The Role of Demographics, Social Deprivation and Ethnicity on Anal Squamous Cell Carcinoma Incidence in England
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Outcomes
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Collection
2.5. Statistical Analysis
3. Results
3.1. Ethnicity
3.2. Social Deprivation
3.3. Staging and Treatment
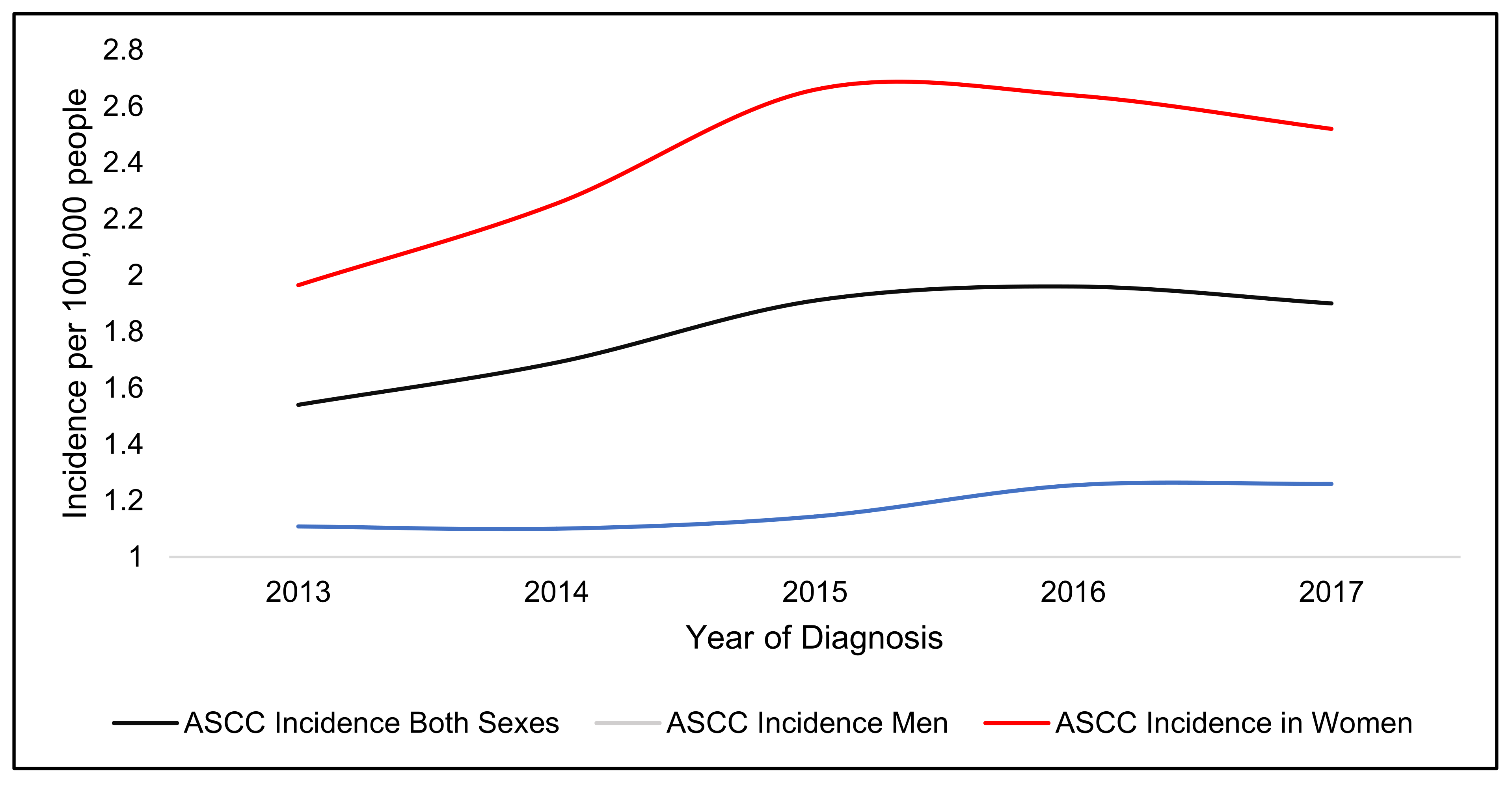
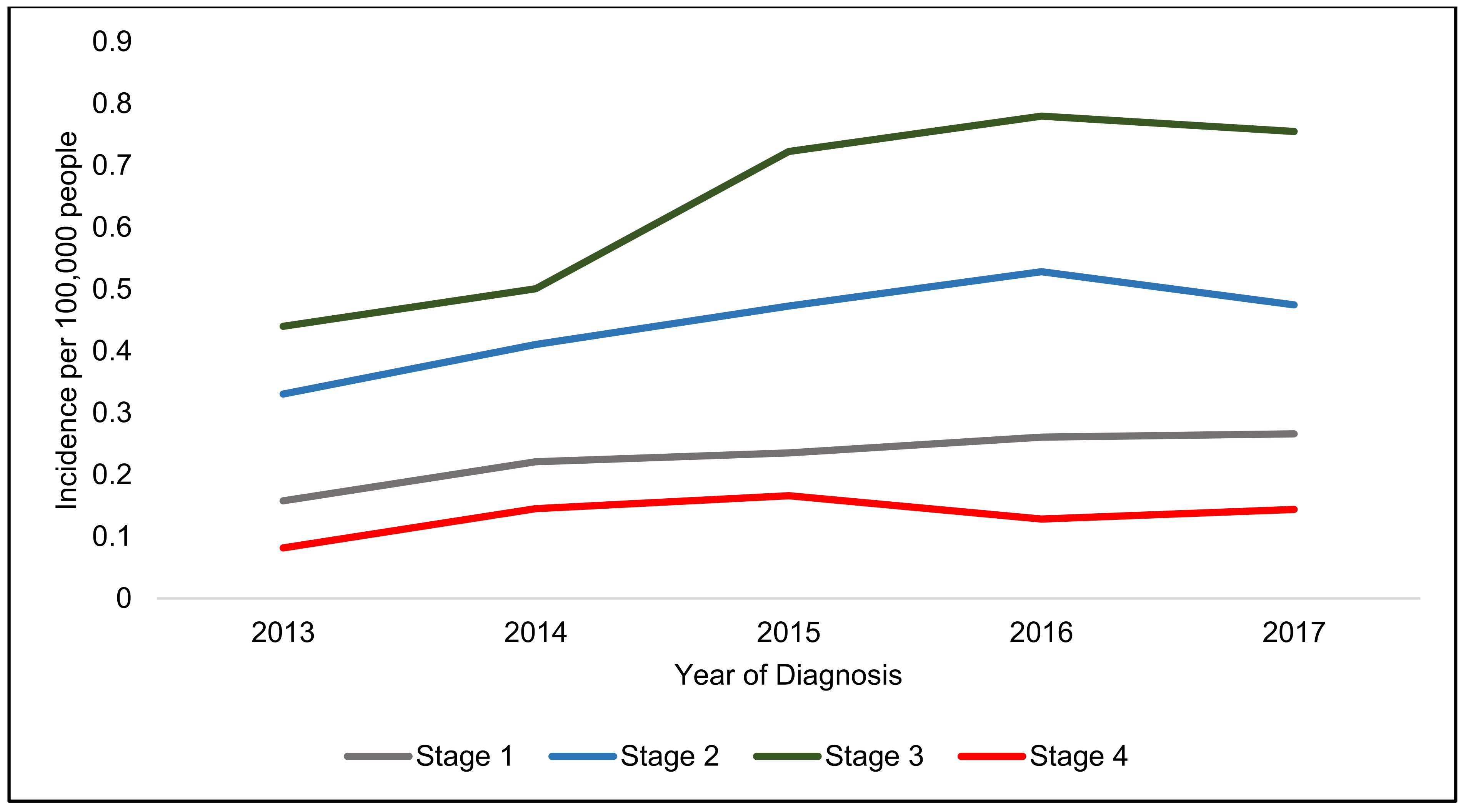
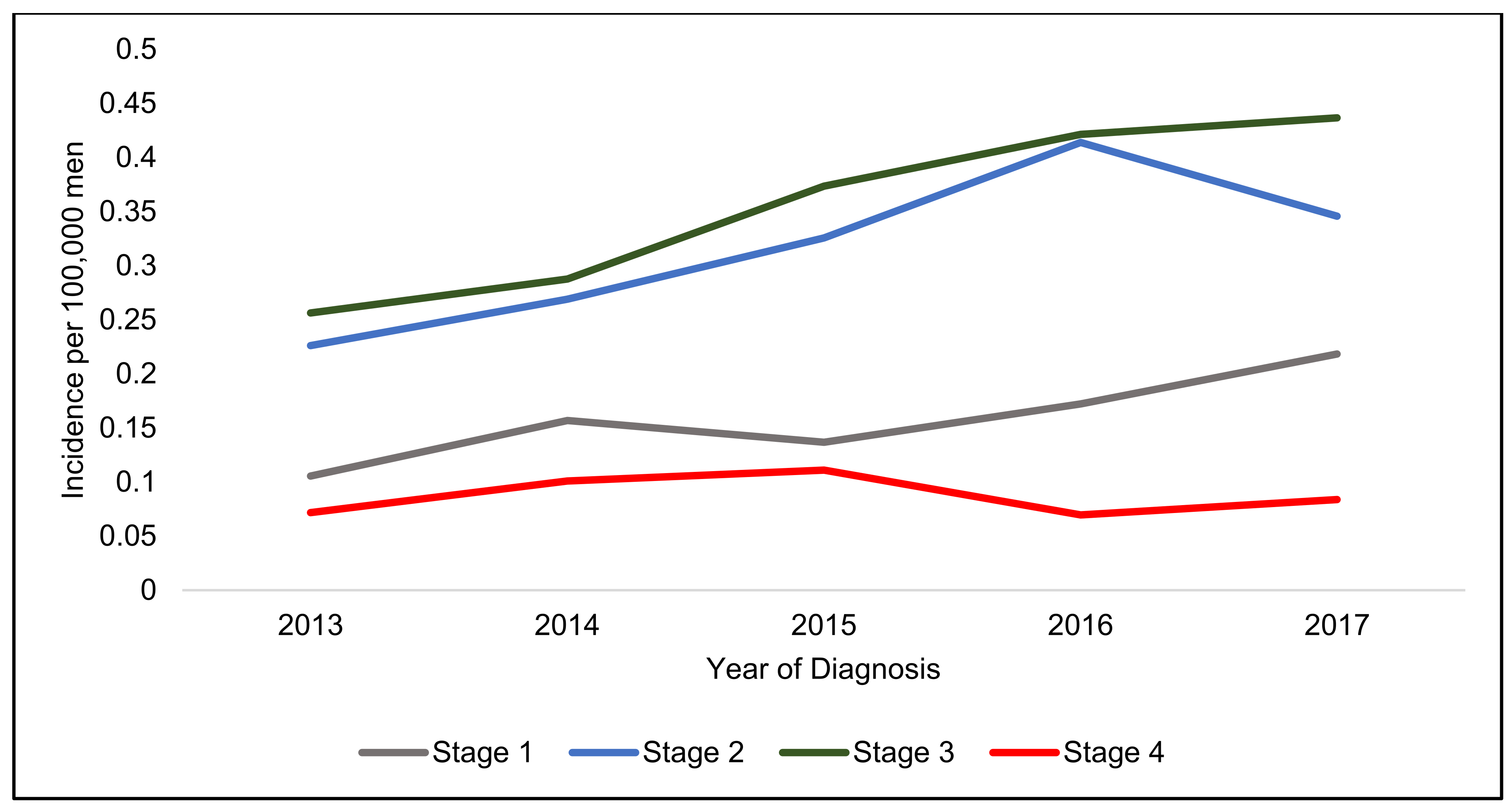
4. Incidence of Anal Squamous Cell Carcinoma
5. Discussion
Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- James, R.D.; Glynne-Jones, R.; Meadows, H.M.; Cunningham, D.; Myint, A.S.; Saunders, M.P.; Maughan, T.; McDonald, A.; Essapen, S.; Leslie, M.; et al. Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): A randomised, phase 3, open-label, 2×2 factorial trial. Lancet Oncol. 2013, 14, 516–524. [Google Scholar] [CrossRef]
- Islami, F.; Ferlay, J.; Lortet-Tieulent, J.; Bray, F.; Jemal, A. International trends in anal cancer incidence rates. Int. J. Epidemiol. 2017, 46, 924–938. [Google Scholar] [CrossRef]
- Tweed, E.J.; Allardice, G.M.; McLoone, P.; Morrison, D.S. Socio-economic inequalities in the incidence of four common cancers: A population-based registry study. Public Health 2018, 154, 1–10. [Google Scholar] [CrossRef]
