Shunt Overdrainage: Reappraisal of the Syndrome and Proposal for an Integrative Model
Abstract
1. Evolution of Concepts and Current Pitfalls in Shunt Overdrainage Syndrome
2. Clinical Manifestations in Shunt Overdrainage
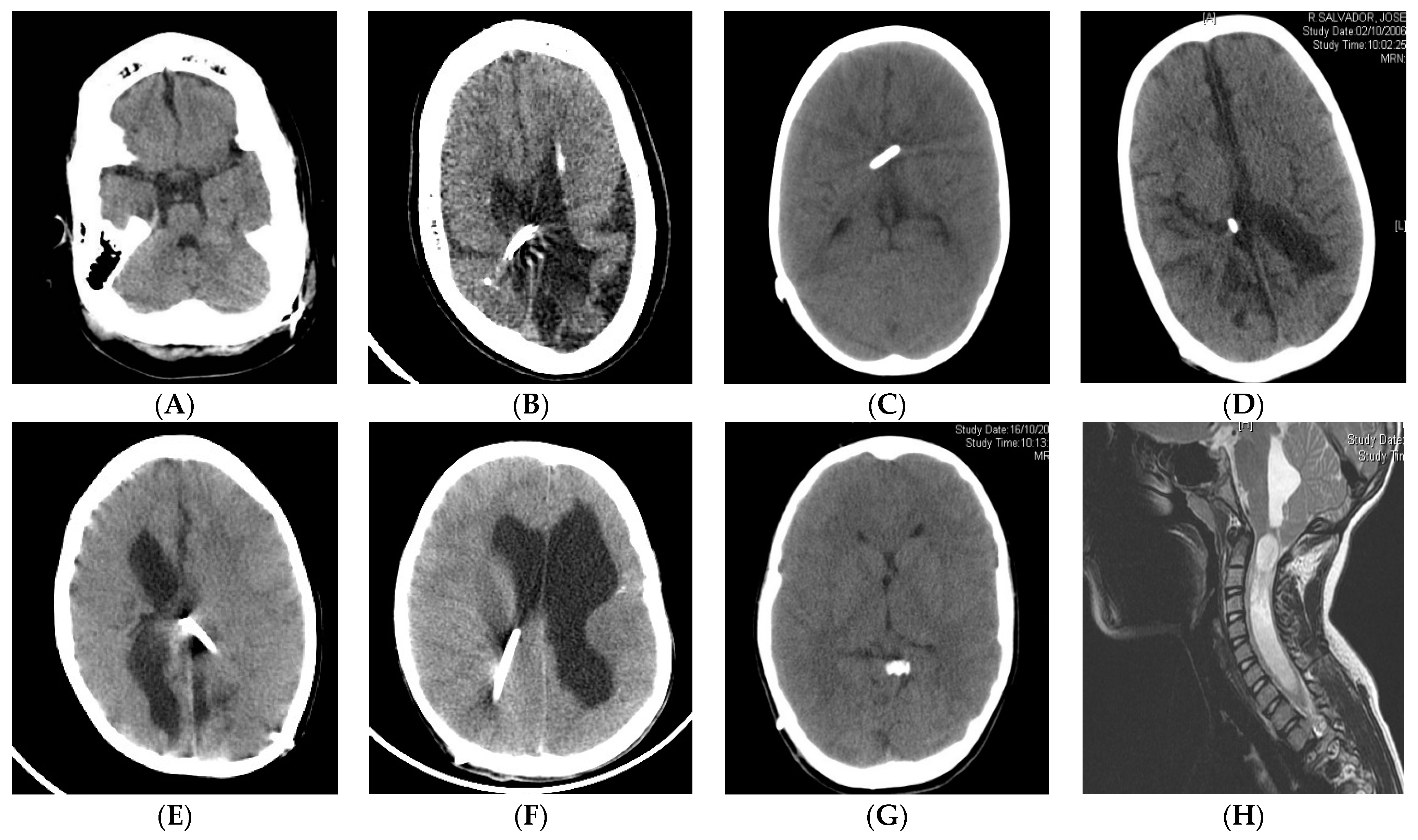
3. Radiology in Shunt Overdrainage
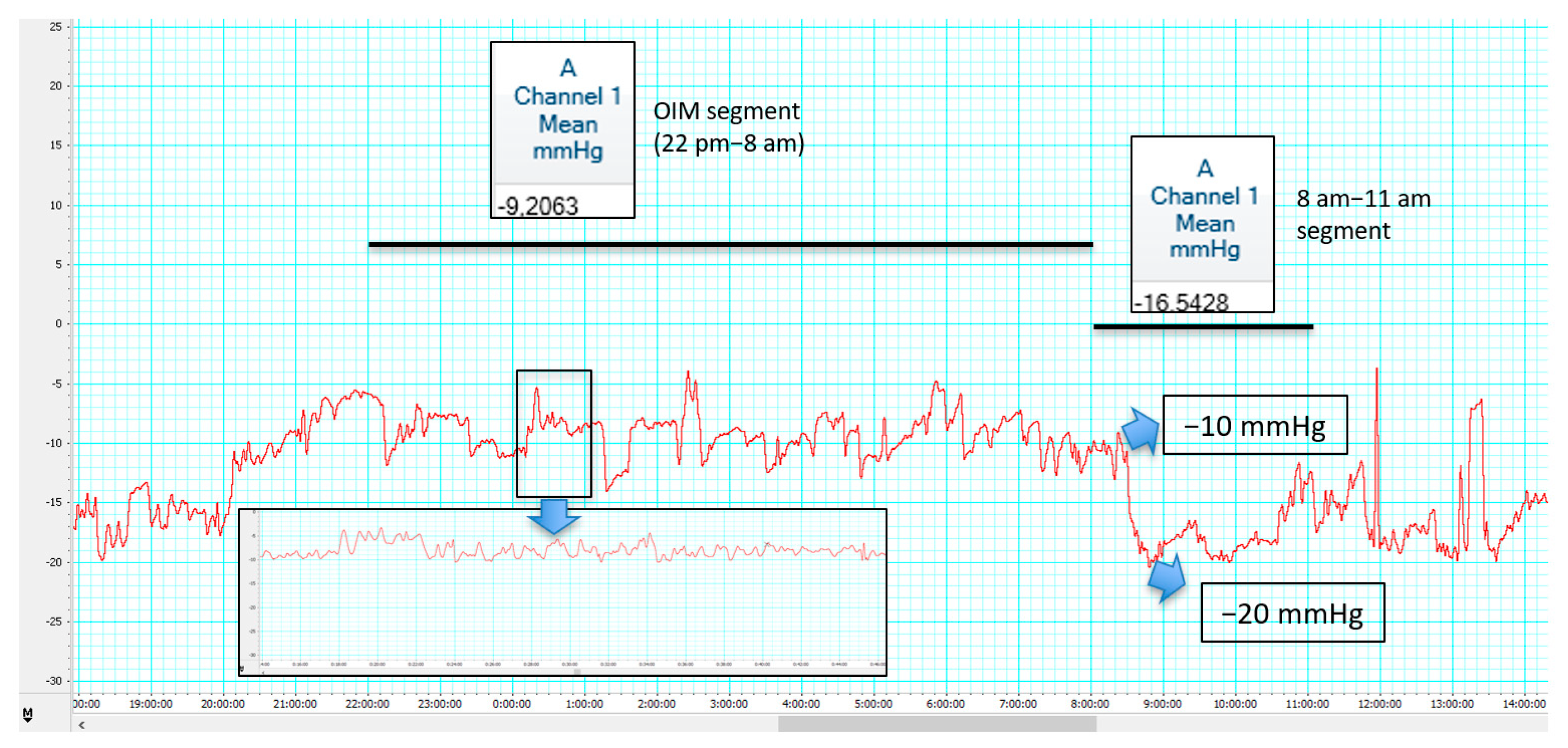
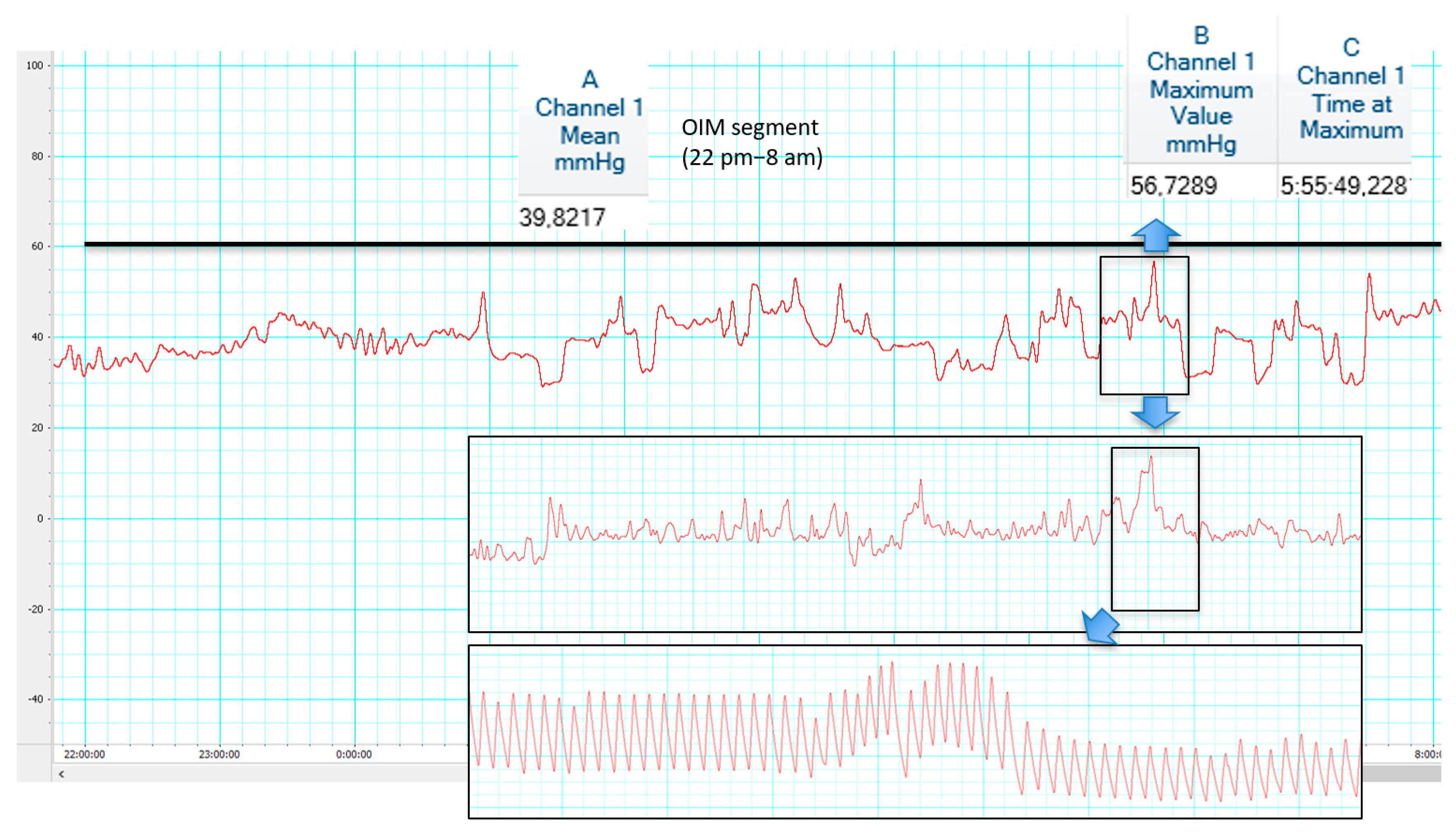
4. ICP Patterns in Shunt Overdrainage
5. Pathophysiological Theories in Shunt Overdrainage
5.1. Siphoning
5.2. Ventricular Collapse and CSF Isolation
5.3. Acquired Craniocerebral Disproportion
5.4. Venous Congestion Theories
6. Management Strategies in Shunt Overdrainage
7. Proposal for an Integrative Model in Shunt Overdrainage
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Grunert, P.; Charalampaki, P.; Ayyad, A. Concept and treatment of hydrocephalus in the Greco-Roman and early Arabic Medicine. Minim. Invasive Neurosurg. 2007, 50, 253–264. [Google Scholar] [CrossRef]
- Rachel, R.A. Surgical treatment of hydrocephalus: A historical perspective. Pediatr. Neurosurg. 1999, 30, 296–304. [Google Scholar] [CrossRef]
- Kompaje, E.J.; Delwel, E.J. The first description of a device for repeated external ventricular drainage in the treatment of congenital hydrocephalus, invented in 1744 by Claude-Nicolas Le Cat. Pediatr. Neurosurg. 2003, 39, 10–13. [Google Scholar] [CrossRef]
- Cheok, S.; Chen, J.; Lazareff, J. The truth and coherence behind the concept of overdrainage of cerebrospinal fluid in hydrocephalic patients. Childs Nerv. Syst. 2014, 30, 599–606. [Google Scholar] [CrossRef]
- Strenger, L. Complications of ventriculovenous shunts. J. Neurosurg. 1963, 20, 219–224. [Google Scholar] [CrossRef]
- Hayward, R. “Casey and Theo”: The children who changed the face of “Water-on-the-brain”. Br. J. Neurosurg. 2009, 23, 347–350. [Google Scholar] [CrossRef]
- Becker, D.P.; Nulsen, F.E. Control of hydrocephalus by valveregulated venous shunt: Avoidance of complications in prolonged shunt maintenance. J. Neurosurg. 1968, 28, 215–226. [Google Scholar] [CrossRef]
- Fox, J.L.; McCullough, D.C.; Green, R.C. Effect of cerebrospinal fluid shunts on intracranial pressure and on cerebrospinal fluid dynamics 2. A new technique of pressure measurements: Results and concepts 3. A concept of hydrocephalus. J. Neurol. Neurosurg. Psychiatry 1973, 36, 302–312. [Google Scholar] [CrossRef][Green Version]
- Portnoy, H.D.; Schulte, R.R.; Fox, J.L.; Croissant, P.D.; Tripp, L. Antisiphon and reversible occlusion valves for shunting in hydrocephalus and preventing post-shunt subdural hematomas. J. Neurosurg. 1973, 38, 729–738. [Google Scholar] [CrossRef]
- Chapman, P.H.; Cosman, E.R.; Arnold, M.A. The relationship between ventricular fluid pressure and body position in normal subjects and subjects with shunts: A telemetric study. Neurosurgery 1990, 26, 181–189. [Google Scholar] [CrossRef]
- Pudenz, R.H.; Foltz, E.L. Hydrocephalus: Overdrainage by ventricular shunts. A review and recommendations. Surg. Neurol. 1991, 35, 200–212. [Google Scholar] [CrossRef]
- Bergsneider, M.; Peacock, W.J.; Mazziotta, J.C.; Becker, D.P. Beneficial effect of siphoning in treatment of adult hydrocephalus. Arch. Neurol. 1999, 56, 1224–1229. [Google Scholar] [CrossRef]
- Epstein, F.J.; Fleischer, A.S.; Hochwald, G.M.; Ransohoff, J. Subtemporal craniectomy for recurrent shunt obstruction secondary to small ventricles. J. Neurosurg. 1974, 41, 29–31. [Google Scholar] [CrossRef]
- Holness, R.O.; Hoffman, H.J.; Hendrick, E.B. Subtemporal decompression for the slit-ventricle syndrome after shunting in hydrocephalic children. Pediatr. Neurosurg. 1979, 5, 137–144. [Google Scholar] [CrossRef]
- Hyde-Rowan, M.D.; Rekate, H.L.; Nulsen, F.E. Reexpansion of previously collapsed ventricles: The slit ventricle syndrome. J. Neurosurg. 1982, 56, 536–539. [Google Scholar] [CrossRef]
- Abbot, R.; Epstein, F.J.; Wisoff, J.H. Chronic headache associated with a functioning shunt. Usefulness of pressure monitoring. Neurosurgery 1991, 28, 72–77. [Google Scholar] [CrossRef]
- Serlo, W.; Heikkinen, E.; Saukkonen, A.L.; Wendt, L.V. Classification and management of the slit ventricle syndrome. Childs Nerv. Syst. 1985, 1, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Epstein, F.; Lapras, C.; Wisoff, J.H. Slit-ventricle syndrome: Etiology and treatment. Pediatr. Neurosci. 1988, 14, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Rekate, H.L. Classification of slit-ventricle syndromes using intracranial pressure monitoring. Pediatr. Neurosurg. 1993, 19, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Baskin, J.J.; Manwaring, K.H.; Rekate, H.L. Ventricular shunt removal: The ultimate treatment of the slit ventricle syndrome. J. Neurosurg. 1998, 88, 478–484. [Google Scholar] [CrossRef]
