Short-Term Efficacy and Safety of Cataract Surgery Combined with Iris-Fixated Phakic Intraocular Lens Explantation: A Multicentre Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Institutions and Institutional Review Board Approval
2.2. Participants
2.3. Surgical Technique
2.4. Ophthalmologic Examinations
2.5. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pascolini, D.; Mariotti, S.P. Global estimates of visual impairment: 2010. Br. J. Ophthalmol. 2012, 96, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Dolgin, E. The myopia boom. Nature 2015, 519, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Morgan, I.G.; French, A.N.; Ashby, R.S.; Guo, X.; Ding, X.; He, M.; Rose, K.A. The epidemics of myopia: Aetiology and prevention. Prog. Retin. Eye Res. 2018, 62, 134–149. [Google Scholar] [CrossRef] [PubMed]
- Morgan, I.G.; Ohno-Matsui, K.; Saw, S.-M. Myopia. Lancet 2012, 379, 1739–1748. [Google Scholar] [CrossRef]
- Guell, J.L.; Morral, M.; Kook, D.; Kohnen, T. Phakic intraocular lenses part 1: Historical overview, current models, selection criteria, and surgical techniques. J. Cataract Refract. Surg. 2010, 36, 1976–1993. [Google Scholar] [CrossRef] [PubMed]
- Kohnen, T.; Kook, D.; Morral, M.; Guell, J.L. Phakic intraocular lenses: Part 2: Results and complications. J. Cataract Refract. Surg. 2010, 36, 2168–2194. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Lim, D.H.; Nam, S.W.; Yang, C.M.; Chung, E.S.; Chung, T.Y. Ten-year clinical outcomes after implantation of a posterior chamber phakic intraocular lens for myopia. J. Cataract Refract. Surg. 2019, 45, 1555–1561. [Google Scholar] [CrossRef]
- Nakamura, T.; Isogai, N.; Kojima, T.; Yoshida, Y.; Sugiyama, Y. Posterior Chamber Phakic Intraocular Lens Implantation for the Correction of Myopia and Myopic Astigmatism: A Retrospective 10-Year Follow-up Study. Am. J. Ophthalmol. 2019, 206, 1–10. [Google Scholar] [CrossRef]
- Tahzib, N.G.; Nuijts, R.M.; Wu, W.Y.; Budo, C.J. Long-term study of Artisan phakic intraocular lens implantation for the correction of moderate to high myopia: Ten-year follow-up results. Ophthalmology 2007, 114, 1133–1142. [Google Scholar] [CrossRef]
- Alio, J.L.; Abdelrahman, A.M.; Javaloy, J.; Iradier, M.T.; Ortuno, V. Angle-supported anterior chamber phakic intraocular lens explantation causes and outcome. Ophthalmology 2006, 113, 2213–2220. [Google Scholar] [CrossRef]
- Alio, J.L.; Toffaha, B.T.; Pena-Garcia, P.; Sadaba, L.M.; Barraquer, R.I. Phakic intraocular lens explantation: Causes in 240 cases. J. Refract. Surg. 2015, 31, 30–35. [Google Scholar] [CrossRef]
