Rituximab Induction and Maintenance in ANCA-Associated Vasculitis: State of the Art and Future Perspectives
Abstract
:1. Clinical Features and Relevance of ANCA in AAV
2. Pathogenesis of AAV: Which Are the Actors and What Is the Role of B Cells?
3. Rituximab in Inducing Remission
4. Rituximab in Maintaining Remission
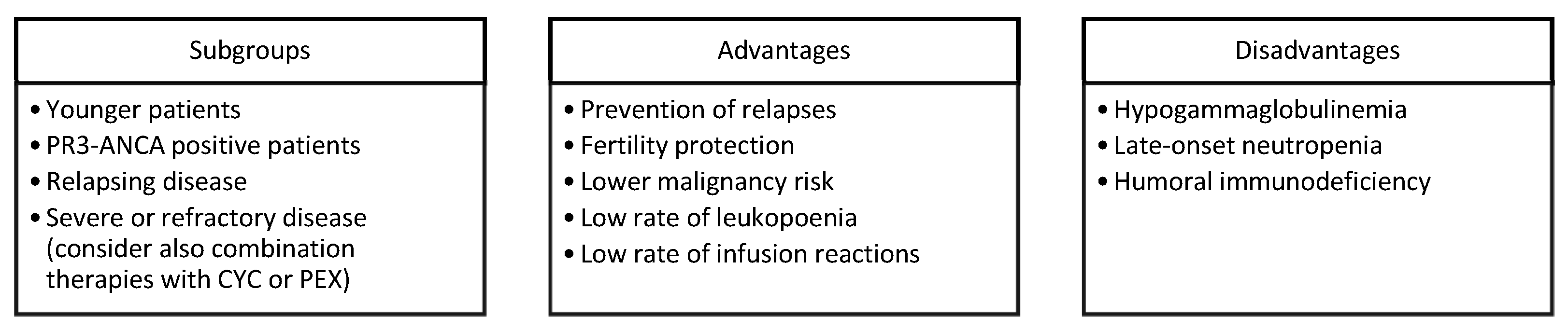
5. Towards a Patient-Tailored Use of RTX in AAV
6. Safety of Rituximab in AAV
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013, 65, 1–11. [Google Scholar] [CrossRef]
- Geetha, D.; Jefferson, J.A. ANCA-Associated Vasculitis: Core Curriculum 2020. Am. J. Kidney Dis. 2020, 75, 124–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvador, F. ANCA Associated Vasculitis. Eur. J. Intern. Med. 2020, 74, 18–28. [Google Scholar] [CrossRef]
- Berti, A.; Dejaco, C. Update on the Epidemiology, Risk Factors, and Outcomes of Systemic Vasculitides. Best Pr. Res. Clin. Rheumatol. 2018, 32, 271–294. [Google Scholar] [CrossRef]
- Mohammad, A.J.; Jacobsson, L.T.H.; Mahr, A.D.; Sturfelt, G.; Segelmark, M. Prevalence of Wegener’s Granulomatosis, Microscopic Polyangiitis, Polyarteritis Nodosa and Churg-Strauss Syndrome within a Defined Population in Southern Sweden. Rheumatology 2007, 46, 1329–1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comarmond, C.; Pagnoux, C.; Khellaf, M.; Cordier, J.-F.; Hamidou, M.; Viallard, J.-F.; Maurier, F.; Jouneau, S.; Bienvenu, B.; Puéchal, X.; et al. Eosinophilic Granulomatosis with Polyangiitis (Churg-Strauss): Clinical Characteristics and Long-Term Followup of the 383 Patients Enrolled in the French Vasculitis Study Group Cohort. Arthritis Rheum. 2013, 65, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Watts, R.A.; Mahr, A.; Mohammad, A.J.; Gatenby, P.; Basu, N.; Flores-Suárez, L.F. Classification, Epidemiology and Clinical Subgrouping of Antineutrophil Cytoplasmic Antibody (ANCA)-Associated Vasculitis. Nephrol. Dial. Transpl. 2015, 30 (Suppl. S1), i14–i22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quartuccio, L.; Treppo, E.; Valent, F.; De Vita, S. Healthcare and Economic Burden of ANCA-Associated Vasculitis in Italy: An Integrated Analysis from Clinical and Administrative Databases. Intern. Emerg. Med. 2020, 16, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Ungprasert, P.; Koster, M.J.; Cheungpasitporn, W.; Wijarnpreecha, K.; Thongprayoon, C.; Kroner, P.T. Inpatient Epidemiology and Economic Burden of Granulomatosis with Polyangiitis: A 10-Year Study of the National Inpatient Sample. Rheumatology 2020, 59, 3685–3689. [Google Scholar] [CrossRef] [PubMed]
- Quartuccio, L.; Bond, M.; Isola, M.; Monti, S.; Felicetti, M.; Furini, F.; Murgia, S.; Berti, A.; Silvestri, E.; Pazzola, G.; et al. Alveolar Haemorrhage in ANCA-Associated Vasculitis: Long-Term Outcome and Mortality Predictors. J. Autoimmun. 2020, 108, 102397. [Google Scholar] [CrossRef]
- Mukhtyar, C.; Lee, R.; Brown, D.; Carruthers, D.; Dasgupta, B.; Dubey, S.; Flossmann, O.; Hall, C.; Hollywood, J.; Jayne, D.; et al. Modification and Validation of the Birmingham Vasculitis Activity Score (Version 3). Ann. Rheum. Dis. 2009, 68, 1827–1832. [Google Scholar] [CrossRef] [Green Version]
- Exley, A.R.; Bacon, P.A.; Luqmani, R.A.; Kitas, G.D.; Gordon, C.; Savage, C.O.; Adu, D. Development and Initial Validation of the Vasculitis Damage Index for the Standardized Clinical Assessment of Damage in the Systemic Vasculitides. Arthritis Rheum. 