- Eide, P.K.; Helseth, E.; Due-Tønnessen, B.; Lundar, T. Changes in Intracranial Pressure after Calvarial Expansion Surgery in Children with Slit Ventricle Syndrome. Pediatr. Neurosurg. 2001, 35, 195–204. [Google Scholar] [CrossRef]
- Eide, P.K. Quantitative analysis of continuous intracranial pressure recordings in symptomatic patients with extracranial shunts. J. Neurol. Neurosurg. Psychiatry 2003, 74, 231–237. [Google Scholar] [CrossRef]
- Khorasani, L.; Sikorski, C.W.; Frim, D.M. Lumbar CSF shunting preferentially drains the cerebral subarachnoid over the ventricular spaces: Implications for the treatment of slit ventricle syndrome. Pediatr. Neurosurg. 2004, 40, 270–276. [Google Scholar] [CrossRef]
- Olson, S. The problematic slit ventricle syndrome. A review of the literature and proposed algorithm for treatment. Pediatr. Neurosurg. 2004, 40, 264–269. [Google Scholar] [CrossRef]
- Rekate, H.L. Slit ventricle syndrome. Diagnosis and management. In Pediatric Hydrocephalus; Cinalli, G., Maxner, W.J., Sainte-Rose, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 335–349. [Google Scholar]
- Rekate, H.L. Shunt-related headaches: The slit ventricle syndromes. Childs Nerv. Syst. 2008, 24, 423–430. [Google Scholar] [CrossRef]
- Ros, B.; Iglesias, S.; Martín, A.; Carrasco, A.; Ibáñez, G.; Arráez, M.A. Shunt overdrainage syndrome: Review of the literature. Neurosurg. Rev. 2018, 41, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Sainz, L.V.; Hockel, K.; Schuhmann, M.U. Chronic overdrainage syndrome: Pathophysiological insights based on ICP analysis: A case-based review. Childs Nerv. Syst. 2018, 34, 401–408. [Google Scholar] [CrossRef]
- Hart, M.G.; Czosnyka, M.; Czosnyka, Z.H.; Fernandes, H.M. Combined Intracranial Pressure Monitoring and Cerebrospinal Fluid Infusion Study to Guide Management of Slit Ventricle Syndrome. Pediatr. Neurosurg. 2013, 49, 113–118. [Google Scholar] [CrossRef]
- Jang, M.; Yoon, S.H. Hypothesis for intracranial hypertension in slit ventricle syndrome: New concept of capillary absorption laziness in the hydrocephalic patients with long-term shunts. Med. Hypotheses 2013, 81, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Piatt, J.H., Jr. Physical examination of patients with cerebrospinal fluid shunts: Is there useful information in pumping the shunt? Pediatrics 1992, 89, 470–473. [Google Scholar]
- Engel, M.; Carmel, P.W.; Chutorian, A.M. Increased intraventricular pressure without ventriculomegaly in children with shunts: “normal volumen” hydrocephalus. Neurosurgery 1979, 5, 549–552. [Google Scholar] [CrossRef]
- McNatt, S.A.; Kim, A.; Hohuan, D.; Krieger, M.; McComb, J.G. Pediatric shunt malfunction without ventricular dilatation. Pediatr. Neurosurg. 2008, 44, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Beez, T.; Munoz-Bendix, C.; Ahmadi, S.A.; Messing-Jünger, M.; Steiger, H.K.; Röhrig, A. Conservative and operative management of iatrogenic craniocerebral disproportion-a case-based review. Childs Nerv. Syst. 2019, 35, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Bergsneider, M.; Egnor, M.R.; Johnston, M.; Kranz, D.; Madsen, J.R.; McAllister, J.P., 2nd; Stewart, C.; Walker, M.L.; Williams, M.A. What we don’t (but should) know about hydrocephalus. J. Neurosurg. 2006, 104 (Suppl. S3), 157–159. [Google Scholar] [CrossRef] [PubMed]
- Di Rocco, C.; Massimi, L.; Tamburrini, G. Shunts vs endoscopic third ventriculostomy in infants: Are there different types and/or rates of complications? A review. Childs Nerv. Syst. 2006, 22, 1573–1589. [Google Scholar] [CrossRef]
- Kraemer, M.R.; Sandoval-Garcia, C.; Bragg, T.; Iskandar, B.J. Shunt-dependent hydrocephalus: Management style among members of the American Society of Pediatric Neurosurgeons. J. Neurosurg. Pediatr. 2017, 20, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Breimer, G.E.; Sival, D.A.; Hoving, E.W. Low-pressure valves in hydrocephalic children: A retrospective analysis. Childs Nerv. Syst. 2012, 28, 469–473. [Google Scholar] [CrossRef][Green Version]
- Sainte-Rose, C.; Piatt, J.H.; Renier, D.; Pierre-Kahn, A.; Hirsch, J.F.; Hoffman, H.J.; Humphreys, R.P.; Hendrick, E.B. Mechanical complications in shunts. Pediatr. Neurosurg. 1992, 17, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, S.; Ros, B.; Martín, A.; Carrasco, A.; Segura, M.; Delgado, A.; Rius, F.; Arráez, M.A. Surgical outcome of the shunt: 15-year experience in a single institution. Childs Nerv. Syst. 2016, 32, 2377–2385. [Google Scholar] [CrossRef]
- Iglesias, S.; Ros, B.; Martín, A.; Carrasco, A.; Rius, F.; Arráez, M.A. Functional outcome in pediatric hydrocephalus: Results of applying the Spanish version of the Hydrocephalus Outcome Questionnaire. J. Neurosurg. Pediatr. 2018, 21, 224–235. [Google Scholar] [CrossRef]