- Sucu, M.E.; Cakmak, S.; Yildirim, Y.; Yildiz, B.K.; Yalcinkaya, G.; Besek, N.K.; Yasar, T. Explantation of phakic intraocular lenses: Causes and outcomes. Int. Ophthalmol. 2021, 41, 265–271. [Google Scholar] [CrossRef]
- Bleckmann, H.; Keuch, R.J. Results of cataract extraction after implantable contact lens removal. J. Cataract Refract. Surg. 2005, 31, 2329–2333. [Google Scholar] [CrossRef]
- de Vries, N.E.; Tahzib, N.G.; Budo, C.J.; Webers, C.A.; de Boer, R.; Hendrikse, F.; Nuijts, R.M. Results of cataract surgery after implantation of an iris-fixated phakic intraocular lens. J. Cataract Refract. Surg. 2009, 35, 121–126. [Google Scholar] [CrossRef]
- Kamiya, K.; Shimizu, K.; Igarashi, A.; Aizawa, D.; Ikeda, T. Clinical outcomes and patient satisfaction after Visian Implantable Collamer Lens removal and phacoemulsification with intraocular lens implantation in eyes with induced cataract. Eye 2010, 24, 304–309. [Google Scholar] [CrossRef][Green Version]
- Meier, P.G.; Majo, F.; Othenin-Girard, P.; Bergin, C.; Guber, I. Refractive outcomes and complications after combined copolymer phakic intraocular lens explantation and phacoemulsification with intraocular lens implantation. J. Cataract Refract. Surg. 2017, 43, 748–753. [Google Scholar] [CrossRef]
- Morales, A.J.; Zadok, D.; Tardio, E.; Anzoulatous, G., Jr.; Litwak, S.; Mora, R.; Martinez, E.; Chayet, A.S. Outcome of simultaneous phakic implantable contact lens removal with cataract extraction and pseudophakic intraocular lens implantation. J. Cataract Refract. Surg. 2006, 32, 595–598. [Google Scholar] [CrossRef]
- Agarwal, P.; Navon, S.E.; Mithal, N. Novel technique of explantation of rigid phakic iris-claw lens and cataract extraction by sutureless manual small-incision surgery. BMJ Case Rep. 2019, 12, e233128. [Google Scholar] [CrossRef]
- Khokhar, S.; Mahabir, M. Phacoemulsification in phakic iris-claw lens with cataract. Indian J. Ophthalmol. 2018, 66, 1609–1610. [Google Scholar]
- Vargas, V.; Alio, J.L.; Barraquer, R.I.; D’Antin, J.C.; Garcia, C.; Duch, F.; Balgos, J.; Alio Del Barrio, J.L. Safety and visual outcomes following posterior chamber phakic intraocular lens bilensectomy. Eye Vis. 2020, 7, 34. [Google Scholar] [CrossRef]
- van Rijn, G.A.; Gaurisankar, Z.S.; Ilgenfritz, A.P.; Lima, J.E.E.; Haasnoot, G.W.; Beenakker, J.M.; Cheng, Y.Y.Y.; Luyten, G.P.M. Middle-and long-term results after iris-fixated phakic intraocular lens implantation in myopic and hyperopic patients: A meta-analysis. J. Cataract Refract. Surg. 2020, 46, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Baayen, R.H.; Davidson, D.J.; Bates, D.M. Mixed-effects modeling with crossed random effects for subjects and items. J. Mem. Lang. 2008, 59, 390–412. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67. [Google Scholar] [CrossRef]
