Abstract
Leukocyte telomere length (LTL) represents a key integrating component of the cumulative effects of environmental, lifestyle, and genetic factors. A question, however, remains on whether LTL can be considered predictive for a longer and healthier life. Within the elderly prospective TRELONG cohort (n = 612), we aimed to investigate LTL as a predictor of longevity and identify the main determinants of LTL among many different factors (physiological and lifestyle characteristics, physical performance and frailty measures, chronic diseases, biochemical measurements and apolipoprotein E genotyping). We found an ever-increasing relationship between LTL quartiles and survival. Hazard ratio analysis showed that for each unit increase in LTL and Short Physical Performance Battery (SPPB) scores, the mortality risk was reduced by 22.41% and 8.78%, respectively. Conversely, male gender, Charlson Comorbidity Index, and age threatened survival, with mortality risk growing by 74.99%, 16.57% and 8.5%, respectively. Determinants of LTL elongation were SPPB scores (OR = 1.1542; p = 0.0066) and years of education (OR = 1.0958; p = 0.0065), while male gender (OR = 0.4388; p = 0.0143) and increased Disease Count Index (OR = 0.6912; p = 0.0066) were determinants of LTL attrition. Longer LTL predicts a significant survival advantage in elderly people. By identifying determinants of LTL elongation, we provided additional knowledge that could offer a potential translation into prevention strategies.
1. Introduction
The aging of the population, also named the “gray” tsunami, is a growing social and public health issue. The impact of the phenomenon is forecasted by worrying data in Italy, one of the most elderly nations in the world [1]. In particular, the Northeast Veneto region area of Treviso is characterized by the highest longevity in Italy, especially among women [2]. The reasons are scantily known.
Aging is a peculiar and manifold process characterized by a gradual deterioration of the body’s capability to react to internal and/or external stress factors, resulting in an increased risk of illness and death [3]. This decline is the key risk factor of many human diseases, including cancer, diabetes, cardiovascular and neurodegenerative disorders. Not all people become eldery in the same manner [4]. Consequently, chronological age is not a consistent marker of the physiological decay of an individual [5], while biological age is expected to reflect the continuing changes within a person [6].
Accumulating evidence has revealed that leukocyte telomere length (LTL) reduction can be regarded as the early pillar of biological aging and may be the origin of cellular dysfunction [3]. Telomeres are short sequences of DNA repeated (TTAGGG) in association with various proteins forming a complex that is essential in preserving the genome stability of the cells [7]. In normal somatic cells, telomeric repeats are reduced by 30–200 bp after each mitotic division. However, telomeres, such as triple G-containing sequences, represent a susceptible target for genotoxic compounds, oxidative stress, and inflammation damages that directly accelerate telomere shortening. In our previous works, we showed that LTL erosion is related to risk factors for age-related diseases, including obesity [8], oxidative stress [9] and inflammatory responses [10], exposure to lifestyle (smoke, alcohol) [11], occupational pollutants [12] and cancer [13]. More recently, we found that in 585 residents in Northeast Italy, the everyday life exposure to polluted air, in particular indoors, loses LTL, particularly in males [14]. Therefore, the rate at which telomeres shorten provides a sturdy biomarker of aging and general health.
However, one of the most discussed questions in gerontology research is whether LTL is prognostic for a long and healthy life. The problem in interpreting the results of several studies arises from the heterogeneity of the factors that affect LTL, including genetics and environment, and in particular the availability of follow-up data both of LTL and survival of enrolled subjects.
The Treviso Longeva (TRELONG) study is a longitudinal cohort study initiated in 2003, which encompassed 668 seniors, women and men, over the age of 70 years, living in Treviso, the town with the highest longevity in North-East Italy [2]. The Trelong Study has investigated different topics such as frailty, cognitive impairment, Mediterranean diet and genetic aspects [15,16,17,18,19,20].
In view of the above, we investigated LTL within the prospective TRELONG cohort with two main aims: to ascertain whether this biomarker is predictive of longevity and to identify the main determinants in LTL among the personal characteristics (age, sex, marital status, education, occupation, etc.), physiological and lifestyle features, physical performance and frailty measures, chronic diseases, biochemical measurements and apolipoprotein E (APOE) genotyping.
2. Materials and Methods
2.1. Study Population
A complete description of the TRELONG study has been previously reported [15]. Eligible participants lived in the municipality of Treviso in North-East Italy. At the beginning of this study in 2003, Treviso had a resident population of 81,700. After the selection of the 13,861 inhabitants over 70 years of age (17% of total inhabitants) from the residents listed in the Registry Office of Treviso, participants were systematically sampled, planning to include 100 participants according to gender and 10-year-age group, 125 women and 125 men in the 70–79-year-age group, and all people over 100 years. A total of 668 people were selected: 311 men and 357 women (mean age 84.0 ± 8.0 years, range 70.0–105.5 years) [15]. Seven- and ten-year follow-ups were then performed. Using an interview, we collected data assessing clinical, lifestyle and demographic information, along with measures of physical performance and frailty, and a blood sample (30 mL). All were performed at home in three time points: in 2003 (baseline wave 1), in 2010 (wave 2) and 2013 (wave 3) [15]. Survival data were collected annually since 2003. In the present work, a total of 613 subjects for whom a sufficient DNA aliquot was still available for the telomere analysis in at least one of the three waves (2003, 2010 and 2013) were studied. All three measurements of LTL (at wave 1, wave 2, and wave 3) were available for only 162 people, because of mortality, impracticability of venous sampling or, rarely, refusal. Ours is, therefore, an observational cohort prospective study. The study protocol was approved by the Ethical Committee of the National Institute on Research and Care of the Elderly (INRCA, Ancona, Italy, prot. n. 50/02 on 22 March 2002). All participants and/or their caregivers provided a written consent.
2.2. Demographic, Physiological and Lifestyle Characteristics
Gender, age at the time of baseline in 2003, marital status, education in years, institutionalization, Body Mass Index (BMI), hypovisus, hearing loss, smoking status and alcohol intake were detected through home interview. To assess physical activity, participants were asked whether they took walks and/or did gardening every day. Data on eating habits were collected in 2003 (baseline) by questioning the participants about the regular intake (yes/no) of a list of foods. The statistical analyses showed that answers were substantially the same for the whole sample, suggesting all participants had the same eating habits [15].
2.3. Assessment of Physical Performance and Frailty Measures
The Short Physical Performance Battery (SPPB) was used to measure frailty via standing balance, walking and chair stand tests [21]. Standing balance tests included tandem, semi tandem and side-by-side stands. To test walking speed, each participant was timed for two walks on an 8-foot walking course. The faster of the two walks was used. The chair stand test assesses the ability to rise from a chair. Participants were asked to stand up from a chair one time. If successful, they were further requested to stand up and sit down five times as quickly as possible and were timed from the initial sitting position to the final standing position at the end of the fifth stand. Performance categories were created for each set of performance measures to permit analyses that included those unable to perform a task. For the 8-foot walk and repeated chair stands, those who could not complete the task were assigned a score of 0. Those completing the task were assigned scores of 1–4 corresponding to the quartiles of time needed to complete the task, with the fastest times scored as 4. The three tests of standing balance were considered as hierarchical in difficulty and assigned a single score of 0–4 for standing balance. A summary performance scale was created by adding up the category scores for walking, chair stand and balance tests [21]. The physical frailty index encompassed the chair stand and walking speed tests [22]. In particular, a severe frailty state was defined when the subject was unable to stand up from a chair and showed a walking speed slower than 0.6 m/s (2.16 km/h). Moderate frailty-state was when the subject showed impairment in one of the two tests. A robust (non-frail) state was defined when the subject was able to stand up from a chair without the use of the arms and showed a walking speed faster than 0.6 m/s.