1997, 40, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Guillevin, L.; Pagnoux, C.; Seror, R.; Mahr, A.; Mouthon, L.; Le Toumelin, P. French Vasculitis Study Group (FVSG) The Five-Factor Score Revisited: Assessment of Prognoses of Systemic Necrotizing Vasculitides Based on the French Vasculitis Study Group (FVSG) Cohort. Medicine 2011, 90, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Robson, J.C.; Dawson, J.; Doll, H.; Cronholm, P.F.; Milman, N.; Kellom, K.; Ashdown, S.; Easley, E.; Gebhart, D.; Lanier, G.; et al. Validation of the ANCA-Associated Vasculitis Patient-Reported Outcomes (AAV-PRO) Questionnaire. Ann. Rheum. Dis. 2018, 77, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Geetha, D.; Jin, Q.; Scott, J.; Hruskova, Z.; Hanouneh, M.; Little, M.A.; Tesar, V.; Seo, P.; Jayne, D.; Pagnoux, C. Comparisons of Guidelines and Recommendations on Managing Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Kidney Int. Rep. 2018, 3, 1039–1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flossmann, O.; Berden, A.; de Groot, K.; Hagen, C.; Harper, L.; Heijl, C.; Höglund, P.; Jayne, D.; Luqmani, R.; Mahr, A.; et al. Long-Term Patient Survival in ANCA-Associated Vasculitis. Ann. Rheum. Dis. 2011, 70, 488–494. [Google Scholar] [CrossRef]
- Berden, A.E.; Ferrario, F.; Hagen, E.C.; Jayne, D.R.; Jennette, J.C.; Joh, K.; Neumann, I.; Noël, L.-H.; Pusey, C.D.; Waldherr, R.; et al. Histopathologic Classification of ANCA-Associated Glomerulonephritis. J. Am. Soc. Nephrol. 2010, 21, 1628–1636. [Google Scholar] [CrossRef] [Green Version]
- Sinico, R.A.; Di Toma, L.; Radice, A. Renal Involvement in Anti-Neutrophil Cytoplasmic Autoantibody Associated Vasculitis. Autoimmun. Rev. 2013, 12, 477–482. [Google Scholar] [CrossRef]
- Heijl, C.; Mohammad, A.J.; Westman, K.; Höglund, P. Long-Term Patient Survival in a Swedish Population-Based Cohort of Patients with ANCA-Associated Vasculitis. RMD Open 2017, 3, e000435. [Google Scholar] [CrossRef] [Green Version]
- Kimmoun, A.; Baux, E.; Das, V.; Terzi, N.; Talec, P.; Asfar, P.; Ehrmann, S.; Geri, G.; Grange, S.; Anguel, N.; et al. Outcomes of Patients Admitted to Intensive Care Units for Acute Manifestation of Small-Vessel Vasculitis: A Multicenter, Retrospective Study. Crit. Care 2016, 20, 27. [Google Scholar] [CrossRef] [Green Version]
- Demiselle, J.; Auchabie, J.; Beloncle, F.; Gatault, P.; Grangé, S.; Du Cheyron, D.; Dellamonica, J.; Boyer, S.; Beauport, D.T.; Piquilloud, L.; et al. Patients with ANCA-Associated Vasculitis Admitted to the Intensive Care Unit with Acute Vasculitis Manifestations: A Retrospective and Comparative Multicentric Study. Ann. Intensive Care 2017, 7, 39. [Google Scholar] [CrossRef] [Green Version]
- Lionaki, S.; Blyth, E.R.; Hogan, S.L.; Hu, Y.; Senior, B.A.; Jennette, C.E.; Nachman, P.H.; Jennette, J.C.; Falk, R.J. Classification of Antineutrophil Cytoplasmic Autoantibody Vasculitides: The Role of Antineutrophil Cytoplasmic Autoantibody Specificity for Myeloperoxidase or Proteinase 3 in Disease Recognition and Prognosis. Arthritis Rheum 2012, 64, 3452–3462. [Google Scholar] [CrossRef] [Green Version]
- Mahr, A.; Katsahian, S.; Varet, H.; Guillevin, L.; Hagen, E.C.; Höglund, P.; Merkel, P.A.; Pagnoux, C.; Rasmussen, N.; Westman, K.; et al. Revisiting the Classification of Clinical Phenotypes of Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis: A Cluster Analysis. Ann. Rheum. Dis. 2013, 72, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Cornec, D.; Gall, E.C.-L.; Fervenza, F.C.; Specks, U. ANCA-Associated Vasculitis—Clinical Utility of Using ANCA Specificity to Classify Patients. Nat. Rev. Rheumatol. 2016, 12, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Greco, A.; De Virgilio, A.; Rizzo, M.I.; Gallo, A.; Magliulo, G.; Fusconi, M.; Ruoppolo, G.; Tombolini, M.; Turchetta, R.; de Vincentiis, M. Microscopic Polyangiitis: Advances in Diagnostic and Therapeutic Approaches. Autoimmun. Rev. 2015, 14, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Furuta, S.; Iwamoto, T.; Nakajima, H. Update on Eosinophilic Granulomatosis with Polyangiitis. Allergol. Int. 2019, 68, 430–436. [Google Scholar] [CrossRef]
- Greco, A.; Marinelli, C.; Fusconi, M.; Macri, G.F.; Gallo, A.; De Virgilio, A.; Zambetti, G.; de Vincentiis, M. Clinic Manifestations in Granulomatosis with Polyangiitis. Int. J. Immunopathol. Pharm. 2016, 29, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Pagnoux, C. Updates in ANCA-Associated Vasculitis. Eur. J. Rheumatol. 2016, 3, 122–133. [Google Scholar] [CrossRef]