- Niimura, M.; Takai, K.; Taniguchi, M. Postoperative epidural haematomas associated with hydrocephalus caused by intraoperative overdrainage of cerebrospinal fluid: Two case reports with a literature review of 19 cases. BMJ Case Rep. 2015, 9, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Niwa, R.; Oya, S.; Nakamura, T.; Hana, T.; Matsui, T. Rapid intracranial pressure drop as a cause for posterior reversible encephalopathy syndrome: Two case reports. Surg. Neurol. Int. 2017, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Weerakkody, R.A.; Czosnyka, M.; Schuhmann, M.U.; Schmidt, E.; Keong, N.; Santarius, T.; Pickard, J.D.; Czosnyka, Z. Clinical assessment of cerebrospinal fluid dynamics in hydrocephalus. Guide to interpretation based on observational study. Acta Neurol. Scand. 2011, 124, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Schuhmann, M.U.; Sood, S.; McAllister, J.P.; Jaeger, M.; Ham, S.D.; Czosnyka, Z.; Czosnyka, M. Value of Overnight Monitoring of Intracranial Pressure in Hydrocephalic Children. Pediatr. Neurosurg. 2008, 44, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Han, P.Y.; Kim, J.H.; Kang, H.I.; Kim, J.S. “Syndrome of the sinking skin-flap” secondary to the ventriculoperitoneal shunt after craniectomy. J. Korean Neurosurg. Soc. 2008, 43, 51–53. [Google Scholar] [CrossRef]
- de Quintana-Schmidt, C.; Clavel-Laria, P.; Asencio-Cortes, C.; Vendrell-Brucet, J.M.; Molet-Teixido, J. Sinking skin flap syndrome. Rev. Neurol. 2011, 52, 661–664. [Google Scholar]
- Gómez-Esteban, J.C.; Berganzo, K.; Tijero, B.; Barcena, J.; Zarranz, J.J. Orthostatic hypotension associated with an epidermoid tumor of the IV ventricle. J. Neurol. 2009, 256, 1357–1359. [Google Scholar] [CrossRef] [PubMed]
- Ruchoux, M.M.; Gray, F.; Gherardi, R.; Schaeffer, A.; Comoy, J.; Poirier, J. Orthostatic hypotension from a cerebellar gangliocytoma (Lhermitte-Duclos disease). Case report. J. Neurosurg. 1986, 65, 245–248. [Google Scholar] [CrossRef]
- Schatz, I.J. Orthostatic hypotension I. Functional and neurogenic causes. Arch. Intern. Med. 1984, 144, 773–777. [Google Scholar] [CrossRef]
- Mokri, B.; Parisi, J.E.; Scheithauer, B.W.; Piepgras, D.G.; Miller, G.M. Meningeal biopsy in intracranial hypotension: Meningeal enhancement on MRI. Neurology 1995, 45, 1801–1807. [Google Scholar] [CrossRef]
- Ulrich, N.H.; Maier, M.; Bernays, R.L.; Krayenbuhl, N.; Kollias, S. Cervical myelopathy due to chronic overshunting in a pediatric patient: Case report and review of the literature. Turk. Neurosurg. 2013, 23, 410–414. [Google Scholar] [CrossRef]
- Howard, B.M.; Sribnick, E.A.; Dhall, S.S. Over-shunting associated myelopathy. J. Clin. Neurosci. 2014, 21, 2242–2244. [Google Scholar] [CrossRef]
- Liu, J.K.; Gottfried, O.N.; Brockmeyer, D.L. Epidural venous engorgement resulting in progressive cervical myelopathy from shunt-related intracranial hypotension. Case report and review of the literature. J. Neurosurg. 2006, 105, 499–503. [Google Scholar] [CrossRef]
- Martínez-Lage, J.F.; Ruíz-Espejo, A.M.; Almagro, M.J.; Alfaro, R.; Felipe-Murcia, M.; López-Guerrero, A.L. CSF overdrainage in shunted intracranial arachnoid cysts: A series and review. Childs Nerv. Syst. 2009, 25, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Adib, S.D.; Hauser, T.K.; Engel, D.C.; Tatagiba, M.; Skardelly, M.; Ramina, K. Overshunting-Associated Myelopathy (OSAM) in a Patient with Bilateral Jugular Vein Occlusion. World Neurosurg. 2018, 116, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Antes, S.; Eymann, R.; Schmitt, M.; Kiefer, M. Pathophysiology of brainstem lesions due to overdrainage. Acta Neurochir. Suppl. 2012, 113, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Mencser, Z.; Kopnickzky, Z.; Kis, D.; Barzo, P. Slit Ventricle as a Neurosurgical Emergency: Case Report and Review of the Literature. World Neurosurg. 2019, 130, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, P.S.; Suriano, I.C.; Neto, H.M. Slit like ventricle syndrome: A life-threatening presentation. Pediatr. Emerg. Care 2009, 25, 674–676. [Google Scholar] [CrossRef]
- Martinez-Lage, J.F.; Pérez-Espejo, M.A.; Almagro, M.J.; Ros de San Pedro, J.; López, F.; Piqueras, C.; Tortosa, J. Síndromes de hiperdrenaje de las válvulas en hidrocefalia infantil. Neurocirugía 2005, 16, 124–133. [Google Scholar] [CrossRef]
- Barami, K.; Sood, S.; Ham, S.D.; Canady, A.I. Postural Changes in Intracranial Pressure in Chronically Shunted Patients. Pediatr. Neurosurg. 2000, 33, 64–69. [Google Scholar] [CrossRef]
- Moayeri, N.N.; Henson, J.W.; Schaefer, P.W.; Zervas, N.T. Spinal dural enhancement on magnetic resonance imaging associated with spontaneous intracranial hypotension. Report of three cases and review of the literature. J. Neurosurg. 1998, 88, 912–918. [Google Scholar] [CrossRef]