Physical strength was also measured via handgrip using a Collins dynamometer (Witte GMBH Chirurgische Instrumente, Adult Size, 12.7 × 5.7 cm, Solingen, Germany), lifting a 2 kg weight over the head and taking the average of three assessments with the stronger hand [17]. Physical frailty was also measured through carrying out other exercises with the hands such as lifting a weight of 2 kg above the head using both arms and also bringing both hands behind the head and behind the shoulders [15]. Weight lifting was classified as: (1) not lifted; (2) lifted to the height of the shoulder; (3) lifted to the height of the head; or (4) lifted over the head. Disability was measured using the Activities of daily living (ADL) and Instrumental activities of daily living (IADL) score according to Lawton and Brody (1969) [23].
2.4. Assessment of Chronic Disease and Other Covariates
In-person interviews and examination of medical records provided information on the history of chronic diseases such as cerebrovascular diseases, chronic renal insufficiency, tumors and others (Table 1). Using this information, a Charlson comorbidity index (CCI) [24] as well as a Disease count index (DCI) [25] were determined. DCI was obtained by summing up the number of coexisting diseases for each subject.

Table 1.
Characteristic of TRELONG study population at the beginning of the study/at the first wave n = 613.
The presence of cognitive decline was measured globally using the Mini-Mental State Examination (MMSE), range 0/30 [18]. Raw scores were corrected for age and education. Depression was assessed with the Geriatric Depression Scale (GDS) [18].
2.5. Biochemical Measurements
A fasted morning blood was collected at home from each participant and transported to the Clinical Chemistry Laboratory of Treviso Hospital, within 30 min before 9.00 a.m. (12h fasting) as previously described [15]. Briefly, to evaluate the general health status of participants, routine hematological and clinical chemistry tests were performed immediately using standard laboratory methods. Serum, plasma (tubes containing anticoagulant Na-citrate) and whole blood (tubes containing anticoagulant K2 EDTA) were prepared, and blood leukocytes were isolated to be stored in the biobank of the Transfusional Center Unit.
Serum biochemical markers, such as albumin, total and high-density lipoprotein (HDL)-Cholesterol, creatinine, etc., were determined by commercial methods (Abbot Laboratories, Abbot Park, IL, USA) on an Aeroseth Analyzer. Hematologic indices were assessed with the ADVIA 120 Hematologic System (Siemens Healthcare Diagnostics, Deerfield, IL, USA). Coagulation tests were performed using a plasma-citrate CA-7000 (Sysmex Corporation, Kobe, Japan) and erythrocyte sedimentation rate (ESR) using an automatic analyzer Test 1 TH (Alifax S.p.A, Polverara, Italy). Immunoglobulins and high sensitivity C-reactive protein (hsCRP) in serum were measured with Behring Nephelometer Analyzer II (BNA) (SimensHealtcare Diagnostics, Deerfield, IL, USA). Plasma interleukin-6 (IL-6) [19] was quantified using ELISA (UltraSensitive ELISA kit, BioSource, San Diego, CA, USA). The minimum detectable amount was 0.10 pg/mL. Plasma insulin-like growth factor-1 (IGF-1) was quantified using ELISA (Nonextraction IGF-1 ELISA kit, Diagnostic Systems Laboratories, Webster, TX, USA), with a minimum detectable amount of 10 ng/mL.
2.6. DNA Extraction from Mononuclear Cells (PBMC)
Fasting peripheral blood samples (30 mL) were collected by venipuncture; one aliquot was used to separate mononuclear cells (PBMC) by a standard Ficoll centrifugation procedure as previously described [15,19]. PBMC pellets were washed with ice-cold PBS, divided into aliquots and stored at −80 °C for further analysis. Genomic DNA was extracted from PBMC pellet using a vacuum-based semi-automated nucleic acid extractor (AB6100, Applied Biosystems, Foster City, CA, USA), checked for concentration by a UV-spectrophotometer (Eppendorf, Hamburg, Germany) and stored at −20 °C. Samples of genomic DNA available derived from the three waves were sent to the Laboratory of Environmental Mutagenesis and Genomic—Occupational Medicine, Department of Cardiac, Thoracic, and Vascular Sciences and Public Health, University Hospital of Padua, for the analysis of leukocyte telomere length (LTL), with a file reporting all information regarding qualitative (260/280 nm) and quantitative (ng/μL) description and volume of each sample (μL).
2.7. APOE Genotyping
APOE genotype was determined by PCR using the following primers: for 5_-tcggccgcagggcgctgatgg-3_; rev 5_-ctcgcgggcccccggccccggcctggta-3_; the resulting 332-bp PCR product was digested with CfoI (Roche, Basel, Switzerland) as previously described [26]. Briefly, the APOE genotype was then assessed after loading the corresponding enzymatic digestions on a capillary electrophoresis unit alongside with standard reference marker to allow the instrumental lining-up (Agilent Technologies, Santa Clara, CA, USA).
2.8. Leukocyte Telomere Length (LTL) Analysis
LTL in genomic DNA was appraised by the real-time quantitative polymerase chain reaction (PCR) as previously described [14,27] according to the reference method [28]. In brief, this method measures the relative LTL by estimating in genomic DNA the proportion of telomere repeat copy number (T) in relation to a single copy gene (S) (T/S ratio). T/S ratio is therefore the unit of measurement of LTL. The single-copy gene employed in this investigation was the human β-globin (hbg) [27]. A “six-point” reference curve was built from a serially diluted DNA pool, varying from 15 to 0.47 ng in each plate, in order to calculate the relative quantities of T and S in ng of samples to be examined. The DNA pool was realized by taking an aliquot of genomic DNA from samples at random designated and derived from the three waves: baseline collection (T0) and consecutive collection after a 7-years (T1) and 10-years (T2) follow up. All samples and standards were analyzed in triplicate and the average of the 3 T/S ratio measurements was considered in the statistical analyses. In brief, Qiagility (QIAGEN, Milano, Italy), which enables a high-precision PCR set up, was used for transferring 10 μL of reaction mix and 5 μL of DNA (5 ng/μL) in a 96-well plate. All PCR reactions were performed on a SteponePlus Real-Time PCR System (Applied Biosystems, Monza, Italy). A primer pair for a beta-globin single copy gene (hbgu and hbgd) [14], as well as a telomere primer pair (telg and telc) as described in Cawthon [28], were used in the reaction mix. The thermal cycling profile for both amplicons began with incubation at 95 °C for 10 min to activate the AmpliTaq DNA polymerase. For telomere PCR, activation was followed by 2 cycles of 15 s at 95 °C, 15 s at 49 °C, and 35 cycles of 15 s at 95 °C, which was then followed by 10 s at 62 °C, and 15 s at 74 °C. For hbg, activation was followed by 35 cycles of 15 s at 95 °C and 1 min at 58 °C. LTL was analyzed in triplicate for all samples of genomic DNA available and replicated on different days. The average of the three T measurements was divided by the average of the three S measurements to calculate the average T/S ratio, i.e., the relative telomere length. A measure was considered acceptable if the standard deviation among triplicate measures was <0.25. The coefficient of variation for the average T/S ratio of samples analyzed over three consecutive days was <9%, which was similar to the reproducibility originally reported for this method.
2.9. Statistical Analysis
The distribution of LTL at the first wave (2003) according to characteristics of the study population was appraised with Mann–Whitney U Test and Kruskal–Wallis test.
2.9.1. Dependence of LTL on Age
Considering the subgroup of n = 162 subjects with available three telomere measurements (in 2003, 2010, 2013), we used the Friedman test to compare the longitudinal distribution of the LTL at the three waves.