- Jennette, J.C.; Falk, R.J.; Gasim, A.H. Pathogenesis of Antineutrophil Cytoplasmic Autoantibody Vasculitis. Curr. Opin. Nephrol. Hypertens. 2011, 20, 263–270. [Google Scholar] [CrossRef] [Green Version]
- Popa, E.R.; Tervaert, J.W.C. The Relation between Staphylococcus Aureus and Wegener’s Granulomatosis: Current Knowledge and Future Directions. Intern. Med. 2003, 42, 771–780. [Google Scholar] [CrossRef] [Green Version]
- Rhee, R.L.; Lu, J.; Bittinger, K.; Lee, J.-J.; Mattei, L.M.; Sreih, A.G.; Chou, S.; Miner, J.J.; Cohen, N.A.; Kelly, B.J.; et al. Dynamic Changes in the Nasal Microbiome Associated with Disease Activity in Patients with Granulomatosis with Polyangiitis. Arthritis Rheumatol. 2021. [Google Scholar] [CrossRef]
- Lyons, P.A.; Rayner, T.F.; Trivedi, S.; Holle, J.U.; Watts, R.A.; Jayne, D.R.W.; Baslund, B.; Brenchley, P.; Bruchfeld, A.; Chaudhry, A.N.; et al. Genetically Distinct Subsets within ANCA-Associated Vasculitis. N. Engl. J. Med. 2012, 367, 214–223. [Google Scholar] [CrossRef] [Green Version]
- Mahr, A.; Guillevin, L.; Poissonnet, M.; Aymé, S. Prevalences of Polyarteritis Nodosa, Microscopic Polyangiitis, Wegener’s Granulomatosis, and Churg-Strauss Syndrome in a French Urban Multiethnic Population in 2000: A Capture-Recapture Estimate. Arthritis Rheum. 2004, 51, 92–99. [Google Scholar] [CrossRef]
- Hogan, S.L.; Cooper, G.S.; Savitz, D.A.; Nylander-French, L.A.; Parks, C.G.; Chin, H.; Jennette, C.E.; Lionaki, S.; Jennette, J.C.; Falk, R.J. Association of Silica Exposure with Anti-Neutrophil Cytoplasmic Autoantibody Small-Vessel Vasculitis: A Population-Based, Case-Control Study. Clin. J. Am. Soc. Nephrol. 2007, 2, 290–299. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Yao, L.-P.; Dong, M.-J.; Xu, Q.; Zhang, J.; Weng, W.-W.; Chen, F. Clinical Characteristics and Outcomes of Propylthiouracil-Induced Antineutrophil Cytoplasmic Antibody-Associated Vasculitis in Patients with Graves’ Disease: A Median 38-Month Retrospective Cohort Study from a Single Institution in China. Thyroid 2017, 27, 1469–1474. [Google Scholar] [CrossRef]
- Deshayes, S.; Dolladille, C.; Dumont, A.; Martin Silva, N.; Chretien, B.; De Boysson, H.; Alexandre, J.; Aouba, A. A worldwide pharmacoepidemiological update of drug-associated ANCA-associated vasculitis at the time of targeted therapies. Arthritis Rheumatol. 2021. [Google Scholar] [CrossRef]
- Matsumoto, K.; Kurasawa, T.; Yoshimoto, K.; Suzuki, K.; Takeuchi, T. Identification of neutrophil β2-integrin LFA-1 as a potential mechanistic biomarker in ANCA-associated vasculitis via microarray and validation analyses. Arthritis Res. Ther. 2021, 23, 136. [Google Scholar] [CrossRef]
- Quartuccio, L.; Lombardi, S.; Fabris, M.; Masolini, P.; Saracco, M.; Pellerito, R.; Vita, S.D. Long-Term Effects of Rituximab in Rheumatoid Arthritis. Ann. N. Y. Acad. Sci. 2009, 1173, 692–700. [Google Scholar] [CrossRef] [PubMed]
- De Vita, S.; Quartuccio, L.; Isola, M.; Mazzaro, C.; Scaini, P.; Lenzi, M.; Campanini, M.; Naclerio, C.; Tavoni, A.; Pietrogrande, M.; et al. A Randomized Controlled Trial of Rituximab for the Treatment of Severe Cryoglobulinemic Vasculitis. Arthritis Rheumatol. 2012, 64, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.H.; Merkel, P.A.; Spiera, R.; Seo, P.; Langford, C.A.; Hoffman, G.S.; Kallenberg, C.G.M.; St Clair, E.W.; Turkiewicz, A.; Tchao, N.K.; et al. Rituximab versus Cyclophosphamide for ANCA-Associated Vasculitis. N. Engl. J. Med. 2010, 363, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.B.; Tervaert, J.W.C.; Hauser, T.; Luqmani, R.; Morgan, M.D.; Peh, C.A.; Savage, C.O.; Segelmark, M.; Tesar, V.; van Paassen, P.; et al. Rituximab versus Cyclophosphamide in ANCA-Associated Renal Vasculitis. N. Engl. J. Med. 2010, 363, 211–220. [Google Scholar] [CrossRef] [Green Version]