- Matsumoto, K.; Ohta, M.; Takeshita, I. Symptomatic spinal extramedullary mass lesion secondary to chronic overdrainage of ventricular fluid-case report. Neurol. Med. Chir. 2002, 42, 140–142. [Google Scholar] [CrossRef]
- Tuli, S.; O’Hayon, B.; Drake, J.; Clarke, M.; Kestle, J. Change in ventricular size and effect of ventricular catheter placement in pediatric patients with shunted hydrocephalus. Neurosurgery 1999, 45, 1329–1333. [Google Scholar] [CrossRef]
- Sandler, A.L.; Goodrich, J.T.; Wagshul, M.E.; Abbott, R. A Comment on the Article by Hart et al. Entitled ‘Combined Intracranial Pressure Monitoring and Cerebrospinal Fluid Infusion Study to Guide Management of Slit Ventricle Syndrome’. Pediatr. Neurosurg. 2013, 49, 258–259. [Google Scholar] [CrossRef]
- Oi, S.; Matsumoto, S. Slit ventricles as a cause of isolated ventricles after shunting. Childs Nerv. Syst. 1985, 1, 189–193. [Google Scholar] [CrossRef]
- Hoffman, H.J.; Tucker, W.S. Cephalocranial disproportion. A complication of the treatment of hydrocephalus in children. Childs Brain 1976, 2, 167–176. [Google Scholar] [PubMed]
- Albright, A.; Tyler-Kabara, E. Slit-ventricle syndrome secondary to shunt-induced suture ossification. Neurosurgery 2001, 48, 764–769. [Google Scholar] [CrossRef]
- Payner, T.D.; Prenger, E.; Berger, T.S.; Crone, K.R. Acquired Chiari malformations: Incidence, diagnosis, and management. Neurosurgery 1994, 34, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Di Rocco, C.; Velardi, F. Acquired Chiari type I malformation managed by supratentorial cranial enlargement. Childs Nerv. Syst. 2003, 19, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Hashi, K. Secondary spinal canal stenosis associaed with long term VP shunting. J. Neurosurg. 1983, 59, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.; Fujii, M.; Kajiwara, K.; Ishihara, H.; Suehiro, E.; Goto, H.; Suzuki, M. Factors influencing spinal canal stenosis in patients with long-term controlled hydrocephalus treated with cerebrospinal fluid shunt. Childs Nerv. Syst. 2010, 26, 931–935. [Google Scholar] [CrossRef]
- Sæhle, T.; Eide, P.K. Intracranial pressure monitoring in pediatric and adult patients with hydrocephalus and tentative shunt failure: A single-center experience over 10 years in 146 patients. J. Neurosurg. 2015, 122, 1076–1086. [Google Scholar] [CrossRef]
- Horcajadas, A.; Román, A.; Olivares, G.; Saura, E.; Jorques, A.; Cordero, N.; Ibáñez, B.; Sánchez, C.; Roldán, M.A. Utilidad de la monitorizacion de la PIC en pacientes con sospecha de disfunción valvular. Neurocirugía 2011, 22, 310–323. [Google Scholar]
- Sood, S.; Kumar, C.R.; Jamous, M.; Schuhmann, M.U.; Ham, S.D.; Canady, A.I. Pathophysiological changes in cerebrovascular distensibility in patients undergoing chronic shunt therapy. J. Neurosurg. 2004, 100 (Suppl. S5), 447–453. [Google Scholar] [CrossRef]
- Eide, P.K.; Sroka, M.; Wozniak, A.; Saehle, T. Morphological characterization of cardiac induced intracranial pressure (ICP) waves in patients with overdrainage of cerebrospinal fluid and negative ICP. Med. Eng. Phys. 2012, 34, 1066–1070. [Google Scholar] [CrossRef]
- Czosnyka, M.; Czosnyka, Z.H. Overdrainage of cerebrospinal fluid and hydrocephalus shunts. Acta Neurochir. 2017, 159, 1387–1388. [Google Scholar] [CrossRef] [PubMed]
- Oi, S.; Shimoda, M.; Shibata, M.; Honda, Y.; Togo, K.; Shinoda, M.; Tsugane, R.; Sato, O. Pathophysiology of long-standing overt ventriculomegaly in adults. J. Neurosurg. 2000, 92, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Pinto, F.C.; Pereira, R.M.; Saad, F.; Teixeira, M.J. Performance of fixed-pressure valve with antisiphon device SPHERA® in hydrocephalus treatment and overdrainage prevention. Arq. Neuro-Psiquiatr. 2012, 70, 704–709. [Google Scholar] [CrossRef]
- Zachenhofer, I.; Donat, M.; Roessler, K. The combination of a programmable valve and a subclavicular antigravity device in hydrocephalus patients at high risk for hygromas. Neurol. Res. 2012, 34, 219–222. [Google Scholar] [CrossRef]
- Sood, S.; Barrett, R.J.; Powell, T.; Ham, S.D. The role of lumbar shunts in the management of slit ventricles: Does the slit-ventricle syndrome exist? J. Neurosurg. 2005, 103 (Suppl. S2), 119–123. [Google Scholar] [CrossRef]
- Atalay, B.; Yilmaz, C.; Cekinmez, M.; Altinors, N.; Caner, H. Treatment of hydrocephalus with functionally isolated ventricles. Acta Neurochir. 2006, 148, 1293–1296. [Google Scholar] [CrossRef] [PubMed]
- Fattal-Valevski, A.; Beni-Adani, L.; Constantini, S. Short-term dexamethasone treatment for symptomatic slit ventricle syndrome. Childs Nerv. Syst. 2005, 21, 981–984. [Google Scholar] [CrossRef]