Since LTL could be measured more than once on the same person (repeated measures taken over time) to account for both within-person and across-person variability, we used the linear mixed model to appraise the influence of chronological age and gender (independent variables) on the logarithmically-transformed LTL (dependent variable), since the telomere distribution was non-Gaussian. The addition of the random effects for each subject was useful to take into account the individual heterogeneity and the dependence between the measurements made on the same subject.
2.9.2. Survival Analysis: Kaplan–Meier Curves
Survival analysis was performed using the non-parametric Kaplan–Meier curves analysis on all 613 subjects. The survival time was calculated for each person starting from the date of the interview of the year corresponding to the first LTL measurement available until the date of death or end of follow-up (25 June 2019). The survival curves were estimated stratifying the subjects in two groups based on the median LTL, or in four groups based on the quartiles (1° quartile: 0.46 ≤ LTL < 1.57; 2° quartile: 1.57 ≤ LTL < 2.11; 3° quartile: 2.11 ≤ LTL < 2.86; 4° quartile: 2.86 ≤ LTL < 7.44). The difference between survival curves was estimated by the log-rank test and the Peto–Peto log-rank test, which is preferable when there are intersections among the survival curves.
2.9.3. Survival Analysis: Cox Proportional Hazards Model
To evaluate the effect of LTL on survival adjusting for other factors, a semi-parametric Cox proportional hazards model was constructed using the same sample of 613 subjects. Before, the adequacy of the proportionality assumption of risks was assessed by analyzing the Schoenfeld residues. The analysis of the Schoenfeld residuals gave a non-significant result (p = 0.92) for each covariate and for the model as a whole; therefore, the hypothesis of proportionality of the risks was accepted. Based on the relevant literature, gender, age, CCI and SPPB were used as covariates besides LTL in the Cox regression model. For the interpretation of the estimates, the hazard ratios of each variable were reported in the tables. Since LTL varied over time and there was more than one measurement for some subjects over the three waves, we used an extension of the Cox model that allows for the inclusion of time-dependent variables by the technique of episode splitting.
This model was further improved considering for all 613 subjects a statistical weight computed so that the TRELONG study population was representative per gender and age of the Italian population over seventy.
2.9.4. Characterization of Subjects with Elongated Telomeres
To find the factors associated with an increase in LTL in all 613 subjects, we created a dummy variable, which was 1 if the subject had an increase in LTL between the first (2003) and the second (2010) waves or between the second (2010) and the third (2013) waves and 0 in absence of elongation. This was the outcome variable of a logistic regression model where the predictors were the variables reported in Table 1. A stepwise approach based on Rao’s score tests was applied to eliminate non-significant variables from the model.
Results were considered significant when a p-Value < 0.05 was obtained. The analyses were performed using the statistical software R version 3.6.3 [29].
3. Results
3.1. Descriptive Results
3.1.1. Characteristic of Population at the Baseline and LTL Distribution
Table 1 reports the main TRELONG variables/characteristics at the time of baseline in 2003 (clinical, lifestyle and demographic information acquired through questionnaires, physical examination, and laboratory tests).
The distribution of LTL (2003) according to the characteristics of the study population is reported in Table 2. LTL decreased (p < 0.05) with age, institutionalization, hearing loss, moderate and severe physical frailty and presence of chronic diseases (DCI and CCI). LTL increased (p < 0.05) with increasing physical activity, better cognitive profile (MMSE score > 26), greater functional autonomy (ADL score = 6 and IADL score = 8), better physical performance (SPPB score ≥ 6 and effective placement of one’s hands behind the shoulders) and physical strength (hand gripscore ≥ 12.2 kg and full lifting of the weight of 2 kg above head). Furthermore, LTL increased with blood chemistry variables such as albumin, hemoglobin and insulin-like growth factor-1 (IGF-1).

Table 2.
Distribution of telomere length according to characteristics of the study population.
3.1.2. LTL and Age in the Longitudinal Study
Figure 1 shows the distribution of LTL in the subgroup of 162 subjects in the three waves. A reduction in both mean and variance is observed, indicating a reduction in telomeres with increasing age. Friedman’s test in fact leads to the rejection of the null hypothesis of equality on the average in the three groups (p < 0.001).
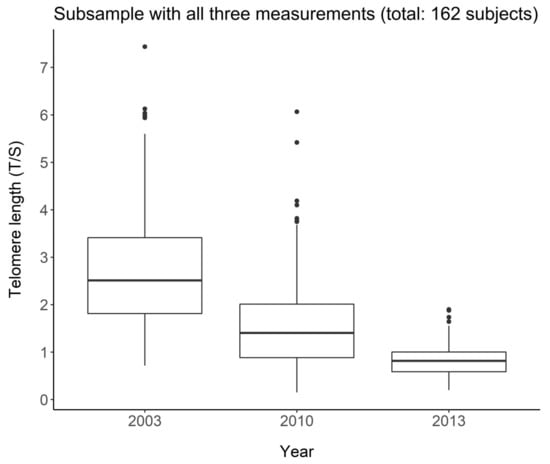
Figure 1.
Telomere length change over the three waves in the group of 162 subjects with all three measurements.
Table 3 shows the results of the mixed linear model on the logarithmically transformed LTL. The gender variable was removed from the model because it was not significant (p = 0.594). The estimated model indicates that for each additional year of age, LTL dropped by 7.96% (p < 0.001). The average trend estimated by the model on the original scale is shown in Figure 2. The residuals of the estimated model satisfy the assumption of normality according to the Shapiro–Wilk test (p = 0.064).

Table 3.
Estimated mixed linear model on the logarithmically transformed LTL (table of fixed effects).
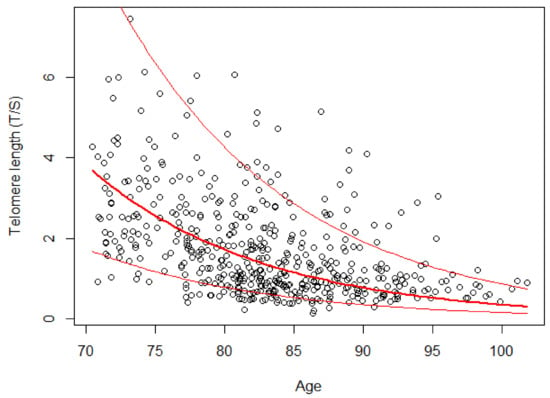
Figure 2.
Telomere length versus age in the group of 162 subjects. The red lines indicate the random-effects model estimated on a logarithmic scale and the range of variation due to the addition of the individual random effects.
3.1.3. LTL and Survival: Kaplan–Meier Curves
Figure 3 shows that the group with longer LTL (T/S > 2.11; curve in blue) had a consistently higher survival than the group with shorter LTL (T/S ≤ 2.11; curve in green). The median survival time, (corresponding to the survival time of 50% of subjects in each group), was 7.57 years for subjects with telomeres longer than 2.11 (T/S) and only 5.41 years for subjects with telomeres equal or shorter than 2.11 (T/S). The two adjacent 95% confidence interval limits did not overlap for most of the curve. The difference between the two survival curves was significant using both the log-rank test (p < 0.001) and the Peto–Peto log-rank test (p < 0.001), which was better because a slight intersection between the two survival curves was observed in the first period (see Figure 3).