- Guillevin, L.; Pagnoux, C.; Karras, A.; Khouatra, C.; Aumaître, O.; Cohen, P.; Maurier, F.; Decaux, O.; Ninet, J.; Gobert, P.; et al. Rituximab versus Azathioprine for Maintenance in ANCA-Associated Vasculitis. N. Engl. J. Med. 2014, 371, 1771–1780. [Google Scholar] [CrossRef] [Green Version]
- Charles, P.; Terrier, B.; Perrodeau, É.; Cohen, P.; Faguer, S.; Huart, A.; Hamidou, M.; Agard, C.; Bonnotte, B.; Samson, M.; et al. Comparison of Individually Tailored versus Fixed-Schedule Rituximab Regimen to Maintain ANCA-Associated Vasculitis Remission: Results of a Multicentre, Randomised Controlled, Phase III Trial (MAINRITSAN2). Ann. Rheum. Dis. 2018, 77, 1143–1149. [Google Scholar] [CrossRef]
- Charles, P.; Perrodeau, É.; Samson, M.; Bonnotte, B.; Néel, A.; Agard, C.; Huart, A.; Karras, A.; Lifermann, F.; Godmer, P.; et al. Long-Term Rituximab Use to Maintain Remission of Antineutrophil Cytoplasmic Antibody-Associated Vasculitis: A Randomized Trial. Ann. Intern. Med. 2020, 173, 179–187. [Google Scholar] [CrossRef]
- Smith, R.M.; Jones, R.B.; Specks, U.; Bond, S.; Nodale, M.; Aljayyousi, R.; Andrews, J.; Bruchfeld, A.; Camilleri, B.; Carette, S.; et al. Rituximab as Therapy to Induce Remission after Relapse in ANCA-Associated Vasculitis. Ann. Rheum. Dis. 2020, 79, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Jayne, D.; Merkel, P. A Randomized, Controlled Trial of Rituximab versus Azathioprine After Induction of Remission with Rituximab for Patients with ANCA-Associated Vasculitis and Relapsing Disease. Arthritis Rheumatol 2019, 71 (Suppl. S10). Available online: https://acrabstracts.org/abstract/a-randomized-controlled-trial-of-rituximab-versus-azathioprine-after-induction-of-remission-with-rituximab-for-patients-with-anca-associated-vasculitis-and-relapsing-disease/ (accessed on 19 July 2021).
- Hassan, R.I.; Gaffo, A.L. Rituximab in ANCA-Associated Vasculitis. Curr. Rheumatol. Rep. 2017, 19, 6. [Google Scholar] [CrossRef]
- Specks, U.; Merkel, P.A.; Seo, P.; Spiera, R.; Langford, C.A.; Hoffman, G.S.; Kallenberg, C.G.M.; St Clair, E.W.; Fessler, B.J.; Ding, L.; et al. Efficacy of Remission-Induction Regimens for ANCA-Associated Vasculitis. N. Engl. J. Med. 2013, 369, 417–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, R.B.; Furuta, S.; Tervaert, J.W.C.; Hauser, T.; Luqmani, R.; Morgan, M.D.; Peh, C.A.; Savage, C.O.; Segelmark, M.; Tesar, V.; et al. Rituximab versus Cyclophosphamide in ANCA-Associated Renal Vasculitis: 2-Year Results of a Randomised Trial. Ann. Rheum. Dis. 2015, 74, 1178–1182. [Google Scholar] [CrossRef]
- Walsh, M.; Merkel, P.A.; Peh, C.A.; Szpirt, W.; Guillevin, L.; Pusey, C.D.; De Zoysa, J.; Ives, N.; Clark, W.F.; Quillen, K.; et al. Plasma Exchange and Glucocorticoid Dosing in the Treatment of Anti-Neutrophil Cytoplasm Antibody Associated Vasculitis (PEXIVAS): Protocol for a Randomized Controlled Trial. Trials 2013, 14, 73. [Google Scholar] [CrossRef] [Green Version]
- Menditto, V.G.; Rossetti, G.; Olivari, D.; Angeletti, A.; Rocchi, M.; Gabrielli, A.; Pomponio, G. Rituximab for Eosinophilic Granulomatosis with Polyangiitis: A Systematic Review of Observational Studies. Rheumatol. 2021, 60, 1640–1650. [Google Scholar] [CrossRef]
- Akiyama, M.; Kaneko, Y.; Takeuchi, T. Rituximab for the Treatment of Eosinophilic Granulomatosis with Polyangiitis: A Systematic Literature Review. Autoimmun. Rev. 2021, 20, 102737. [Google Scholar] [CrossRef] [PubMed]
- Tieu, J.; Smith, R.; Basu, N.; Brogan, P.; D’Cruz, D.; Dhaun, N.; Flossmann, O.; Harper, L.; Jones, R.B.; Lanyon, P.C.; et al. Rituximab for Maintenance of Remission in ANCA-Associated Vasculitis: Expert Consensus Guidelines. Rheumatology 2020, 59, e24–e32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terrier, B.; Pagnoux, C.; Perrodeau, É.; Karras, A.; Khouatra, C.; Aumaître, O.; Cohen, P.; Decaux, O.; Desmurs-Clavel, H.; Maurier, F.; et al. Long-Term Efficacy of Remission-Maintenance Regimens for ANCA-Associated Vasculitides. Ann. Rheum. Dis. 2018, 77, 1150–1156. [Google Scholar] [CrossRef] [Green Version]
