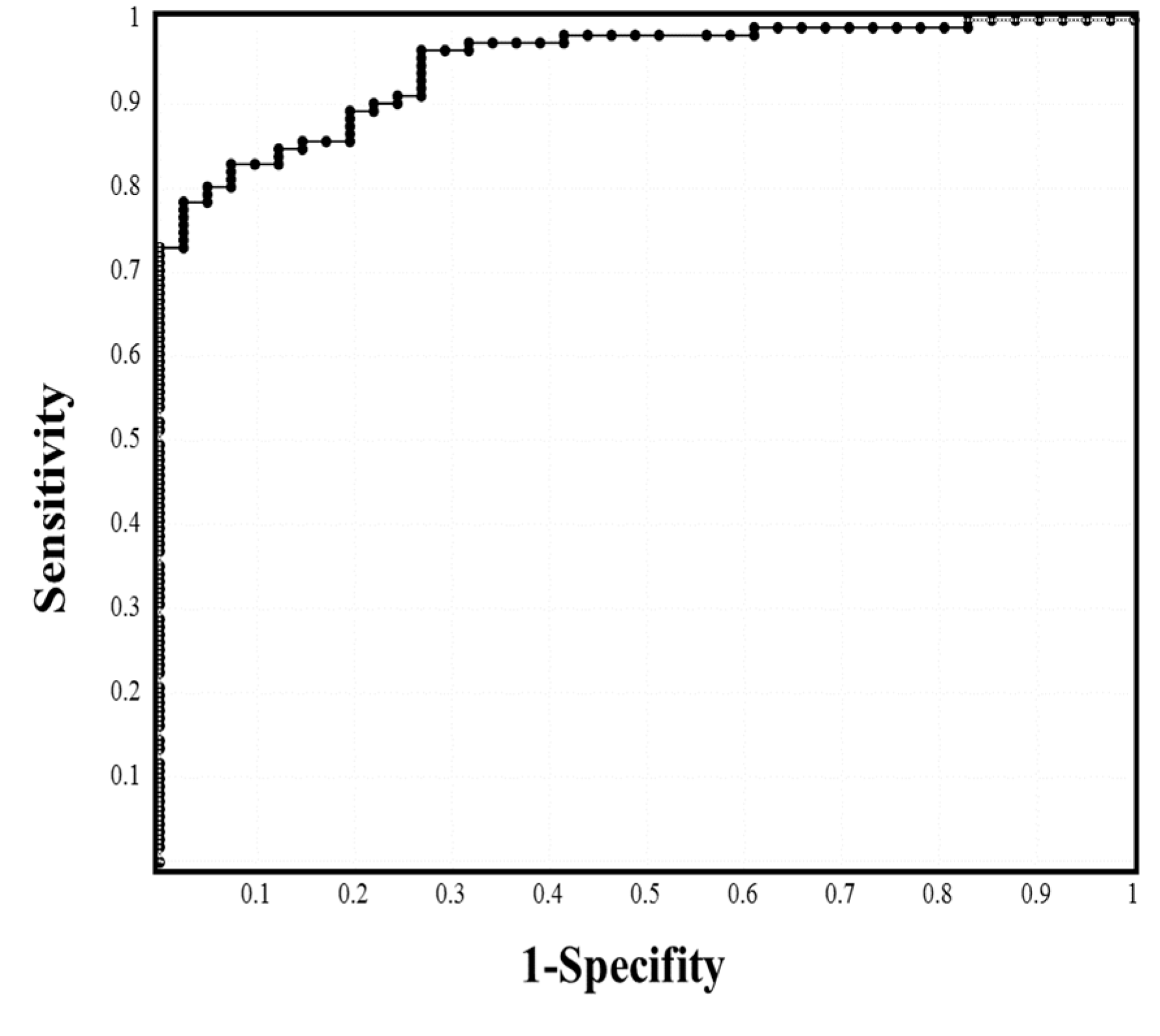
| Cutoff Value | Sensitivity | Odds Ratio | AUC | |
|---|---|---|---|---|
| WBC | 5.5 × 109/L | 55.0% | 1.491 | 0.580 |
| Neutrophil | 3.5 × 109/L | 68.5% | 5.067 | 0.734 |
| Lymphocyte | 1.1 × 109/L | 65.8% | 3.990 | 0.695 |
| Hemoglobin | 14.0 g/dL | 57.0% | 1.898 | 0.580 |
| Platelet | 20.1 × 1010/L | 59.3% | 2.269 | 0.662 |
| PT-INR | 0.997 | 61.4% | 2.557 | 0.676 |
| APTT | 32.8 s | 53.2% | 1.316 | 0.530 |
| D-dimer | 0.60 μg/mL | 67.4% | 4.438 | 0.697 |
| CRP | 2.36 mg/mL | 85.0% | 33.65 | 0.946 |
| Procalcitonin | 0.069 ng/mL | 74.6% | 9.387 | 0.853 |
| Ferritin | 379 ng/mL | 78.3% | 14.12 | 0.872 |
| Total protein | 6.89 g/dL | 62.2% | 2.558 | 0.703 |
| Albumin | 3.73 g/dL | 81.7% | 20.44 | 0.882 |
| A/G ratio | 1.10 | 84.6% | 30.67 | 0.897 |
| T-Bil | 0.67 mg/dL | 66.5% | 4.248 | 0.711 |
| AST | 27.8 U/L | 76.2% | 11.86 | 0.794 |
| LDH | 222 U/L | 76.9% | 3.939 | 0.886 |
| Creatinine | 0.74 mg/dL | 62.3% | 3.163 | 0.630 |
| eGFR | 75.5 | 65.3% | 3.571 | 0.690 |
| Hemoglobin A1c | 6.11% | 73.9% | 10.63 | 0.788 |
| Admission Data | Worsening Data | Worsening Period (Day) | p-Value | |
|---|---|---|---|---|
| WBC (×109/L) | 6.0 (5.4–8.5) | 18.6 (15.4–23.7) | 10.0; 5.3–12.3 | 0.046 |
| Neutrophile (×109/L) | 4.5 (3.8–5.2) | 16.5 (13.1–21.1) | 10.0; 6.0–11.5 | 0.044 |
| Lymphocyte (×109/L) | 0.6 (0.2–0.9) | 0.4 (0.2–0.5) | 3.0; 1.0–7.3 | 0.229 |
| Hemoglobin (g/dL) | 14.6 (13.0–14.7) | 10.2 (9.0–11.4) | 13.0; 10.5–15.5 | <0.001 |
| Platelet (×1010/L) | 13.8 (10.6–19.0) | 7.3 (5.3–11.5) | 1.0; 1.0–10.8 | 0.020 |
| PT-INR | 1.02 (0.99–1.11) | 1.29 (1.17–1.37) | 12.0; 3.8–12.5 | 0.006 |
| APTT (sec) | 33.0 (28.0–33.5) | 81.5 (58.5–180) | 2.0; 1.8–9.3 | 0.005 |
| D-dimer (μg/mL) | 1.2 (0.7–1.8) | 3.7 (1.7–8.1) | 6.0; 1.0–10.3 | 0.155 |
| CRP (mg/mL) | 9.21 (7.53–13.02) | 11.67 (9.65–15.27) | 2.0; 1.0–6.5 | 0.728 |
| Total protein (g/dL) | 6.55 (6.30–6.95) | 4.20 (4.15–4.55) | 7.0; 5.5–15.0 | <0.001 |