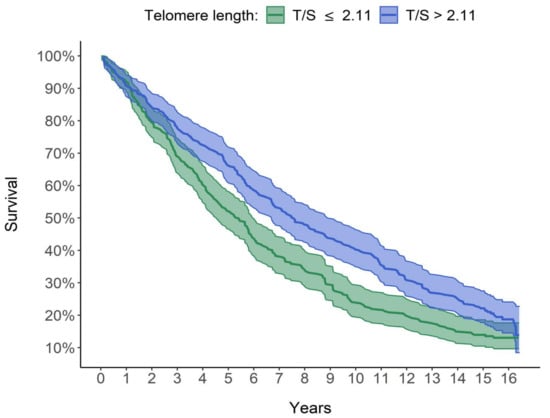
Figure 3.
Graphic comparison of the survival of the 2 groups. Survival curves estimated using Kaplan–Meier method. The median survival time is 5.41 years for subjects with T/S ≤ 2.11 and 7.57 years for subjects with T/S > 2.11.
Figure 4 shows that the median survival time was 4.67, 5.87, 6.73 and 8.79 years, respectively, for LTL quartiles 1 to 4 (1 ≥ 0.46 and <1.57; 2 ≥ 1.57 and <2.11; 3 ≥ 2.11 and <2.86; 4 ≥ 2.86 and <7.44). The Peto–Peto log-rank test rejected the hypothesis of equality between the survival curves in the four groups (p <0.001). The median survival time was over 4 years longer in the group with the longest telomeres (in purple in Figure 4) than in the group with the shortest telomeres (in red in Figure 4). In conclusion, there was an ever-increasing dependence between LTL and estimated survival: the longer the LTL, the longer the survival and therefore the lower the estimated mortality risk.
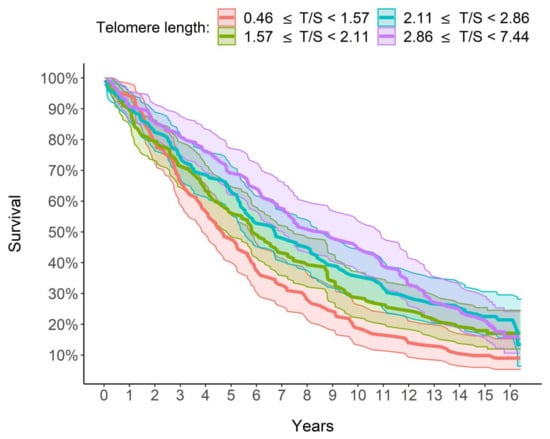
Figure 4.
Graphic comparison of the survival of the 4 groups/quartiles. Survival curves were estimated using Kaplan–Meier method. From the group with the shortest LTL to the one with the longest LTL, the median survival time is, respectively, 4.67, 5.87, 6.73 and 8.79 years.
3.2. Outcome Results
3.2.1. Determinants of Survival
Table 4 shows the results of the semi-parametric Cox proportional hazards model, estimated with the episode splitting technique. It can be seen that LTL had a significant effect on survival even in a multivariate analysis taking into account the effect of other factors such as age, gender, the CCI and the SPPB. In fact, all the variables included in the model (age, LTL, gender, CCI, SPPB) were very significant (p < 0.001). The coefficient associated with LTL and SPPB had a negative sign, indicating a protective effect of these variables with respect to mortality risk, while the sign was positive for other variables (age, gender (male), CCI), indicating an increase in risk. The interpretation of the hazard ratios for each variable can be the following: (1) age, for each additional year of the subject, there is an increase of 7.75% in the risk of mortality, all other factors being equal; (2) LTL, for each unit increase in the LTL, the risk of mortality decreases by 16.99% with the other explanatory variables being equal, age included; (3) Charlson index score, for each unit increase in the index, the risk of mortality increases by 9.65%, all other factors being equal; (4) SPPB index score, for each unit increase of the index, the mortality risk, all other factors being equal, is reduced by 10.01%; (5) gender, males have a 73.16% higher risk of mortality, all other factors being equal, compared to females.

Table 4.
Determinants of survival. Estimated semi-parametric Cox’s proportional hazards model for survival time.
Table 5 shows that after re-weighting the sample, the significant effects of all the explanatory variables did persist and the interpretation remained substantially the same. However, in this second model, the protective effect of LTL was slightly stronger (22.41% decrease per unit increase in the risk of mortality), while the protective effect of SPPB was slightly weaker (8.78% decrease per unit increase). Risk factors such as age (8.5% increase for each additional year), gender (74.99% higher risk for males) and CCI (16.57% growth per unit increase) all appeared to have a stronger effect when compared with those obtained from the model on the unweighted sample (Table 4).

Table 5.
Determinants of survival. Estimated semi-parametric Cox’s proportional hazards model for survival-time with episode splitting and weighed sample.
3.2.2. Determinants of LTL Elongation
Out of 613 subjects, overall 73 subjects (11.9%) presented an increase in LTL: they were 38 in the first period (2003–2010) and 35 in the second period (2010–2013). No subject registered an increase during the whole observation period (2003–2013).
Table 6 reports the results of fitting of logistic regression model based on Rao’s score test. Male gender was a risk factor for telomere attrition (OR = 0.4388; p = 0.0143) along with increase in the DCI values (OR = 0.6912; p = 0.0066). By contrast, SPPB scores (OR = 1.1542; p = 0.0066) and years of education (OR = 1.0958; p = 0.0065) were determinant of telomere elongation.

Table 6.
Results from logistic regression model exploring the relationship between telomere elongation (dichotomous variable) and characteristics of the study population. Model selection based on Rao test (n = 613).
4. Discussion
4.1. LTL and Survival
A key finding from our study is the steadily increasing relationship between LTL and survival: the longer the LTL, the greater the survival and, therefore, a lower mortality risk is estimated. In particular, as shown in Figure 3, the group with the longest quartile LTL (T/S >2.86) had 8.79 median years of survival, which is over 4 years higher than that of the group with the shortest quartile LTL (<1.57 T/S). Furthermore, in a multivariate analysis taking into account the effect of other factors, LTL increased survival and therefore decreased mortality. In detail, for each unit increase in the LTL, the risk of mortality decreased by 22.41%, with the other explanatory variables being equal, age included (Table 5).
LTL has often been related to life expectancy, but contradictory results have been reported in older adults. Whereas some studies have reported that LTL attrition is a marker of mortality [30,31,32,33], others have not found such a relationship [34,35,36,37], making the relationship between LTL and survival in humans uncertain. Those authors that did not find this association mentioned variation in methods of LTL measurements [35] and variations among investigated populations, including a short age range (>75 years) [30,35,37] and diseases examined, where the elongation of telomeres could be a characteristic of the disease [36].
4.2. Determinants of Survival
Key survival determinants were not only LTL but also SPPB: for each unit increase in the index, the mortality risk was reduced by 8.78%, considering all other factors being equal, age included (Table 5). It is known that physical activity is correlated with reduced risks of all cause and cause-specific mortality [38]. Furthermore, a prospective cohort study showed that middle-aged and older adults achieved considerable longevity advantages by becoming more physically active, regardless of past physical activity [39]. In our study, we used the SPPB that may be more likely to capture the real health status of older persons. This test, with three different evaluations (walking speed, chair stand and balance time) [21,40], was thought an extremely sensitive indicator of global health status or vulnerability, revealing numerous underlying physiological disorders [41]. Our results are in line with one of the most complete meta-analyses with a proper sample size that definitively discovered the association between SPPB score and all-cause mortality [42].
On the other hand, the male gender, CCI and age were factors that threatened the survival, with a risk of mortality growing by 74.99% for males, by 16.57% per unit increase in CCI and by 8.5% for each additional year of age (Table 5). These data confirmed the current literature on gender inequity: men achieve higher mortality rates than women [43]. Human females’ longevity benefit may derive from the hormonal effect on inflammatory and immunological reactions [44]. CCI is a validated, simple and easily applicable method for appraising the risk of death and has been broadly applied as a predictor of long-term prognosis and survival [24,45]. In our study, CCI score gave an HR of 1.2 (Table 5) similar to that reported by Fraccaro et al. [46]; i.e., 1.3 HR in an adult cohort study from Salford (UK) from 2005 to 2014.