- Gopaluni, S.; Smith, R.M.; Lewin, M.; McAlear, C.A.; Mynard, K.; Jones, R.B.; Specks, U.; Merkel, P.A.; Jayne, D.R.W. RITAZAREM Investigators Rituximab versus Azathioprine as Therapy for Maintenance of Remission for Anti-Neutrophil Cytoplasm Antibody-Associated Vasculitis (RITAZAREM): Study Protocol for a Randomized Controlled Trial. Trials 2017, 18, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Dam, L.S.; Oskam, J.M.; Kamerling, S.W.A.; Arends, E.J.; Bredewold, O.W.; Berkowska, M.A.; van Dongen, J.J.M.; Rabelink, T.J.; van Kooten, C.; Teng, Y.K.O. Highly Sensitive Flow Cytometric Detection of Residual B-Cells After Rituximab in Anti-Neutrophil Cytoplasmic Antibodies-Associated Vasculitis Patients. Front. Immunol. 2020, 11, 566732. [Google Scholar] [CrossRef]
- Md Yusof, M.Y.; Vital, E.M.; Das, S.; Dass, S.; Arumugakani, G.; Savic, S.; Rawstron, A.C.; Emery, P. Repeat Cycles of Rituximab on Clinical Relapse in ANCA-Associated Vasculitis: Identifying B Cell Biomarkers for Relapse to Guide Retreatment Decisions. Ann. Rheum. Dis. 2015, 74, 1734–1738. [Google Scholar] [CrossRef] [Green Version]
- von Borstel, A.; Land, J.; Abdulahad, W.H.; Rutgers, A.; Stegeman, C.A.; Diepstra, A.; Heeringa, P.; Sanders, J.S. CD27+CD38hi B Cell Frequency During Remission Predicts Relapsing Disease in Granulomatosis with Polyangiitis Patients. Front. Immunol. 2019, 10, 2221. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.M.; Jones, R.B.; Jayne, D.R.W. Progress in Treatment of ANCA-Associated Vasculitis. Arthritis Res. Ther. 2012, 14, 210. [Google Scholar] [CrossRef] [Green Version]
- Yates, M.; Watts, R.A.; Bajema, I.M.; Cid, M.C.; Crestani, B.; Hauser, T.; Hellmich, B.; Holle, J.U.; Laudien, M.; Little, M.A.; et al. EULAR/ERA-EDTA Recommendations for the Management of ANCA-Associated Vasculitis. Ann. Rheum. Dis. 2016, 75, 1583–1594. [Google Scholar] [CrossRef] [Green Version]
- McAdoo, S.P.; Medjeral-Thomas, N.; Gopaluni, S.; Tanna, A.; Mansfield, N.; Galliford, J.; Griffith, M.; Levy, J.; Cairns, T.D.; Jayne, D.; et al. Long-Term Follow-up of a Combined Rituximab and Cyclophosphamide Regimen in Renal Anti-Neutrophil Cytoplasm Antibody-Associated Vasculitis. Nephrol. Dial. Transpl. 2019, 34, 63–73. [Google Scholar] [CrossRef]
- Cortazar, F.B.; Muhsin, S.A.; Pendergraft, W.F.; Wallace, Z.S.; Dunbar, C.; Laliberte, K.; Niles, J.L. Combination Therapy with Rituximab and Cyclophosphamide for Remission Induction in ANCA Vasculitis. Kidney Int. Rep. 2018, 3, 394–402. [Google Scholar] [CrossRef] [Green Version]
- Jayne, D.R.; Chapel, H.; Adu, D.; Misbah, S.; O’Donoghue, D.; Scott, D.; Lockwood, C.M. Intravenous Immunoglobulin for ANCA-Associated Systemic Vasculitis with Persistent Disease Activity. QJM 2000, 93, 433–439. [Google Scholar] [CrossRef] [Green Version]
- Jayne, D.R.W.; Bruchfeld, A.N.; Harper, L.; Schaier, M.; Venning, M.C.; Hamilton, P.; Burst, V.; Grundmann, F.; Jadoul, M.; Szombati, I.; et al. Randomized Trial of C5a Receptor Inhibitor Avacopan in ANCA-Associated Vasculitis. J. Am. Soc. Nephrol. 2017, 28, 2756–2767. [Google Scholar] [CrossRef] [Green Version]
- Little, M.A.; Nightingale, P.; Verburgh, C.A.; Hauser, T.; De Groot, K.; Savage, C.; Jayne, D.; Harper, L.; European Vasculitis Study (EUVAS) Group. Early mortality in systemic vasculitis: Relative contribution of adverse events and active vasculitis. Ann. Rheum. Dis. 2010, 69, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Tieu, J.; Smith, R.M.; Gopaluni, S.; Kumararatne, D.S.; McClure, M.; Manson, A.; Houghton, S.; Jayne, D.R.W. Rituximab Associated Hypogammaglobulinemia in Autoimmune Disease. Front. Immunol. 2021, 12, 671503. [Google Scholar] [CrossRef] [PubMed]