- Dunnett, C.W. A multiple comparison procedure for comparing several treatments with a control. Am. Stat. Assoc. 1955, 50, 1096–1211. [Google Scholar] [CrossRef]
- Grzybowski, A.; Kanclerz, P. Early postoperative intraocular pressure elevation following cataract surgery. Curr. Opin. Ophthalmol. 2019, 30, 56–62. [Google Scholar] [CrossRef]
- Levkovitch-Verbin, H.; Habot-Wilner, Z.; Burla, N.; Melamed, S.; Goldenfeld, M.; Bar-Sela, S.M.; Sachs, D. Intraocular pressure elevation within the first 24 hours after cataract surgery in patients with glaucoma or exfoliation syndrome. Ophthalmology 2008, 115, 104–108. [Google Scholar] [CrossRef]
- Syed, Z.A.; Moayedi, J.; Mohamedi, M.; Tashter, J.; Anthony, T.; Celiker, C.; Khazen, G.; Melki, S.A. Cataract surgery outcomes at a UK independent sector treatment centre. Br. J. Ophthalmol. 2015, 99, 1460–1465. [Google Scholar] [CrossRef]
- Amro, M.; Chanbour, W.; Arej, N.; Jarade, E. Third- and fourth-generation formulas for intraocular lens power calculation before and after phakic intraocular lens insertion in high myopia. J. Cataract Refract. Surg. 2018, 44, 1321–1325. [Google Scholar] [CrossRef]
- Yasa, D.; Kose, B.; Sucu, M.E.; Agca, A. Intraocular lens power calculation in a posterior chamber phakic intraocular lens implanted eye. Int. Ophthalmol. 2020, 40, 2017–2022. [Google Scholar] [CrossRef]
- Goles, N.; Nerancic, M.; Konjik, S.; Pajic-Eggspuehler, B.; Pajic, B.; Cvejic, Z. Phacoemulsification and IOL-Implantation without Using Viscoelastics: Combined Modeling of Thermo Fluid Dynamics, Clinical Outcomes, and Endothelial Cell Density. Sensors 2021, 21, 2399. [Google Scholar] [CrossRef]
- Hayashi, K.; Yoshida, M.; Manabe, S.; Hirata, A. Cataract surgery in eyes with low corneal endothelial cell density. J. Cataract Refract. Surg. 2011, 37, 1419–1425. [Google Scholar] [CrossRef]
- Kim, D.H.; Wee, W.R.; Hyon, J.Y. The pattern of early corneal endothelial cell recovery following cataract surgery: Cellular migration or enlargement? Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 2211–2216. [Google Scholar] [CrossRef]

| Variable | Value |
|---|---|
| No. eyes | 96 eyes/91 patients |
| Right/left eyes | 48/48 |
| Women/men | 69/27 |
| Age at cataract surgery (years) | 55.0 ± 7.5 |
| Age at pIOL implantation (years) | 45.3 ± 7.4 |
| Duration between surgeries (years) | 9.7 ± 3.6 |
| Emery-Little classification of nuclear cataract (eyes) | Grade I (16), grade II (46), grade III (31), grade IV (3) |
| pIOL material (eyes) | PMMA (53), silicone (43) |
| UCVA (logMAR) | 0.29 ± 0.34 |
| BCVA (logMAR) | −0.01 ± 0.17 |