- Chernov, M.F.; Kamikawa, S.; Yamane, F.; Ishihara, S.; Hori, T. Neurofiberscope-guided management of slit-ventricle syndrome due to shunt placement. J. Neurosurg. 2005, 102 (Suppl. S3), 260–267. [Google Scholar] [CrossRef]
- Kan, P.; Walker, M.L.; Drake, J.M.; Kestle, J.R. Predicting slitlike ventricles in children on the basis of baseline characteristics at the time of shunt insertion. J. Neurosurg. 2007, 106 (Suppl. S5), 347–349. [Google Scholar] [CrossRef] [PubMed]
- Di Rocco, C.; Iannelli, A. Complications of CSF shunting. In The Treatment of Infantile Hydrocephalus; Di Rocco, C., Ed.; CRC: Boca Raton, FL, USA, 1987; Volume II, pp. 79–153. [Google Scholar]
- Chumas, P.D.; Armstrong, D.C.; Drake, J.M.; Kulkarni, A.V.; Hoffman, H.J.; Humphreys, R.P.; Rutka, J.T.; Hendrick, E.B. Tonsillar herniation: The rule rather than the exception after lumboperitoneal shunting in the pediatric population. J. Neurosurg. 1993, 78, 568–573. [Google Scholar] [CrossRef]
- Lazareff, J.A.; Kelly, J.; Saito, M. Herniation of cerebellar tonsils following supratentorial shunt placement. Childs Nerv. Syst. 1998, 14, 394–397. [Google Scholar] [CrossRef]
- Rekate, H.L.; Wallace, D. Lumboperitoneal shunts in children. Pediatr. Neurosurg. 2003, 38, 41–46. [Google Scholar] [CrossRef]
- Major, O.; Fedorcsák, I.; Sipos, L.; Hantos, P.; Kónya, E.; Dobronyi, I.; Paraicz, E. Slit-ventricle syndrome in shunt operated children. Acta Neurochir. 1994, 127, 69–72. [Google Scholar] [CrossRef]
- Buxton, N.; Punt, J. Subtemporal decompression: The treatment of noncompliant ventricle syndrome. Neurosurgery 1999, 44, 513–518. [Google Scholar] [CrossRef]
- Chumas, P.D.; Drake, J.M.; Del Bigio, M.R. Death from chronic tonsillar herniation in a patient with lumboperitoneal shunt and Crouzon’s disease. Br. J. Neurosurg. 1992, 6, 595–599. [Google Scholar] [CrossRef]
- Rekate, H.L. Brain turgor (kb): Intrinsic property of the brain to resist distortion. Pediatr. Neurosurg. 1992, 18, 257–262. [Google Scholar] [CrossRef]
- Park, S.W.; Yoon, S.H.; Cho, K.H.; Shin, Y.S. Valve pressure upgrade may produce progressive deterioration of vision in children with slit ventricle syndrome. Pediatr. Neurosurg. 2007, 43, 428–432. [Google Scholar] [CrossRef]
- Bateman, G.A. Hypertensive slit ventricle syndrome: Pseudotumor cerebri with a malfunctioning shunt? J. Neurosurg. 2013, 119, 1503–1510. [Google Scholar] [CrossRef]
- Preuss, M.; Hoffmann, K.T.; Reiss-Zimmermann, M.; Hirsch, W.; Merkenschlager, A.; Meixensberger, J.; Dengl, M. Updated physiology and pathophysiology of CSF circulation—the pulsatile vector theory. Childs Nerv. Syst. 2013, 29, 1811–1825. [Google Scholar] [CrossRef]
- Barami, K.; Sood, S. The cerebral venous system and the postural regulation of intracranial pressure: Implications in the management of patients with cerebrospinal fluid diversion. Childs Nerv. Syst. 2016, 32, 599–607. [Google Scholar] [CrossRef]
- Liniger, P.; Marchand, S.; Kaiser, G.L. Flow control versus antisiphon valves: Late results concerning slit ventricles and slitventricle syndrome. Eur. J. Pediatr. Surg. 2003, 13 (Suppl. S1), S3–S6. [Google Scholar] [CrossRef] [PubMed]
- Alavi, S.; Schulz, M.; Schaumann, A.; Schwarz, K.; Thomale, U.W. Valve exchange towards an adjustable differential pressure valve with gravitational unit, clinical outcome of a single-center study. Childs Nerv. Syst. 2017, 33, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Thomale, U.W.; Gebert, A.F.; Haberl, H.; Schulz, M. Shunt survival rates by using the adjustable differential pressure valve combined with a gravitational unit (proGAV) in pediatric neurosurgery. Childs Nerv. Syst. 2013, 29, 425–431. [Google Scholar] [CrossRef]
- Rohde, V.; Mayfrank, L.; Ramakers, V.T.; Gilsbach, J.M. Four year experience with the routine use of the programmable Hakim valve in the management of children with hydrocephalus. Acta Neurochir. 1998, 140, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Aschoff, A.; Kremer, P.; Hashemi, B.; Kunze, S. The scientific history of hydrocephalus and its treatment. Neurosurg. Rev. 1999, 22, 67–93. [Google Scholar] [CrossRef]
- Gruber, R.W.; Roehrig, B. Prevention of ventricular catheter obstruction and slit ventricle syndrome by the prophylactic use of the Integra antisiphon device in shunt therapy for pediatric hypertensive hydrocephalus: A 25-year follow-up study. J. Neurosurg. Pediatr. 2010, 5, 14–16. [Google Scholar] [CrossRef]
- Sotelo, J. The hydrokinetic parameters of shunts for hydrocephalus might be inadequate. Surg. Neurol. Int. 2012, 3, 40. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gehlen, M.; Eklund, A.; Kurtcuoglu, V.; Malm, J.; Daners, M.S. Comparison of anti-siphon devices—how do they affect CSF dynamics in supine and upright posture? Acta Neurochir. 2017, 159, 1389–1397. [Google Scholar] [CrossRef]