- Wijetilleka, S.; Jayne, D.R.; Mukhtyar, C.; Ala, A.; Bright, P.D.; Chinoy, H.; Harper, L.; Kazmi, M.A.; Kiani-Alikhan, S.; Li, C.K.; et al. Recommendations for the management of secondary hypogammaglobulinaemia due to B cell targeted therapies in autoimmune rheumatic diseases. Rheumatology 2019, 58, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Kronbichler, A.; Kerschbaum, J.; Gopaluni, S.; Tieu, J.; Alberici, F.; Jones, R.B.; Smith, R.M.; Jayne, D.R.W. Trimethoprim-Sulfamethoxazole Prophylaxis Prevents Severe/Life-Threatening Infections Following Rituximab in Antineutrophil Cytoplasm Antibody-Associated Vasculitis. Ann. Rheum. Dis. 2018, 77, 1440–1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monti, S.; Delvino, P.; Riboli, M.; Rebuffi, C.; Xoxi, B.; De Silvestri, A.; Montecucco, C. The role of Trimethoprim/sulfametoxazole in reducing relapses and risk of infec-tions in ANCA-associated vasculitis: A meta-analysis. Rheumatology 2021, 22, keab267. [Google Scholar] [CrossRef]
- Sparks, J.A.; Wallace, Z.S.; Seet, A.M.; Gianfrancesco, M.A.; Izadi, Z.; Hyrich, K.L.; Strangfeld, A.; Gossec, L.; Carmona, L.; Mateus, E.F.; et al. Associations of baseline use of biologic or targeted synthetic DMARDs with COVID-19 severity in rheumatoid arthritis: Results from the COVID-19 Global Rheumatology Alliance physician registry. Ann. Rheum. Dis. 2021, 80, 1137–1146. [Google Scholar] [CrossRef]
- Quartuccio, L.; Treppo, E.; Binutti, M.; Del Frate, G.; De Vita, S. Timing of Rituximab and Immunoglobulin Level Influence the Risk of Death for COVID-19 in ANCA-Associated Vasculitis. Rheumatology 2021, 60, 3476–3477. [Google Scholar] [CrossRef] [PubMed]
- Benucci, M.; Quartuccio, L.; Li Gobbi, F.; Damiani, A.; Grossi, V.; Infantino, M.; Manfredi, M. Persistence of RT-PCR-SARS-CoV-2 Infection and Delayed Serological Response, as a Possible Effect of Rituximab According to the Hypothesis of Schulze-Koops et Al. Ann. Rheum. Dis. 2020. [Google Scholar] [CrossRef]
- McClure, M.E.; Zhu, Y.; Smith, R.M.; Gopaluni, S.; Tieu, J.; Pope, T.; Kristensen, K.E.; Jayne, D.R.W.; Barrett, J.; Jones, R.B. Long-term maintenance rituximab for ANCA-associated vasculitis: Relapse and infection prediction models. Rheumatology 2021, 60, 1491–1501, PMID: 33141217; PMCID: PMC7937025. [Google Scholar] [CrossRef] [PubMed]
- Furuta, S.; Nakagomi, D.; Kobayashi, Y.; Hiraguri, M.; Sugiyama, T.; Amano, K.; Umibe, T.; Kono, H.; Kurasawa, K.; Kita, Y.; et al. Effect of Reduced-Dose vs High-Dose Glucocorticoids Added to Rituximab on Remission Induction in ANCA-Associated Vasculitis: A Randomized Clinical Trial. JAMA 2021, 325, 2178–2187. [Google Scholar] [CrossRef] [PubMed]
- Paul, F.; Cartron, G. Infusion-Related Reactions to Rituximab: Frequency, Mechanisms and Predictors. Expert. Rev. Clin. Immunol. 2019, 15, 383–389. [Google Scholar] [CrossRef]
- Tesfa, D.; Ajeganova, S.; Hägglund, H.; Sander, B.; Fadeel, B.; Hafström, I.; Palmblad, J. Late-Onset Neutropenia Following Rituximab Therapy in Rheumatic Diseases: Association with B Lymphocyte Depletion and Infections. Arthritis Rheum. 2011, 63, 2209–2214. [Google Scholar] [CrossRef] [Green Version]
- Heijl, C.; Harper, L.; Flossmann, O.; Stücker, I.; Scott, D.G.I.; Watts, R.A.; Höglund, P.; Westman, K.; Mahr, A. European Vasculitis Study Group (EUVAS) Incidence of Malignancy in Patients Treated for Antineutrophil Cytoplasm Antibody-Associated Vasculitis: Follow-up Data from European Vasculitis Study Group Clinical Trials. Ann. Rheum. Dis. 2011, 70, 1415–1421. [Google Scholar] [CrossRef]
- Choi, S.T.; Ahn, S.V.; Lee, P.H.; Moon, C.M. The Cancer Risk According to Three Subtypes of ANCA-Associated Vasculitis: A Propensity Score-Matched Analysis of a Nationwide Study. Semin. Arthritis Rheum. 2021, 51, 692–699. [Google Scholar] [CrossRef]