- Teng, A.M.; Atkinson, J.; Disney, G.; Wilson, N.; Blakely, T. Changing socioeconomic inequalities in cancer incidence and mortality: Cohort study with 54 million person-years follow-up 1981-2011. Int. J. Cancer 2017, 140, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Woods, L.M.; Rachet, B.; Coleman, M.P. Origins of socio-economic inequalities in cancer survival: A review. Annals Oncol. Off. J. Eur. Soc. Med. Oncol. 2006, 17, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Lyratzopoulos, G.; Abel, G.A.; Brown, C.H.; Rous, B.A.; Vernon, S.A.; Roland, M.; Greenberg, D.C. Socio-demographic inequalities in stage of cancer diagnosis: Evidence from patients with female breast, lung, colon, rectal, prostate, renal, bladder, melanoma, ovarian and endometrial cancer. Annals Oncol. Off. J. Eur. Soc. Med. Oncol. 2013, 24, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Bryere, J.; Dejardin, O.; Launay, L.; Colonna, M.; Grosclaude, P.; Launoy, G.; French Network of Cancer, R. Socioeconomic status and site-specific cancer incidence, a Bayesian approach in a French Cancer Registries Network study. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. (ECP) 2018, 27, 391–398. [Google Scholar] [CrossRef]
- Celie, K.-B.; Jackson, C.; Agrawal, S.; Dodhia, C.; Guzman, C.; Kaufman, T.; Hellenthal, N.; Monie, D.; Monzon, J.; Oceguera, L. Socioeconomic and gender disparities in anal cancer diagnosis and treatment. Surg. Oncol. 2017, 26, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Gold, H.T.; Schreiber, D.; Leichman, L.P.; Sherman, S.E.; Becker, D.J. Impact of socioeconomic status on survival for patients with anal cancer. Cancer 2018, 124, 1791–1797. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.R.; Susko, M.; Lindquist, K.; Anwar, M. Socioeconomic disparities in timeliness of care and outcomes for anal cancer patients. Cancer Med. 2019, 8, 7186–7196. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. PLoS Med. 2007, 4, 296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ministry of Housing, Communities and Local Government. English Indices of Deprivation. Available online: https://www.gov.uk/government/statistics/english-indices-of-deprivation-2019 (accessed on 3 January 2021).
- Goffredo, P.; Utria, A.; Engelbart, J.; Masson, A.; Kalakoti, P.; Hassan, I. The impact of patient demographics vs tumor factors on the prognosis of anal squamous cell carcinoma treated with standard chemoradiation therapy. Dis. Colon Rectum 2018, 61, 196. [Google Scholar] [CrossRef]
- Brogden, D.R.L.; Walsh, U.; Pellino, G.; Kontovounisios, C.; Tekkis, P.; Mills, S.C. Evaluating the efficacy of treatment options for anal intraepithelial neoplasia: A systematic review. Int. J. Colorectal Dis. 2021, 36, 213–226. [Google Scholar] [CrossRef]