4.3. LTL in the Longitudinal Study
LTL distribution at the baseline in 2003 showed that LTL attrition was related to age (Table 2). Another key finding, by analyzing LTL at the three waves in the subgroup of 162 subjects, was LTL drop by 7.96% for each additional year of age (Table 3). The rate of LTL shortening we found was similar to that observed in the longitudinal population-based Bruneck Study (age 45–85 years), in which the telomeres lost on average 455 bp in length after 10 years, corresponding to an average loss of 45.5 bp per year that is equal to about 10% [47]. An overall lesser decrease (13.6% after 7 years follow-up) than our study was, however, found in a cohort study of 516 subjects aged 65–106 from Southern Italy [48]. This could be attributed to the major number of centenarians in whose LTL may be expected to increase after 90 years [49]. Lastly, our LTL drop is comparable to the 7.6% decrease in LTL reported by McCracken et al. [50] associated with an annual increase in air pollution. It could be speculated that our decrease in LTL may be attributed to the air pollution of the Veneto region, being part of the Pianura Padana, one of the most polluted regions in Europe.
Another noteworthy key finding of our study was the paradoxical increase in LTL in almost 12% of the study population. This finding, with almost exactly the same percentage, has been previously reported [34]. Loss of telomeric DNA caused by deterioration or incomplete replication seems to be balanced by telomere elongation, which involves the telomerase, a DNA polymerase that synthesizes TTAGGG repeats, inducing the de novo synthesis of additional repeats. This specific reverse transcriptase complex counters the replication-associated telomere shortening with the addition of G-rich telomeric repeats to the chromosome ends, thus efficiently stabilizing TL [51]. That the gain in LTL is merely a consequence of a high degree of measurement error can be rejected by the accurate quality controls with measurements in triplicate of T/S ratios that resulted in a standard deviation < 0.25 and with a coefficient of variation (CV), on average, less than 9%, in samples analyzed during three successive days, which is comparable to the reproducibility formerly described for this method [28].
4.4. Determinants of LTL Elongation
Among determinants eliciting LTL elongation were SPPB and years of education, while being male and DCI were factors that contributed to reduced LTL. Until now, few studies have explored the association between LTL and SPPB, with conflicting results. Pereira et al. [52], in a study with a small number of subjects, reported unexpectedly that shorter LTL was correlated with better physical capability evaluated by SPPB score. No association was found in a cohort of 136 patients aged 45–85 years with knee osteoarthritis pain, in which the SPPB test is considered a clinical measure for assessing physical function [53]. We can assert that, in general, in observational studies, greater physical activity or exercise was associated with longer LTL in various populations, whereas in a small number of longitudinal studies in which exercise interventions were performed to investigate the potential effect on LTL, such a relationship was not fully established [54]. It has been reported that physical exercise, affecting the balance between oxidative stress and antioxidants and altering methylation patterns, buffers LTL shortening [54]. Furthermore, physical training increases telomerase activity in myocytes and circulating mononuclear cells in rats [55] and in human leukocytes [55], thus providing another possible and potentially testable pathway to clarify how physical activity protects LTL [56].
The positive relationship between LTL and years of education agrees with previous findings on a positive association between educational attainment and LTL [57,58]. The implication of this finding suggests that education confers health benefits by limiting biological aging. Education, in fact, is one of the leading determinants of overall social status over the course of a person’s lifetime and can reduce exposure to chronic stress and/or increase one’s capacity to effectively deal with potential threats or stressors [59].
Instead, the role of the male sex as a negative factor for telomere elongation is consistent with the majority of previous cross-sectional and longitudinal studies establishing that women have longer telomeres than men [60] and that the rate of LTL shortening is slower in women than men [60]. Several realistic biological reasons can be formulated to elucidate this, including the action of estrogens [61], which can promote the production of telomerase [62] and protect against damage caused by reactive oxygen species [63].
Convincing evidence in the literature suggests the association between shorter LTL and several age-related diseases (such as cardiovascular diseases, neurodegenerative diseases, type 2 diabetes mellitus, premature aging syndromes and cancers) [30,64,65]. Therefore, it is not surprising that the concomitant existence of a higher number of diseases, indicated by a higher DCI, is a risk factor that contributes to reduce LTL.
4.5. Strengths and Limitations
Whether LTL can be considered a predictor for longevity and healthy life is one of the most questioned issues in the field of gerontological research. Problems in results’ interpretation stem from the heterogeneity of factors influencing LTL, including genetics and environment, and in particular from the available follow-up data for both LTL and survival for recruited samples.
Our study was conducted in Treviso, in Veneto, a region of North-East Italy, which is characterized by the highest longevity, in particular among women [2]. Prevalence of the APOEε4 allele in Treviso is equal to 16.3%, which is lower than the 25% mostly observed for northern European populations without dementia [26], and this can explain, at least in part, the greater longevity of women in the Treviso area. It should be noted that the Northern population, including Venetians, represents a quite homogeneous population from the genetics point of view, which is different from other populations studied so far in Italy. In the ancestors of Northern Italians, climate-related selective pressures have influenced an adaptive evolution that make Northern Italian people less prone to develop accelerating aging disorders, including diabetes type 2 and obesity [66].
In our study, we determined LTL during ten-year follow up in the prospective cohort “Treviso Longeva (TRELONG)”, a very well-characterized study population. In particular, this is a systematic sample of subjects aged 70–106 years, drawn from the list of residents of the municipality of Treviso. Therefore, the study sample is highly representative of the community and of the real world [15]. Furthermore, the cohort is well characterized with several types of information, including physiological variables and lifestyles, physical performance and frailty measures, assessment of chronic disease and other covariates besides biochemical measurements and APOE genotyping [26].
We used the q-RT PCR technique to assess LTL in triplicate. This fast and highly sensitive method requires relatively small quantities of DNA and allows high throughput. Since an almost totally automated workflow from DNA extraction to LTL analysis was used, pre-analytical factors and analytical factors did not vary amongst analyses and the same pool DNA was used as a reference standard in all q-RT PCR reactions. In this way, the interbach variation was under control, minimizing measurement error and warranting a CV of less than 9%.
Moreover, in this study, the start date of the survey was the day of the interview rather than the date of birth so that the measurements relating to the lengths of the telomeres of the interviewees who had very different ages were comparable. In this case, then, the duration of the episode indicates the remaining survival time for the individual with that LTL.
Another strong point is that the Cox model estimate for time-dependent variables was further improved considering that for all 613 subjects a statistical weight was computed so that the TRELONG study population was representative per gender and age of the Italian population over seventy. Therefore, the results could be more easily extended to the entire population over seventy at the national level. It should be noted, however, that while the sample of the present study was found in a single city, a sample detected at the national level would be affected by the different geographical distribution of the subjects recruited.
5. Conclusions
Our results demonstrate that LTL could be a powerful tool for predicting survival in our elderly population. By identifying SPPB as a key survival determinant, our study suggests that the regular application of the SPPB in clinical practice may offer helpful prognostic information regarding the risk of all-cause mortality. This study also confirms that male gender, CCI, and, therefore chronological age, are threats for survival.
Furthermore, by identifying SPPB scores and years of education as positive and DCI as a negative determinant of LTL elongation, we recognized factors on which to converge to improve prevention strategies.