- Gomes Pinto, F.C.; Fernandes de Oliveira, M.; Souza de Castro, J.P.; Rocha Morais, J.V.; Gomes Pinto, F.M.; Teixeira, M.J. Clinical performance of fixed-pressure Sphera Duo® hydrocephalus shunt. Arq. Neuro-Psiquiatr. 2020, 78, 9–12. [Google Scholar] [CrossRef]
- Bozhkov, Y.; Roessler, K.; Hore, N.; Buchfelder, M.; Brandner, S. Neurological outcome and frequency of overdrainage in normal pressure hydrocephalus directly correlates with implanted ventriculo-peritoneal shunt valve type. Neurol. Res. 2017, 39, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Freimann, F.B.; Kimura, T.; Stockhammer, F.; Schulz, M.; Rohde, V.; Thomale, U.W. In vitro performance and principles of anti-siphoning devices. Acta Neurochir. 2014, 156, 2191–2199. [Google Scholar] [CrossRef]
- Freimann, F.B.; Luhdo, M.L.; Rohde, V.; Vajkoczy, P.; Wolf, S.; Sprung, C. The Frankfurt horizontal plane as a reference for the implantation of gravitational units: A series of 376 adult patients. Acta Neurochir. 2014, 156, 1351–1356. [Google Scholar] [CrossRef]
- Lemke, J.; Meier, U. Improved outcome in shunted iNPH with a combination of a Codman Hakim programmable valve and an Aeskulap–Miethke ShuntAssistant. Cent. Eur. Neurosurg. 2010, 71, 113–116. [Google Scholar] [CrossRef][Green Version]
- Lemcke, J.; Meier, U.; Müller, C.; Fritsch, M.; Kiefer, M.; Eymann, R.; Kehler, U.; Langer, N.; Schuhmann, M.U.; Speil, A.; et al. On the method of a randomised comparison of programmable valves with and without gravitational units: The SVASONA study. Acta Neurochir. Suppl. 2012, 114, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Desai, V.R.; Sadrameli, S.S.; Jenson, A.V.; Asante, S.K.; Daniels, B.; Trask, T.W.; Britz, G. Ventriculoperitoneal shunt complications in an adult population: A comparison of various shunt designs to prevent overdrainage. Surg. Neurol. Int. 2020, 11, 269. [Google Scholar] [CrossRef] [PubMed]
- Kaestner, S.; Kruschat, T.; Nitzsche, N.; Deinsberger, W. Gravitational shunt units may cause under-drainage in bedridden patients. Acta Neurochir. 2009, 151, 217–221. [Google Scholar] [CrossRef]
- Alvarado, A.; Boyle, J.; Martinez, D.; Avellino, A.M.; Lin, J. Commentary to Postural Headache Associated with Ventriculo-Peritoneal Shunt Overdrainage: What are Our Options. Neurosurgery 2017, 80, E247–E248. [Google Scholar] [CrossRef][Green Version]
- Freimann, F.P.; Schulz, M.; Haberl, H.; Thomale, U.W. Feasibility of telemetric ICP-guided valve adjustments for complex shunt therapy. Childs Nerv. Syst. 2014, 30, 689–697. [Google Scholar] [CrossRef]
- Diesner, N.; Freimann, F.; Clajus, C.; Kallenberg, K.; Rohde, V.; Stockhammer, F. Female gender predisposes for cerebrospinal fluid overdrainage in ventriculoperitoneal shunting. Acta Neurochir. 2016, 158, 1273–1278. [Google Scholar] [CrossRef]
- Antes, S.; Stadie, A.; Müller, S.; Linsler, S.; Breuskin, D.; Oertel, J. Intracranial Pressure Guided Shunt Valve Adjustments with the Miethke Sensor Reservoir. World Neurosurg. 2018, 109, e642–e650. [Google Scholar] [CrossRef]
- Tschan, C.A.; Antes, S.; Huthmann, A.; Vulcu, S.; Oertel, J.; Wagner, W. Overcoming CSF overdrainage with the adjustable gravitational valve proSA. Acta Neurochir. 2014, 156, 767–776. [Google Scholar] [CrossRef]
- Suchorska, B.; Kunz, M.; Schniepp, R.; Jahn, K.; Goetz, C.; Tonn, J.C.; Peraud, A. Optimized surgical treatment for normal pressure hydrocephalus: Comparison between gravitational and differential pressure valve. Acta Neurochir. 2015, 157, 703–709. [Google Scholar] [CrossRef]
- Meier, U.; Kiefer, M.; Neumann, U.; Lemcke, J. On the optimal opening pressure of hydrostatic valves in cases of idiopathic normal-pressure hydrocephalus: A prospective randomized study with 123 patients. Acta Neurochir. Suppl. 2006, 96, 358–363. [Google Scholar] [CrossRef]
- Freimann, F.B.; Vajkoczy, P.; Sprung, C. Patients benefit from low-pressure settings enabled by gravitational valves in normal pressure hydrocephalus. Clin. Neurol. Neurosurg. 2013, 115, 1982–1986. [Google Scholar] [CrossRef]
- Trinh, V.T.; Duckworth, E.A. Revision to an adjustable nonsiphon control valve in low pressure hydrocephalus: Therapeutic siphoning and a new perspective on NPH: Series of 3 cases and review of the literature. Clin. Neurol. Neurosurg. 2013, 115, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Funnell, J.P.; D’Antona, L.; Craven, C.L.; Thorne, L.; Watkins, L.D.; Toma, A.K. Ultra-low-pressure hydrocephalic state in NPH: Benefits of therapeutic siphoning with adjustable antigravity valves. Acta Neurochir. 2020, 162, 2967–2974. [Google Scholar] [CrossRef] [PubMed]