| Albumin (g/dL) | 3.45 (2.80–3.55) | 1.90 (1.75–2.15) | 10.0; 6.5–15.0 | <0.001 |
| A/G ratio | 1.05 (0.90–1.10) | 0.80 (0.75–0.90) | 3.0; 3.0–7.3 | 0.005 |
| T-Bil (mg/dL) | 0.69 (0.50–0.98) | 1.20 (0.61–1.61) | 8.0; 1.0–12.8 | 0.110 |
| AST (U/L) | 43.0 (34.0–139.5) | 65.0 (52.0–119.5) | 3.0; 1.0–9.0 | 0.900 |
| LDH (U/L) | 428 (317–731) | 476 (395–723) | 2.0; 1.0–7.8 | 0.803 |
| Creatinine (mg/dL) | 1.24 (0.68–1.51) | 1.28 (0.75–1.51) | 3.0; 1.0–4.3 | 0.879 |
| eGFR | 45.0 (36.5–75.0) | 43.0 (36.0–75.0) | 1.0; 1.0–3.3 | 0.155 |
| Hemoglobin ≤ 10 g/dL | Platelet Count ≤ 10 × 1010/L | PT-INR ≥ 1.26 | APTT ≥ 50 s | D-Dimer ≥ 3.0 μg/mL | |
|---|---|---|---|---|---|
| Case (%) | 4/9 (44.4%) | 5/9 (55.5%) | 4/9 (44.4%) | 7/9 (77.8%) | 5/9 (44.4%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto, A.; Wada, H.; Ichikawa, Y.; Mizuno, H.; Tomida, M.; Masuda, J.; Makino, K.; Kodama, S.; Yoshida, M.; Fukui, S.; et al. Evaluation of Biomarkers of Severity in Patients with COVID-19 Infection. J. Clin. Med. 2021, 10, 3775. https://doi.org/10.3390/jcm10173775
Yamamoto A, Wada H, Ichikawa Y, Mizuno H, Tomida M, Masuda J, Makino K, Kodama S, Yoshida M, Fukui S, et al. Evaluation of Biomarkers of Severity in Patients with COVID-19 Infection. Journal of Clinical Medicine. 2021; 10(17):3775. https://doi.org/10.3390/jcm10173775
Chicago/Turabian StyleYamamoto, Akitaka, Hideo Wada, Yuhuko Ichikawa, Hikaru Mizuno, Masaki Tomida, Jun Masuda, Katsutoshi Makino, Shuji Kodama, Masamichi Yoshida, Shunsuke Fukui, and et al. 2021. "Evaluation of Biomarkers of Severity in Patients with COVID-19 Infection" Journal of Clinical Medicine 10, no. 17: 3775. https://doi.org/10.3390/jcm10173775
APA StyleYamamoto, A., Wada, H., Ichikawa, Y., Mizuno, H., Tomida, M., Masuda, J., Makino, K., Kodama, S., Yoshida, M., Fukui, S., Moritani, I., Inoue, H., Shiraki, K., & Shimpo, H. (2021). Evaluation of Biomarkers of Severity in Patients with COVID-19 Infection. Journal of Clinical Medicine, 10(17), 3775. https://doi.org/10.3390/jcm10173775