Author Contributions
Conceptualization, S.P. and M.G.; methodology, S.P., M.C., A.G., G.M., E.D., A.V. and M.G.; software, A.G. and G.M.; validation, S.P., M.C., A.G., G.M., E.D., A.V. and M.G.; formal analysis, S.P., M.C., A.G., G.M. and M.G.; investigation, S.P., M.C., A.G., G.M., E.D., A.V. and M.G.; resources, S.P.; data curation, S.P., M.C., A.G., G.M. and M.G.; writing—original draft preparation, S.P., M.C., A.G., G.M. and M.G.; writing—review and editing, S.P., M.C., A.G., G.M., E.D., A.V. and M.G.; visualization, S.P., M.C., A.G., G.M., E.D., A.V. and M.G.; supervision, S.P.; project administration, S.P. and M.G.; funding acquisition, S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by BIRD, grant number 175721.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethical Committee of the National Institute on Research and Care of the Elderly (INRCA, Italy, prot. n. 50/02 on 22 March 2002).
Informed Consent Statement
Informed consent was acquired from all participants involved in the study.
Data Availability Statement
The data presented in this study are obtainable on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
Activities of daily living (ADL); Apolipoprotein E (APOE; Body Mass Index (BMI); Charlson Comorbidity Index (CCI); Disease Count Index (DCI); Erythrocyte sedimentation rate (ESR); Geriatric depression scale (GDS); High-sensitivity C-reactive protein(hs-CRP); High-density lipoprotein (HDL) cholesterol; Instrumental activities of daily living (IADL); Insulin-like growth factor-1 (IGF-1); Interleukin-6 (IL-6); Leukocyte telomere length LTL; Low-density lipoprotein (LDL); Mini Mental State Examination (MMSE); Short physical performance battery (SPPB); Treviso Longeva (TRELONG).
References
- Chang, A.Y.; Skirbekk, V.F.; Tyrovolas, S.; Kassebaum, N.J.; Dieleman, J.L. Measuring Population Ageing: An Analysis of the Global Burden of Disease Study 2017. Lancet Public Health 2019, 4, e159–e167. [Google Scholar] [CrossRef] [Green Version]
- Lipsi, R.M. Evoluzione Della Geografia Della Mortalità in Italia: Tavole Provinciali e Probabilità di Morte Per Causa. Anni: 1971–1973, 1981–1983, 1991–1993; Fonti e Strumenti: Rome, Italy, 2002. [Google Scholar]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [Green Version]
- Fulop, T.; Larbi, A.; Witkowski, J.M.; McElhaney, J.; Loeb, M.; Mitnitski, A.; Pawelec, G. Aging, Frailty and Age-Related Diseases. Biogerontology 2010, 11, 547–563. [Google Scholar] [CrossRef]
- Levine, M.E. Modeling the Rate of Senescence: Can Estimated Biological Age Predict Mortality More Accurately than Chronological Age? J. Gerontol. A. Biol. Sci. Med. Sci. 2013, 68, 667–674. [Google Scholar] [CrossRef] [Green Version]
- Peters, A.; Nawrot, T.S.; Baccarelli, A.A. Hallmarks of Environmental Insults. Cell 2021, 184, 1455–1468. [Google Scholar] [CrossRef]
- Blackburn, E.H.; Epel, E.S.; Lin, J. Human Telomere Biology: A Contributory and Interactive Factor in Aging, Disease Risks, and Protection. Science 2015, 350, 1193–1198. [Google Scholar] [CrossRef] [Green Version]
- Pavanello, S.; Angelici, L.; Hoxha, M.; Cantone, L.; Campisi, M.; Tirelli, A.S.; Vigna, L.; Pesatori, A.C.; Bollati, V. Sterol 27-Hydroxylase Polymorphism Significantly Associates With Shorter Telomere, Higher Cardiovascular and Type-2 Diabetes Risk in Obese Subjects. Front. Endocrinol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Pavanello, S.; Stendardo, M.; Mastrangelo, G.; Casillo, V.; Nardini, M.; Mutti, A.; Campisi, M.; Andreoli, R.; Boschetto, P. Higher Number of Night Shifts Associates with Good Perception of Work Capacity and Optimal Lung Function but Correlates with Increased Oxidative Damage and Telomere Attrition. BioMed Res. Int. 2019, 2019, e8327629. [Google Scholar] [CrossRef] [Green Version]
- Pavanello, S.; Stendardo, M.; Mastrangelo, G.; Bonci, M.; Bottazzi, B.; Campisi, M.; Nardini, M.; Leone, R.; Mantovani, A.; Boschetto, P. Inflammatory Long Pentraxin 3 Is Associated with Leukocyte Telomere Length in Night-Shift Workers. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Pavanello, S.; Hoxha, M.; Dioni, L.; Bertazzi, P.A.; Snenghi, R.; Nalesso, A.; Ferrara, S.D.; Montisci, M.; Baccarelli, A. Shortened Telomeres in Individuals with Abuse in Alcohol Consumption. Int. J. Cancer 2011, 129, 983–992. [Google Scholar] [CrossRef] [Green Version]
- Pavanello, S.; Pesatori, A.-C.; Dioni, L.; Hoxha, M.; Bollati, V.; Siwinska, E.; Mielzyńska, D.; Bolognesi, C.; Bertazzi, P.-A.; Baccarelli, A. Shorter Telomere Length in Peripheral Blood Lymphocytes of Workers Exposed to Polycyclic Aromatic Hydrocarbons. Carcinogenesis 2010, 31, 216–221. [Google Scholar] [CrossRef] [Green Version]
- Pavanello, S.; Carta, A.; Mastrangelo, G.; Campisi, M.; Arici, C.; Porru, S. Relationship between Telomere Length, Genetic Traits and Environmental/Occupational Exposures in Bladder Cancer Risk by Structural Equation Modelling. Int. J. Environ. Res. Public. Health 2018, 15, 5. [Google Scholar] [CrossRef] [Green Version]
- Pavanello, S.; Campisi, M.; Mastrangelo, G.; Hoxha, M.; Bollati, V. The Effects of Everyday-Life Exposure to Polycyclic Aromatic Hydrocarbons on Biological Age Indicators. Environ. Health 2020, 19, 1–13. [Google Scholar] [CrossRef]
- Gallucci, M.; Ongaro, F.; Bresolin, F.; Bernardi, U.; Salvato, C.; Minello, A.; Amici, G.P.; Barasciutti, E.; Mazzuco, S.; Gajo, G.B.; et al. The Treviso Longeva (Trelong) Study: A Biomedical, Demographic, Economic and Social Investigation on People 70 Years and over in a Typical Town of North-East of Italy. Arch. Gerontol. Geriatr. 2007, 44, 173–192. [Google Scholar] [CrossRef] [PubMed]
- Gallucci, M.; Antuono, P.; Ongaro, F.; Forloni, P.L.; Albani, D.; Amici, G.P.; Regini, C. Physical Activity, Socialization and Reading in the Elderly over the Age of Seventy: What Is the Relation with Cognitive Decline? Evidence from “The Treviso Longeva (TRELONG) Study”. Arch. Gerontol. Geriatr. 2009, 48, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Gallucci, M.; Ongaro, F.; Meggiolaro, S.; Antuono, P.; Gustafson, D.R.; Forloni, G.L.; Albani, D.; Gajo, G.B.; Durante, E.; Caberlotto, L.; et al. Factors Related to Disability: Evidence from the “Treviso Longeva (TRELONG) Study”. Arch. Gerontol. Geriatr. 2011, 52, 309–316. [Google Scholar] [CrossRef]
- Gallucci, M.; Mazzuco, S.; Ongaro, F.; Di Giorgi, E.; Mecocci, P.; Cesari, M.; Albani, D.; Forloni, G.L.; Durante, E.; Gajo, G.B.; et al. Body Mass Index, Lifestyles, Physical Performance and Cognitive Decline: The “Treviso Longeva (Trelong)” Study. J. Nutr. Health Aging 2013, 17, 378–384. [Google Scholar] [CrossRef]
- Albani, D.; Mazzuco, S.; Chierchia, A.; Fusco, F.; Boeri, L.; Martines, R.; Giorgi, E.D.; Frigato, A.; Durante, E.; Caberlotto, L.; et al. The SIRT1 Promoter Polymorphic Site Rs12778366 Increases IL-6 Related Human Mortality in the Prospective Study “Treviso Longeva (TRELONG)”. Int. J. Mol. Epidemiol. Genet. 2015, 6, 20–26. [Google Scholar]
- Gallucci, M.; Pallucca, C.; Di Battista, M.E.; Fougère, B.; Grossi, E. Artificial Neural Networks Help to Better Understand the Interplay Between Cognition, Mediterranean Diet, and Physical Performance: Clues from TRELONG Study. J. Alzheimers Dis. 2019, 71, 1321–1330. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A Short Physical Performance Battery Assessing Lower Extremity Function: Association with Self-Reported Disability and Prediction of Mortality and Nursing Home Admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Gill, T.M.; Baker, D.I.; Gottschalk, M.; Peduzzi, P.N.; Allore, H.; Byers, A. A Program to Prevent Functional Decline in Physically Frail, Elderly Persons Who Live at Home. Available online: https://www.nejm.org/doi/10.1056/NEJMoa020423 (accessed on 8 July 2021).