| Target refraction (D) | −0.17 ± 0.49 |
| Spherical equivalent (D) | −1.43 ± 1.59 |
| Cylinder (D) | −0.82 ± 0.73 |
| Endothelial cell density (cells/mm2) | 1986 ± 732 |
| Axial length (mm) | 28.39 ± 1.94 |
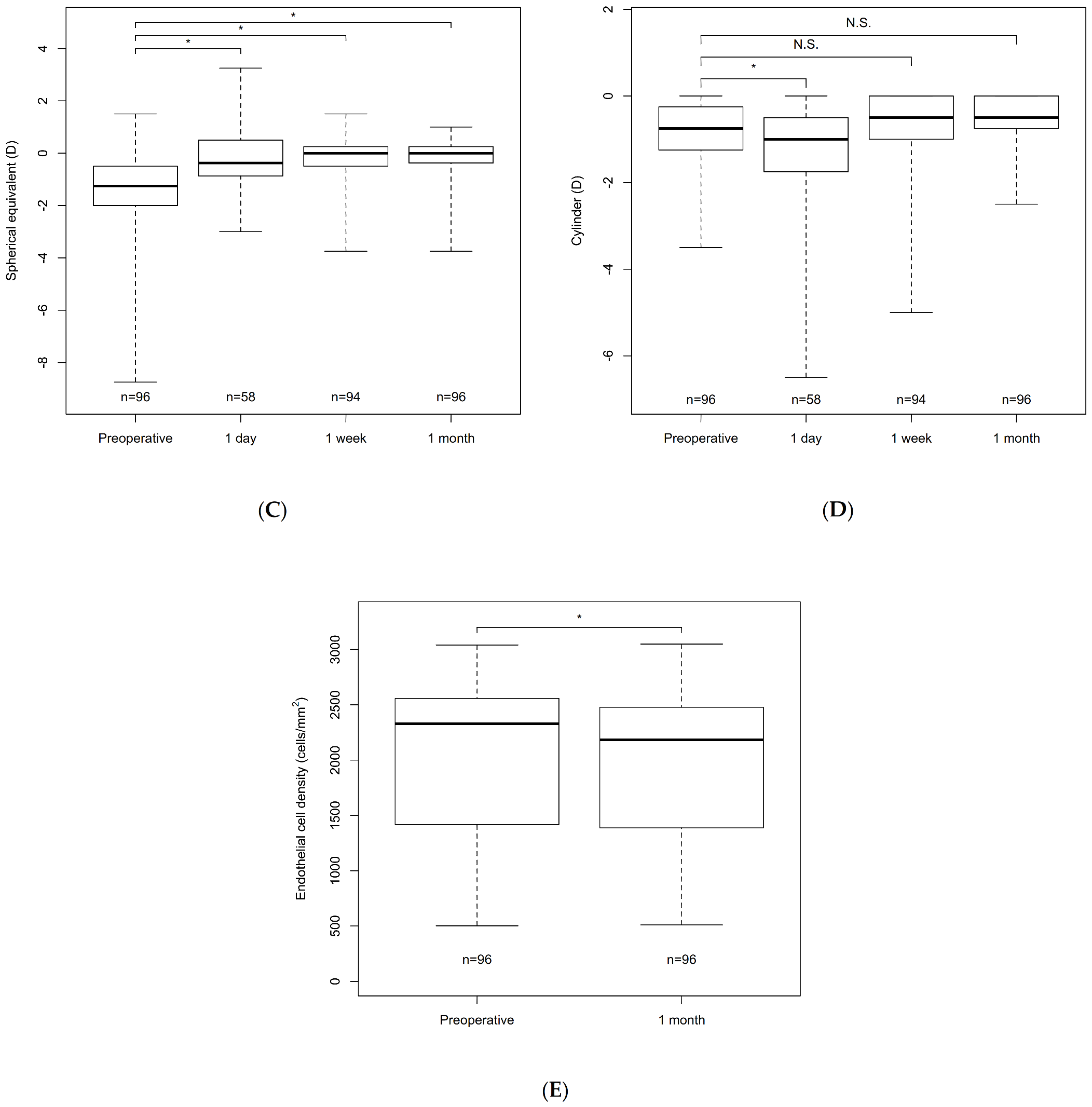
| UCVA (logMAR) | BCVA (logMAR) | SE (D) | Cylinder (D) | ECD (cells/mm2) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Variable | p Value | N | Variable | p Value | N | Variable | p Value | N | Variable | p Value | N | Variable | p Value | |
| Preoperative | 95 | 0.29 ± 0.34 | 96 | −0.01 ± 0.17 | 96 | −1.43 ± 1.59 | 96 | −0.82 ± 0.73 | 96 | 1986 ± 732 | |||||
| Day 1 | 93 | 0.11 ± 0.30 | <0.001 * | 58 | 0.01 ± 0.17 | 1.0 | 58 | −0.32 ± 1.12 | <0.001 * | 58 | −1.33 ± 1.45 | 0.0060 * | |||
| Week 1 | 94 | 0.06 ± 0.23 | <0.001 * | 94 | −0.08 ± 0.12 | <0.001 * | 94 | −0.18 ± 0.78 | <0.001 * | 94 | −0.82 ± 0.97 | 1.0 | |||
| Month 1 | 96 | 0.03 ± 0.19 | <0.001 * | 96 | −0.09 ± 0.10 | <0.001 * | 96 | −0.17 ± 0.84 | <0.001 * | 96 | −0.57 ± 0.59 | 0.078 | 96 | 1897 ± 725 | <0.001 * |
| Complication | % (eyes) |
|---|---|
| IOP elevation exceeding 25 mmHg | 10.4% (10) |
| Corneal edema | 8.3% (8) |
| Iritis | 3.1% (3) |
| Corneal epithelial defect | 2.1% (2) |
| Hyphema | 2.1% (2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omoto, M.K.; Torii, H.; Masui, S.; Ayaki, M.; Toda, I.; Arai, H.; Nakamura, T.; Tsubota, K.; Negishi, K. Short-Term Efficacy and Safety of Cataract Surgery Combined with Iris-Fixated Phakic Intraocular Lens Explantation: A Multicentre Study. J. Clin. Med. 2021, 10, 3672. https://doi.org/10.3390/jcm10163672
Omoto MK, Torii H, Masui S, Ayaki M, Toda I, Arai H, Nakamura T, Tsubota K, Negishi K. Short-Term Efficacy and Safety of Cataract Surgery Combined with Iris-Fixated Phakic Intraocular Lens Explantation: A Multicentre Study. Journal of Clinical Medicine. 2021; 10(16):3672. https://doi.org/10.3390/jcm10163672
Chicago/Turabian StyleOmoto, Miki Kamikawatoko, Hidemasa Torii, Sachiko Masui, Masahiko Ayaki, Ikuko Toda, Hiroyuki Arai, Tomoaki Nakamura, Kazuo Tsubota, and Kazuno Negishi. 2021. "Short-Term Efficacy and Safety of Cataract Surgery Combined with Iris-Fixated Phakic Intraocular Lens Explantation: A Multicentre Study" Journal of Clinical Medicine 10, no. 16: 3672. https://doi.org/10.3390/jcm10163672
APA StyleOmoto, M. K., Torii, H., Masui, S., Ayaki, M., Toda, I., Arai, H., Nakamura, T., Tsubota, K., & Negishi, K. (2021). Short-Term Efficacy and Safety of Cataract Surgery Combined with Iris-Fixated Phakic Intraocular Lens Explantation: A Multicentre Study. Journal of Clinical Medicine, 10(16), 3672. https://doi.org/10.3390/jcm10163672