- van Daalen, E.E.; Rizzo, R.; Kronbichler, A.; Wolterbeek, R.; Bruijn, J.A.; Jayne, D.R.; Bajema, I.M.; Rahmattulla, C. Effect of Rituximab on Malignancy Risk in Patients with ANCA-Associated Vasculitis. Ann. Rheum. Dis. 2017, 76, 1064–1069. [Google Scholar] [CrossRef]
- Mittal, S.; Naidu, G.S.R.S.N.K.; Jha, S.; Rathi, M.; Nada, R.; Minz, R.W.; Sharma, K.; Dhir, V.; Jain, S.; Sharma, A. Experience with Similar Biologic Rituximab in 77 Patients of Granulomatosis with Polyangiitis-a Real-Life Experience. Clin. Rheumatol. 2021, 40, 645–651. [Google Scholar] [CrossRef]
- Kwon, H.C.; Kim, M.K.; Song, J.J.; Park, Y.B.; Lee, S.W. Rituximab Biosimilar Prevents Poor Outcomes of Microscopic Polyangiitis and Granulomatosis with Polyangiitis as Effectively as Rituximab Originator. Yonsei. Med. J. 2020, 61, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Bénard, V.; Farhat, C.; Zarandi-Nowroozi, M.; Durand, M.; Charles, P.; Puéchal, X.; Guillevin, L.; Pagnoux, C.; Makhzoum, J.P. Comparison of Two Rituximab Induction Regimens for Antineutrophil Cytoplasm Antibody-Associated Vasculitis: Systematic Review and Meta-Analysis. ACR Open Rheumatol. 2021, 3, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Fauci, A.S.; Haynes, B.F.; Katz, P.; Wolff, S.M. Wegener’s Granulomatosis: Prospective Clinical and Therapeutic Experience with 85 Patients for 21 Years. Ann. Intern. Med. 1983, 98, 76–85. [Google Scholar] [CrossRef] [PubMed]
| Clinical Manifestations | MPA | GPA | EGPA |
|---|---|---|---|
| Constitutional symptoms | Fever, Weight Loss, Fatigue, Arthralgia, Myalgia | ||
| 55–80% | 70–100% | 30–50% | |
| Skin | Palpable Purpura, Nodules, Pseudourticarial Rash, Livedo Reticularis, Ulcers | ||
| 35–60% | 10–50% | 50–70% | |
| ENT | Infrequent | Frequent (60–80%): Destructive Sinusitis, Saddle-Nose Deformity, Crusting Rhinitis, Nasal Septum Deformity, Otitis Media | Allergic Rhinitis, Sinus Polyposis |
| Lung | Frequent (60–80%): Cough, Haemoptysis, Dyspnoea, Interstitial Lung Pattern, Alveolar Haemorrhage | Frequent (60–80%): Non-Migratory Nodule or Infiltrates, Excavated Nodules, Bronchial And/or Subglottic Stenosis | Asthma (Approximately 100%), Migratory Nodules or Infiltrates, Eosinophil Pleural Effusion |
| Kidney | Proteinuria, Haematuria, Renal Failure | ||
| Frequent (80%): Glomerulonephritis | Frequent (60–80%): Glomerulonephritis | Possible (20%) | |
| Neurologic | Mononeuritis Multiplex, Polyneuropathy, Cranial Nerves Disorders, Pachymeningitis | ||
| Possible (35%) | Possible (25%) | Frequent (65–75%) | |
| Heart | Myocarditis, Pericarditis, Ischemia | ||
| Possible (10–50%): From Asymptomatic to Cardiomyopathy | |||
| Eye | Uveitis, Conjunctivitis, Episcleritis | ||
| Mono or Bilateral Proptosis, Orbital Tumour | |||
| Venous thrombosis | 7–8% | ||
| Laboratory | Increase ESR and CRP, Anaemia, Thrombocytosis | ||
| Eosinophilia | |||
| cANCA/PR3 | 10–20% | 80–90% | |
| pANCA/MPO | 60–85% | 0–10% | 30–60%, usually pANCA/MPO |
| Name | Population | Number of Patients | Primary Endpoint | Results | Other Findings |
|---|---|---|---|---|---|
| RAVE [40] | N = 197 pts; GPA or MPA; new onset (49%) or relapsing disease; ANCA+ | RTX arm: 99 pts received 4 weekly RTX 375 mg/m2; Control arm: 98 pts received PO CYC followed by AZA; same GCs regimen; randomized 1:1 | Remission of disease without GCs at 6 months | (1) RTX was noninferior to CYC (64% vs. 53%) at remission induction at 6 months (p < 0.001) (2) RTX was superior to CYC (67% vs. 42%) in relapsing disease (p = 0.01) | (a) 50% of pts in RTX arm became negative for PR3–ANCA, as compared with only 17% in the control arm; (b) similar AEs |
| RITUXVAS [41] | N = 44 pts; GPA or MPA; new onset of renal AAV | RTX arm: 33 pts received two doses CYC IV plus 4 weekly RTX 375 mg/m2; Control arm: 11 pts received IV CYC followed by AZA; same GCs regimen; randomized 3:1 | Sustained remission rates at 12 months and severe AEs | (1) Equivalent results in achieving sustained remission (76% vs. 82%, p = 0.68) 2)Severe adverse events were similar (42% vs. 36%, p = 0.77) | (a) Sustained remission rates were high in both groups |