- Lawton, M.P.; Brody, E.M. Assessment of Older People: Self-Maintaining and Instrumental Activities of Daily Living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Colinet, B.; Jacot, W.; Bertrand, D.; Lacombe, S.; Bozonnat, M.-C.; Daurès, J.-P.; Pujol, J.-L. A New Simplified Comorbidity Score as a Prognostic Factor in Non-Small-Cell Lung Cancer Patients: Description and Comparison with the Charlson’s Index. Br. J. Cancer 2005, 93, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, D.R.; Mazzuco, S.; Ongaro, F.; Antuono, P.; Forloni, G.; Albani, D.; Gajo, G.B.; Durante, E.; Caberlotto, L.; Zanardo, A.; et al. Body Mass Index, Cognition, Disability, APOE Genotype, and Mortality: The “Treviso Longeva” Study. Am. J. Geriatr. Psychiatry 2012, 20, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Pavanello, S.; Campisi, M.; Fabozzo, A.; Cibin, G.; Tarzia, V.; Toscano, G.; Gerosa, G. The Biological Age of the Heart Is Consistently Younger than Chronological Age. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Cawthon, R.M. Telomere Length Measurement by a Novel Monochrome Multiplex Quantitative PCR Method. Nucleic Acids Res. 2009, 37, e21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing. 2014. Available online: http://www.R-project.org/ (accessed on 17 August 2021).
- Cawthon, R.M.; Smith, K.R.; O’Brien, E.; Sivatchenko, A.; Kerber, R.A. Association between Telomere Length in Blood and Mortality in People Aged 60 Years or Older. Lancet 2003, 361, 393–395. [Google Scholar] [CrossRef]
- Rode, L.; Nordestgaard, B.G.; Bojesen, S.E. Peripheral Blood Leukocyte Telomere Length and Mortality Among 64 637 Individuals From the General Population. JNCI J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef] [Green Version]
- Mons, U.; Müezzinler, A.; Schöttker, B.; Dieffenbach, A.K.; Butterbach, K.; Schick, M.; Peasey, A.; De Vivo, I.; Trichopoulou, A.; Boffetta, P.; et al. Leukocyte Telomere Length and All-Cause, Cardiovascular Disease, and Cancer Mortality: Results From Individual-Participant-Data Meta-Analysis of 2 Large Prospective Cohort Studies. Am. J. Epidemiol. 2017, 185, 1317–1326. [Google Scholar] [CrossRef] [Green Version]
- Arbeev, K.G.; Verhulst, S.; Steenstrup, T.; Kark, J.D.; Bagley, O.; Kooperberg, C.; Reiner, A.P.; Hwang, S.-J.; Levy, D.; Fitzpatrick, A.L.; et al. Association of Leukocyte Telomere Length With Mortality Among Adult Participants in 3 Longitudinal Studies. JAMA Netw. Open 2020, 3, e200023. [Google Scholar] [CrossRef] [Green Version]
- Martin-Ruiz, C.M.; Gussekloo, J.; van Heemst, D.; von Zglinicki, T.; Westendorp, R.G.J. Telomere Length in White Blood Cells Is Not Associated with Morbidity or Mortality in the Oldest Old: A Population-Based Study. Aging Cell 2005, 4, 287–290. [Google Scholar] [CrossRef]
- Bischoff, C.; Petersen, H.C.; Graakjaer, J.; Andersen-Ranberg, K.; Vaupel, J.W.; Bohr, V.A.; Kølvraa, S.; Christensen, K. No Association between Telomere Length and Survival among the Elderly and Oldest Old. Epidemiol. Camb. Mass 2006, 17, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Houben, J.M.J.; Giltay, E.J.; Rius-Ottenheim, N.; Hageman, G.J.; Kromhout, D. Telomere Length and Mortality in Elderly Men: The Zutphen Elderly Study. J. Gerontol. A. Biol. Sci. Med. Sci. 2011, 66, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Svensson, J.; Karlsson, M.K.; Ljunggren, Ö.; Tivesten, Å.; Mellström, D.; Movérare-Skrtic, S. Leukocyte Telomere Length Is Not Associated with Mortality in Older Men. Exp. Gerontol. 2014, 57, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Veeranki, S.P.; Magnussen, C.G.; Xi, B. Recommended Physical Activity and All Cause and Cause Specific Mortality in US Adults: Prospective Cohort Study. BMJ 2020, 370, m2031. [Google Scholar] [CrossRef] [PubMed]
- Mok, A.; Khaw, K.-T.; Luben, R.; Wareham, N.; Brage, S. Physical Activity Trajectories and Mortality: Population Based Cohort Study. BMJ 2019, 365, l2323. [Google Scholar] [CrossRef] [Green Version]
- Pahor, M.; Guralnik, J.M.; Ambrosius, W.T.; Blair, S.; Bonds, D.E.; Church, T.S.; Espeland, M.A.; Fielding, R.A.; Gill, T.M.; Groessl, E.J.; et al. Effect of Structured Physical Activity on Prevention of Major Mobility Disability in Older Adults: The LIFE Study Randomized Clinical Trial. JAMA 2014, 311, 2387–2396. [Google Scholar] [CrossRef]
- Savino, E.; Volpato, S.; Zuliani, G.; Guralnik, J.M. Assessment of Mobility Status and Risk of Mobility Disability in Older Persons. Curr. Pharm. Des. 2014, 20, 3099–3113. [Google Scholar] [CrossRef]
- Pavasini, R.; Guralnik, J.; Brown, J.C.; di Bari, M.; Cesari, M.; Landi, F.; Vaes, B.; Legrand, D.; Verghese, J.; Wang, C.; et al. Short Physical Performance Battery and All-Cause Mortality: Systematic Review and Meta-Analysis. BMC Med. 2016, 14, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Owens, I.P.F. Ecology and Evolution. Sex Differences in Mortality Rate. Science 2002, 297, 2008–2009. [Google Scholar] [CrossRef]
- Austad, S.N.; Fischer, K.E. Sex Differences in Lifespan. Cell Metab. 2016, 23, 1022–1033. [Google Scholar] [CrossRef] [Green Version]
- Radovanovic, D.; Seifert, B.; Urban, P.; Eberli, F.R.; Rickli, H.; Bertel, O.; Puhan, M.A.; Erne, P.; AMIS Plus Investigators. Validity of Charlson Comorbidity Index in Patients Hospitalised with Acute Coronary Syndrome. Insights from the Nationwide AMIS Plus Registry 2002–2012. Heart 2014, 100, 288–294. [Google Scholar] [CrossRef] [Green Version]