- Darragh, T.M.; Colgan, T.J.; Thomas Cox, J.; Heller, D.S.; Henry, M.R.; Luff, R.D.; McCalmont, T.; Nayar, R.; Palefsky, J.M.; Stoler, M.H.; et al. The Lower Anogenital Squamous Terminology Standardization project for HPV-associated lesions: Background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Int. J. Gynecol. Pathol. 2013, 32, 76–115. [Google Scholar] [CrossRef] [Green Version]
- Geh, I.; Gollins, S.; Renehan, A.; Scholefield, J.; Goh, V.; Prezzi, D.; Moran, B.; Bower, M.; Alfa-Wali, M.; Adams, R. Association of Coloproctology of Great Britain & Ireland (ACPGBI): Guidelines for the Management of Cancer of the Colon, Rectum and Anus (2017)—Anal Cancer. Colorectal Dis. 2017, 19, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.B.; Gaertner, W.B.; Glasgow, S.C.; Herzig, D.O.; Feingold, D.; Steele, S.R.; Prepared on Behalf of the Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons; Rectal, S. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for Anal Squamous Cell Cancers (Revised 2018). Dis. Colon Rectum 2018, 61, 755–774. [Google Scholar] [CrossRef]
- Office for National Statistics. National Census. 2011. Available online: https://www.ons.gov.uk/census/2011census/2011censusdata (accessed on 9 January 2021).