- Gutowski, P.; Rot, S.; Fritsch, M.; Meier, U.; Gölz, L.; Lemcke, J. Secondary deterioration in patients with normal pressure hydrocephalus after ventriculoperitoneal shunt placement: A proposed algorithm of treatment. Fluids Barriers CNS 2020, 17, 18. [Google Scholar] [CrossRef] [PubMed]
- Steinbok, P.; Poskitt, K.J.; Cochrane, D.D.; Kestle, J.R. Prevention of postshunting ventricular asymmetry by transseptal placement of ventricular catheters. A randomized study. Pediatr. Neurosurg. 1994, 21, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Gil, Z.; Siomin, V.; Beni-Adani, L.; Sira, B.; Constantini, S. Ventricular catheter placement in children with hydrocephalus and small ventricles: The use of a frameless neuronavigation system. Childs Nerv. Syst. 2002, 18, 26–29. [Google Scholar] [CrossRef]
- Oi, S.; Abott, R. Loculated ventricles and isolated compartments in hydrocephalus: Their pathophysiology and the efficacy of neuroendoscopic surgery. Neurosurg. Clin. N. Am. 2004, 15, 77–87. [Google Scholar] [CrossRef]
- Harter, D.H. Management strategies for treatment of the trapped fourth ventricle. Childs Nerv. Syst. 2004, 20, 710–716. [Google Scholar] [CrossRef]
- Udayakumaran, S.; Biyani, N.; Rosenbaum, D.P.; Ben-Sira, L.; Constantini, S.; Beni-Adani, L. Posterior fossa craniotomy for trapped fourth ventricle in shunt-treated hydrocephalic children: Long-term outcome. J. Neurosurg. Pediatr. 2011, 7, 52–63. [Google Scholar] [CrossRef]
- Butler, W.E.; Khan, S.A. The application of controlled intracranial hypertension in slit ventricle syndrome patients with obstructive hydrocephalus and shunt malfunction. Pediatr. Neurosurg. 2001, 35, 305–310. [Google Scholar] [CrossRef]
- Iglesias, S.; Ros, B.; Ibáñez, G.; Delgado, A.; Ros, A.; Arráez, M.A. Shunt independence in paediatric hydrocephalus: Our 16-year experience and review. Childs Nerv. Syst. 2019, 35, 1547–1555. [Google Scholar] [CrossRef]
- Nadkarni, T.D.; Rekate, H.L.; Wallace, D. Concurrent use of a lumboperitoneal shunt with programable valve and ventricular acceess device in the treatment of pseudotumor cerebri: Review of 40 cases. J. Neurosurg. Pediatr. 2008, 2, 19–24. [Google Scholar] [CrossRef]
- Wang, V.Y.; Barbaro, N.M.; Lawton, M.T.; Pitts, L.; Kunwar, S.; Parsa, A.T.; Gupta, N.; McDermott, M.W. Complications of lumboperitoneal shunts. Neurosurgery 2007, 60, 1045–1048; discussion 1049. [Google Scholar] [CrossRef]
- Yadav, Y.R.; Parihar, V.; Sinha, M. Lumbar peritoneal shunt. Neurol. India 2010, 58, 179–184. [Google Scholar] [CrossRef]
- Yang, T.H.; Chang, C.S.; Sung, W.W.; Liu, J.T. Lumboperitoneal Shunt: A New Modified Surgical Technique and a Comparison of the Complications with Ventriculoperitoneal Shunt in a Single Center. Medicina 2019, 55, 643. [Google Scholar] [CrossRef]
- Rekate, H.L.; Nadkarni, T.; Wallace, D. Severe intracranial hypertension in slit ventricle syndrome managed using a cisterna magna-ventricle-peritoneum shunt. J. Neurosurg. 2006, 104 (Suppl. S4), 240244. [Google Scholar] [CrossRef]
- Obana, W.G.; Raskin, N.H.; Cogen, P.H.; Szymanski, J.A.; Edwards, M.S. Antimigraine treatment for slit ventricle syndrome. Neurosurgery 1990, 27, 760–763. [Google Scholar] [CrossRef]
- Walsh, J.W.; James, H.E. Subtemporal craniectomy and elevation of shunt valve opening pressure in the management of small ventricle-induced cerebrospinal fluid shunt dysfunction. Neurosurgery 1982, 10 Pt 1, 698–703. [Google Scholar] [CrossRef]
- Gough, J.; Walker, D.G.; Theile, R.; Tomlinson, F.H. The role of cranial expansion for craniocephalic disproportion. Pediatr. Neurosurg. 2005, 41, 61–69. [Google Scholar] [CrossRef]
- Martínez-Lage, J.F.; Ruiz-Espejo Vilar, A.; Pérez-Espejo, M.A.; Almagro, M.J.; Ros de San Pedro, J.; Felipe Murcia, M. Shunt-related craniocerebral disproportion: Treatment with cranial vault expanding procedures. Neurosurg. Rev. 2006, 29, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Weinzweig, J.; Bartlett, S.P.; Chen, J.C.; Losee, J.; Sutton, L.; Duhaime, A.C.; Whitaker, L.A. Cranial Vault Expansion in the Management of Postshunt Craniosynostosis and Slit Ventricle Syndrome. Plast. Reconstr. Surg. 2008, 122, 1171–1180. [Google Scholar] [CrossRef]
- Hankinson, T.C.; Mocco, J.; Kimball, B.; Anderson, R.C.; Feldstein, N.A. Internal cranial expansion procedure for the treatment of symptomatic intracranial hypertension. J. Neurosurg. 2007, 107 (Suppl. S5), 402–405. [Google Scholar] [CrossRef]
- Ellis, J.A.; Anderson, R.C.; O’Hanlon, J.; Goodman, R.R.; Feldstein, N.A.; Ghatan, S. Internal cranial expansion surgery for the treatment of refractory idiopathic intracranial hypertension. J. Neurosurg. Pediatr. 2012, 10, 14–20. [Google Scholar] [CrossRef]
- Fabiano, A.J.; Siddiqui, A.H. Spinal cord syrinx expansion following acquired Chiari malformation decompression: Case report. Clin. Neurol. Neurosurg. 2010, 112, 832–834. [Google Scholar] [CrossRef] [PubMed]