| Name | Population | Number of Patients | Primary Endpoint | Results | Other Findings |
|---|---|---|---|---|---|
| MAINRITSAN [42] | N = 115 pts; GPA or MPA in remission of disease after CYC; ANCA+ | RTX arm: 57 pts received RTX (500 mg every 6 months); Control arm: 58 pts received AZA; randomized 1:1 | Rate of major relapse at month 28 | (1) Lower relapse rate in RTX arm (5% vs. 29%, HR for relapse 6.61, IC 95%: 1.56–27.96, p = 0.002) | (a) Similar AEs (p = 0.92) |
| MAINRITSAN2 [43] | N = 162 pts; GPA or MPA in remission of disease; ANCA+ or ANCA- | Tailored-arm: 81 pts patients received a 500 mg RTX infusion at randomisation, then in case of change in ANCA status or CD19+ B cell counts exceeded 0/mm3; Control arm: 81 pts receiveda fixed 500 mg RTX infusion on days 0 and 14 postrandomisation, then 6, 12, and 18 months after the first infusion; randomized 1:1 | Number of relapses or worsening disease (BVAS > 0) at month 28 | (1) Equivalent results in number of relapses [21 pts had suffered 22 relapses: 14/81 (17.3%) in 13 tailored-infusion recipients and 8/81 (9.9%) in 8 fixed-schedule patients (p = 0.22)] | (a) AAV relapse rates did not differ significantly; (b) individually tailored-arm patients received fewer rituximab infusions |
| MAINRITSAN3 [44] | N= 97 pts; GPA or MPA in sustained remission after RTX-maintenance therapy; pts must have successfully completed the MAINRITSAN2 trial without any major relapses | RTX arm: 50 pts received additional 2 years of RTX over 18 months (4 infusions); Control arm: 47 pts received placebo; randomized 1:1 | Relapse-free survival at month 28 | (1) Relapse-free survival was higher in RTX arm at month 28 (96% vs. 74%, HR 7.5, CI: 1.67–33.7, p = 0.008) | (a) Major relapse-free survival estimates at month 28 were 100% in RTX arm versus 87% in control arm (p = 0.009); (b) lower relapse rate in RTX arm (4% versus 26%); (c) no increase in AEs in extended RTX (24% versus 30%) (d) in the placebo arm, relapse is much more common in PR3-ANCA positive pts |
| RITAZAREM [46,55] | N = 190 pts; relapsed GPA or MPA re-induced with RTX (4 weekly RTX 375 mg/m2) and in remission of disease at month 4 (N = 170) | RTX arm: 85 pts received RTX (1000 mg every 4 months for 5 doses); Control arm: 85 pts received AZA; randomized 1:1 | Time to disease relapse reported at 24 months | (1) RTX was superior to AZA in relapsing disease with preliminary overall HR estimate of 0.36 (CI 95%: 0.23–0.57, p < 0.001) | (a) No increase AEs in RTX arm (22% versus 36%); (b) relapse is very common in both arms; (c) the effect of higher-dose RTX is not sustained in long term |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Treppo, E.; Binutti, M.; Agarinis, R.; De Vita, S.; Quartuccio, L. Rituximab Induction and Maintenance in ANCA-Associated Vasculitis: State of the Art and Future Perspectives. J. Clin. Med. 2021, 10, 3773. https://doi.org/10.3390/jcm10173773
Treppo E, Binutti M, Agarinis R, De Vita S, Quartuccio L. Rituximab Induction and Maintenance in ANCA-Associated Vasculitis: State of the Art and Future Perspectives. Journal of Clinical Medicine. 2021; 10(17):3773. https://doi.org/10.3390/jcm10173773
Chicago/Turabian StyleTreppo, Elena, Marco Binutti, Roberto Agarinis, Salvatore De Vita, and Luca Quartuccio. 2021. "Rituximab Induction and Maintenance in ANCA-Associated Vasculitis: State of the Art and Future Perspectives" Journal of Clinical Medicine 10, no. 17: 3773. https://doi.org/10.3390/jcm10173773
APA StyleTreppo, E., Binutti, M., Agarinis, R., De Vita, S., & Quartuccio, L. (2021). Rituximab Induction and Maintenance in ANCA-Associated Vasculitis: State of the Art and Future Perspectives. Journal of Clinical Medicine, 10(17), 3773. https://doi.org/10.3390/jcm10173773