- Fraccaro, P.; Kontopantelis, E.; Sperrin, M.; Peek, N.; Mallen, C.; Urban, P.; Buchan, I.E.; Mamas, M.A. Predicting Mortality from Change-over-Time in the Charlson Comorbidity Index: A Retrospective Cohort Study in a Data-Intensive UK Health System. Medicine 2016, 95, e4973. [Google Scholar] [CrossRef]
- Ehrlenbach, S.; Willeit, P.; Kiechl, S.; Willeit, J.; Reindl, M.; Schanda, K.; Kronenberg, F.; Brandstätter, A. Influences on the Reduction of Relative Telomere Length over 10 Years in the Population-Based Bruneck Study: Introduction of a Well-Controlled High-Throughput Assay. Int. J. Epidemiol. 2009, 38, 1725–1734. [Google Scholar] [CrossRef] [Green Version]
- Crocco, P.; De Rango, F.; Dato, S.; Rose, G.; Passarino, G. Telomere Length as a Function of Age at Population Level Parallels Human Survival Curves. Aging 2021, 13, 204–218. [Google Scholar] [CrossRef]
- Barbi, E.; Lagona, F.; Marsili, M.; Vaupel, J.W.; Wachter, K.W. The Plateau of Human Mortality: Demography of Longevity Pioneers. Science 2018, 360, 1459–1461. [Google Scholar] [CrossRef] [Green Version]
- McCracken, J.; Baccarelli, A.; Hoxha, M.; Dioni, L.; Melly, S.; Coull, B.; Suh, H.; Vokonas, P.; Schwartz, J. Annual Ambient Black Carbon Associated with Shorter Telomeres in Elderly Men: Veterans Affairs Normative Aging Study. Environ. Health Perspect. 2010, 118, 1564–1570. [Google Scholar] [CrossRef] [Green Version]
- Verdun, R.E.; Karlseder, J. Replication and Protection of Telomeres. Nature 2007, 447, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.S.M.; Thomasini, R.L.; Lustosa, L.P.; Pereira, D.S.; Pereira, L.S.M.; Kassab, G.B.I.; Silva, T.J.; Guerra, R.O.; Parentoni, A.N. Is the Leukocyte Telomere Length Associated with Decreased Physical Functional Capacity in the Elderly? Rejuvenation Res. 2020, 23, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Sibille, K.T.; Chen, H.; Bartley, E.J.; Riley, J.; Glover, T.L.; King, C.D.; Zhang, H.; Cruz-Almeida, Y.; Goodin, B.R.; Sotolongo, A.; et al. Accelerated Aging in Adults with Knee Osteoarthritis Pain: Consideration for Frequency, Intensity, Time, and Total Pain Sites. Pain Rep. 2017, 2, e591. [Google Scholar] [CrossRef] [PubMed]
- Arsenis, N.C.; You, T.; Ogawa, E.F.; Tinsley, G.M.; Zuo, L. Physical Activity and Telomere Length: Impact of Aging and Potential Mechanisms of Action. Oncotarget 2017, 8, 45008–45019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werner, C.; Fürster, T.; Widmann, T.; Pöss, J.; Roggia, C.; Hanhoun, M.; Scharhag, J.; Büchner, N.; Meyer, T.; Kindermann, W.; et al. Physical Exercise Prevents Cellular Senescence in Circulating Leukocytes and in the Vessel Wall. Circulation 2009, 120, 2438–2447. [Google Scholar] [CrossRef]
- Puterman, E.; Lin, J.; Blackburn, E.; O’Donovan, A.; Adler, N.; Epel, E. The Power of Exercise: Buffering the Effect of Chronic Stress on Telomere Length. PLoS ONE 2010, 5, e10837. [Google Scholar] [CrossRef]
- Steptoe, A.; Hamer, M.; Butcher, L.; Lin, J.; Brydon, L.; Kivimäki, M.; Marmot, M.; Blackburn, E.; Erusalimsky, J.D. Educational Attainment but Not Measures of Current Socioeconomic Circumstances Are Associated with Leukocyte Telomere Length in Healthy Older Men and Women. Brain. Behav. Immun. 2011, 25, 1292–1298. [Google Scholar] [CrossRef]
- Surtees, P.G.; Wainwright, N.W.J.; Pooley, K.A.; Luben, R.N.; Khaw, K.-T.; Easton, D.F.; Dunning, A.M. Educational Attainment and Mean Leukocyte Telomere Length in Women in the European Prospective Investigation into Cancer (EPIC)-Norfolk Population Study. Brain. Behav. Immun. 2012, 26, 414–418. [Google Scholar] [CrossRef]
- Adler, N.; Pantell, M.S.; O’Donovan, A.; Blackburn, E.; Cawthon, R.; Koster, A.; Opresko, P.; Newman, A.; Harris, T.B.; Epel, E. Educational Attainment and Late Life Telomere Length in the Health, Aging and Body Composition Study. Brain. Behav. Immun. 2013, 27, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Gardner, M.; Bann, D.; Wiley, L.; Cooper, R.; Hardy, R.; Nitsch, D.; Martin-Ruiz, C.; Shiels, P.; Sayer, A.A.; Barbieri, M.; et al. Gender and Telomere Length: Systematic Review and Meta-Analysis. Exp. Gerontol. 2014, 51, 15–27. [Google Scholar] [CrossRef]
- Mayer, S.; Brüderlein, S.; Perner, S.; Waibel, I.; Holdenried, A.; Ciloglu, N.; Hasel, C.; Mattfeldt, T.; Nielsen, K.V.; Möller, P. Sex-Specific Telomere Length Profiles and Age-Dependent Erosion Dynamics of Individual Chromosome Arms in Humans. Cytogenet. Genome Res. 2006, 112, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, T.S.; Staessen, J.A.; Gardner, J.P.; Aviv, A. Telomere Length and Possible Link to X Chromosome. Lancet Lond. Engl. 2004, 363, 507–510. [Google Scholar] [CrossRef]
- Aviv, A. Telomeres, Sex, Reactive Oxygen Species, and Human Cardiovascular Aging. J. Mol. Med. Berl. Ger. 2002, 80, 689–695. [Google Scholar] [CrossRef]
- Aviv, A.; Shay, J.W. Reflections on Telomere Dynamics and Ageing-Related Diseases in Humans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20160436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Ezquerro, J.D.; Rodríguez-Castañeda, A.; Ortiz-Ramírez, M.; Sánchez-García, S.; Rosas-Vargas, H.; Sánchez-Arenas, R.; García-de la Torre, P. Oxidative Stress, Telomere Length, and Frailty in an Old Age Population. Rev. Investig. Clin. Organo Hosp. Enferm. Nutr. 2019, 71, 393–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sazzini, M.; Abondio, P.; Sarno, S.; Gnecchi-Ruscone, G.A.; Ragno, M.; Giuliani, C.; De Fanti, S.; Ojeda-Granados, C.; Boattini, A.; Marquis, J.; et al. Genomic History of the Italian Population Recapitulates Key Evolutionary Dynamics of Both Continental and Southern Europeans. BMC Biol. 2020, 18, 1–19. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).