- Peleggi, A.F.; Lovely, T.J. Treatment of delayed Chiari malformation and syringomyelia after lumboperitoneal shunt placement: Case report and treatment recommendations. Surg. Neurol. Int. 2012, 3, 101. [Google Scholar] [CrossRef] [PubMed]
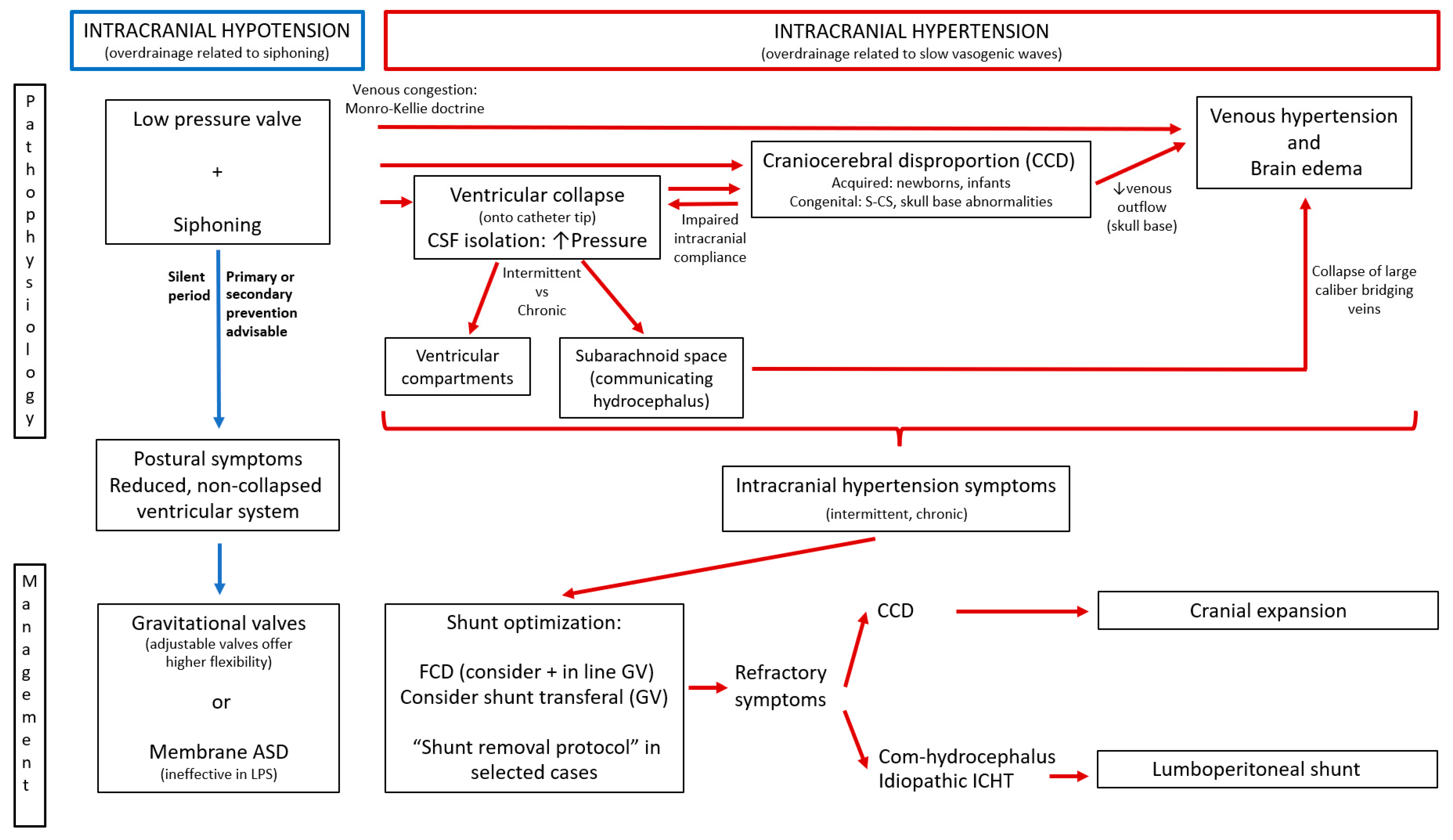
| SOS Integrative Model | Intracranial Hypotension | Intracranial Hypertension | ||
|---|---|---|---|---|
| Pathophysiology (Multifactorial) | Siphoning (Triggers Other Mechanisms) | Ventricular Collapse and CSF Isolation | Acquired CCD | Venous Hypertension |
| Description | Role of postural change (Law of Stevin). Venous congestion appears (Monro-Kellie). CVO when Starling resistor is non-functional | Ventricular collapse, distortion of cerebral structures with CSF isolation in ventricular compartments or in the subarachnoid space | Early suture ossification, microcephaly and acquired Chiari. Impaired cerebral compliance. Venous congestion is present | Increased pressure in the subarachnoid space, collapse of large bridging veins, venous hypertension, brain edema and increased cerebral elastance |
| Predisposing factors | Low brain elastance (brain atrophy). Infants: PHH, AS. Adults: previous SDH, SDHy, PTH, LOVA, NPH | Shunting at first months of life (PHH, neonatal meningitis), good brain elastance, low pressure valves | Early shunting (premature, newborn, infants), S-CS and skull base abnormalities | Hydrocephalus with venous origin, congenital heart disease, pseudotumor cerebri (idiopathic ICHT), S-CS and skull base abnormalities |
| Clinical manifestations | Chronic: variable silent period, “low-pressure postural headache”, etc. Sinking skin flap or OSAM may appear | Intermittent headaches (SVS): severe headache, nausea-vomiting, altered consciousness, etc. Chronic symptoms may appear between symptomatic crises. | Chronic states: weakness, irritability, poor food tolerance, developmental delay, etc. Acute episodes may appear | Symptoms and signs of sustained ICHT: papilledema, altered consciousness, bradycardia or systemic hypertension |
| Radiological findings | Small ventricles (moderate or normal sized also found). Extra-axial collections of fluid or blood. MRI: widened brain sulci, calcifications, etc. | Small ventricles with collapse onto the catheter tip. Occasionally with ventricular asymmetry, isolated compartments or IFV | Small ventricles. Suture sclerosis, laminated thickening, posterior fossa hypoplasia, etc. MRI: obliterated subarachnoid space, Chiari, spinal canal stenosis | Small ventricles with collapse onto the catheter tip. MRI: diffuse meningeal and arachnoid thickening, gadolinium hyperuptake, venous congestion, brain edema |
| ICP patterns | “Siphoning” pattern: Low/negative Pb Decrease < −10 mmHg in tilting tests Infusion tests: Pb < 0, Rcsf < 8, pulse amplitude < 4, RAP < 0.6 | “Overdrainage related to ICP slow vasogenic waves”: Irregular traces, triangular shape of ICP pulse waveform, B waves storms Mean ICP > 20 mmHg, ICP peaks > 25 mmHg, RAP peaks > 0.6 Raised AMP and SLOW during the wave episodes Infusion tests: Pb usually high but pulse wave rarely visible | ||
| Management strategies Primary or secondary prevention advisable | GV or Membrane ASD (ineffective in LPS) | 1st. Shunt optimization: FCD recommended, consider combination with in-line GV. (Consider shunt transferal or “shunt removal protocol” in selected cases) 2nd. Cranial expansion in congenital or acquired CCD, particularly in S-CS and skull base abnormalities 3rd. LPS in communicating hydrocephalus or idiopathic ICHT. High resistance systems recommended | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ros, B.; Iglesias, S.; Linares, J.; Cerro, L.; Casado, J.; Arráez, M.A. Shunt Overdrainage: Reappraisal of the Syndrome and Proposal for an Integrative Model. J. Clin. Med. 2021, 10, 3620. https://doi.org/10.3390/jcm10163620
Ros B, Iglesias S, Linares J, Cerro L, Casado J, Arráez MA. Shunt Overdrainage: Reappraisal of the Syndrome and Proposal for an Integrative Model. Journal of Clinical Medicine. 2021; 10(16):3620. https://doi.org/10.3390/jcm10163620
Chicago/Turabian StyleRos, Bienvenido, Sara Iglesias, Jorge Linares, Laura Cerro, Julia Casado, and Miguel Angel Arráez. 2021. "Shunt Overdrainage: Reappraisal of the Syndrome and Proposal for an Integrative Model" Journal of Clinical Medicine 10, no. 16: 3620. https://doi.org/10.3390/jcm10163620
APA StyleRos, B., Iglesias, S., Linares, J., Cerro, L., Casado, J., & Arráez, M. A. (2021). Shunt Overdrainage: Reappraisal of the Syndrome and Proposal for an Integrative Model. Journal of Clinical Medicine, 10(16), 3620. https://doi.org/10.3390/jcm10163620