- Islami, F.; Fedewa, S.A.; Jemal, A. Trends in cervical cancer incidence rates by age, race/ethnicity, histological subtype, and stage at diagnosis in the United States. Prev. Med. 2019, 123, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Clegg, L.X.; Reichman, M.E.; Miller, B.A.; Hankey, B.F.; Singh, G.K.; Lin, Y.D.; Goodman, M.T.; Lynch, C.F.; Schwartz, S.M.; Chen, V.W.; et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: Selected findings from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer Causes Control CCC 2009, 20, 417–435. [Google Scholar] [CrossRef] [Green Version]
- Beavis, A.L.; Gravitt, P.E.; Rositch, A.F. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer 2017, 123, 1044–1050. [Google Scholar] [CrossRef] [Green Version]
- Akers, A.Y.; Newmann, S.J.; Smith, J.S. Factors underlying disparities in cervical cancer incidence, screening, and treatment in the United States. Curr. Probl. Cancer 2007, 31, 157–181. [Google Scholar] [CrossRef] [PubMed]
- Marlow, L.A.V.; Waller, J.; Wardle, J. Barriers to cervical cancer screening among ethnic minority women: A qualitative study. J. Fam. Plan.Reprod. Health Care 2015, 41, 248–254. [Google Scholar] [CrossRef] [Green Version]
- Vrinten, C.; Gallagher, A.; Waller, J.; Marlow, L.A.V. Cancer stigma and cancer screening attendance: A population based survey in England. BMC Cancer 2019, 19, 566. [Google Scholar] [CrossRef] [Green Version]
- Stier, E.A.; Sebring, M.C.; Mendez, A.E.; Ba, F.S.; Trimble, D.D.; Chiao, E.Y. Prevalence of anal human papillomavirus infection and anal HPV-related disorders in women: A systematic review. Am. J. Obstet. Gynecol. 2015, 213, 278–309. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, A.A.; Suk, R.; Shiels, M.S.; Damgacioglu, H.; Lin, Y.Y.; Stier, E.A.; Nyitray, A.G.; Chiao, E.Y.; Nemutlu, G.S.; Chhatwal, J.; et al. Incidence trends and burden of human papillomavirus-associated cancers among women in the United States, 2001–2017. J. Natl. Cancer Inst. 2021, 113, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Johnson, H.C.; Lafferty, E.I.; Eggo, R.M.; Louie, K.; Soldan, K.; Waller, J.; Edmunds, W.J. Effect of HPV vaccination and cervical cancer screening in England by ethnicity: A modelling study. Lancet Public Health 2018, 3, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Murfin, J.; Irvine, F.; Meechan-Rogers, R.; Swift, A. Education, income and occupation and their influence on the uptake of cervical cancer prevention strategies: A systematic review. J. Clin. Nurs. 2020, 29, 393–415. [Google Scholar] [CrossRef]
- Costas-Muniz, R.; Leng, J.; Aragones, A.; Ramirez, J.; Roberts, N.; Mujawar, M.I.; Gany, F. Association of socioeconomic and practical unmet needs with self-reported nonadherence to cancer treatment appointments in low-income Latino and Black cancer patients. Ethn. Health 2016, 21, 118–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brogden, D.R.L.; Kontovounisios, C.; Pellino, G.; Bower, M.; Mills, S.C.; Tekkis, P.P. Improving outcomes for the treatment of Anal Squamous Cell Carcinoma and Anal Intraepithelial Neoplasia. Tech. Coloproctol. 2019, 23, 1109–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Female (n = 3692) | Male (n = 1765) | Statistical Association | |
|---|---|---|---|
| Age | Median = 60–64 years | Median = 60–64 years | p = 0.21 |
| Mode = 60–64 years | Mode = 65–69 years | ||
| Ethnicity | p = 0.39 | ||
| Black African/Black Caribbean | 45 (1.2%) | 19 (1.1%) | |
| Caucasian | 3439 (93.2%) | 1638 (92.8%) | |
| Indian Subcontinent | 21 (0.6%) | 15 (0.9%) | |
| Mixed Black/White | 6 (0.2%) | 1 (0.1%) | |
| Mixed Asian/White | 5 (0.1%) | 1 (0.1%) | |
| Other Mixed | 3 (0.1%) | 5 (0.3%) | |
| Chinese | 2 (0.1%) | 2 (0.1%) | |
| Other Asian | 4 (0.1%) | 4 (0.2%) | |
| Other Ethnicity | 32 (0.9%) | 21 (1.2%) | |
| Unknown Ethnicity | 129 (3.5%) | 59 (3.3%) | |
| Deprivation score | p = 0.06 | ||
| Score 1 (least deprived) | 605 (16.4%) | 260 (14.7%) | |
| Score 2 | 735 (19.9%) | 324 (18.4%) | |
| Score 3 | 719 (19.5%) | 364 (20.6%) | |
| Score 4 | 791 (21.4%) | 356 (20.2%) | |
| Score 5 (most deprived) | 739 (20.0%) | 403 (22.8%) | |
| Unknown | 103 (2.8%) | 58 (3.3%) | |
| Staging | p < 0.001 | ||
| Stage 1 | 456 (12.4%) | 228 (12.9%) | |
| Stage 2 | 875 (23.7%) | 479 (27.1%) | |
| Stage 3 | 1411 (38.2%) | 552 (31.3%) | |
| Stage 4 | 274 (7.4%) | 135 (7.7%) | |
| Unknown Staging | 676 (18.3%) | 371 (21.0%) |
| Treatment Received | Stage 1 (n = 684) | Stage 2 (n = 1354) | Stage 3 (n = 1963) | Stage 4 (n = 409) | Unknown Staging (n = 1047) | Statistical Association |
|---|---|---|---|---|---|---|
| Surgery alone | 239 (34.9%) | 160 (11.8%) | 127 (6.5%) | 43(10.5%) | 238(22.7%) | p < 0.001 |
| Chemotherapy alone | 10 (1.5%) | 18 (1.3%) | 45 (2.3%) | 32 (7.8%) | 26 (2.5%) | p < 0.001 |
| Radiotherapy alone | 42 (6.1%) | 121 (8.9%) | 183 (9.3%) | 52 (12.7%) | 105 (10.0%) | p = 0.01 |
| Surgery and Chemotherapy | 12 (1.8%) | 11 (0.8%) | 22 (1.1%) | 19 (4.7%) | 14 (1.3%) | p < 0.001 |
| Surgery and Radiotherapy | 23 (3.4%) | 60 (4.4%) | 84 (4.3%) | 20 (4.9%) | 41 (3.9%) | p = 0.72 |
| Chemoradiotherapy | 165 (24.1%) | 615 (45.4%) | 872 (44.4%) | 97 (2.2%) | 274 (26.2%) | p < 0.001 |
| Surgery and Chemoradiotherapy | 117 (17.1%) | 209 (15.4%) | 350 (17.8%) | 49 (12.0%) | 101 (9.7%) | p < 0.001 |
| No treatment | 76 (11.1%) | 160 (11.8%) | 280 (14.3%) | 97 (23.7%) | 248 (23.7%) | p < 0.001 |
| Staging | Incidence Rate 2017 (100,000 People) | Incidence Increase (%) | Incidence Rate 2017 (100,000 Women) | Incidence Increase (%) | Incidence Rate 2017 (100,000 Men) | Incidence Increase (%) |
|---|---|---|---|---|---|---|
| Stage 1 | 0.27 | 68.6 | 0.31 | 50.0 | 0.22 | 106.9 |
| Stage 2 | 0.47 | 43.6 | 0.60 | 39.1 | 0.35 | 52.9 |
| Stage 3 | 0.76 | 71.6 | 1.07 | 72.4 | 0.44 | 70.4 |
| Stage 4 | 0.14 | 76.1 | 0.20 | 121.5 | 0.08 | 16.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brogden, D.R.L.; Kontovounisios, C.; Mandalia, S.; Tekkis, P.; Mills, S.C. The Role of Demographics, Social Deprivation and Ethnicity on Anal Squamous Cell Carcinoma Incidence in England. J. Clin. Med. 2021, 10, 3621. https://doi.org/10.3390/jcm10163621
Brogden DRL, Kontovounisios C, Mandalia S, Tekkis P, Mills SC. The Role of Demographics, Social Deprivation and Ethnicity on Anal Squamous Cell Carcinoma Incidence in England. Journal of Clinical Medicine. 2021; 10(16):3621. https://doi.org/10.3390/jcm10163621
Chicago/Turabian StyleBrogden, Danielle R. L., Christos Kontovounisios, Sundhiya Mandalia, Paris Tekkis, and Sarah C. Mills. 2021. "The Role of Demographics, Social Deprivation and Ethnicity on Anal Squamous Cell Carcinoma Incidence in England" Journal of Clinical Medicine 10, no. 16: 3621. https://doi.org/10.3390/jcm10163621
APA StyleBrogden, D. R. L., Kontovounisios, C., Mandalia, S., Tekkis, P., & Mills, S. C. (2021). The Role of Demographics, Social Deprivation and Ethnicity on Anal Squamous Cell Carcinoma Incidence in England. Journal of Clinical Medicine, 10(16), 3621. https://doi.org/10.3390/jcm10163621







