Reappraisal of Pediatric Normal-Pressure Hydrocephalus
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Case Presentations
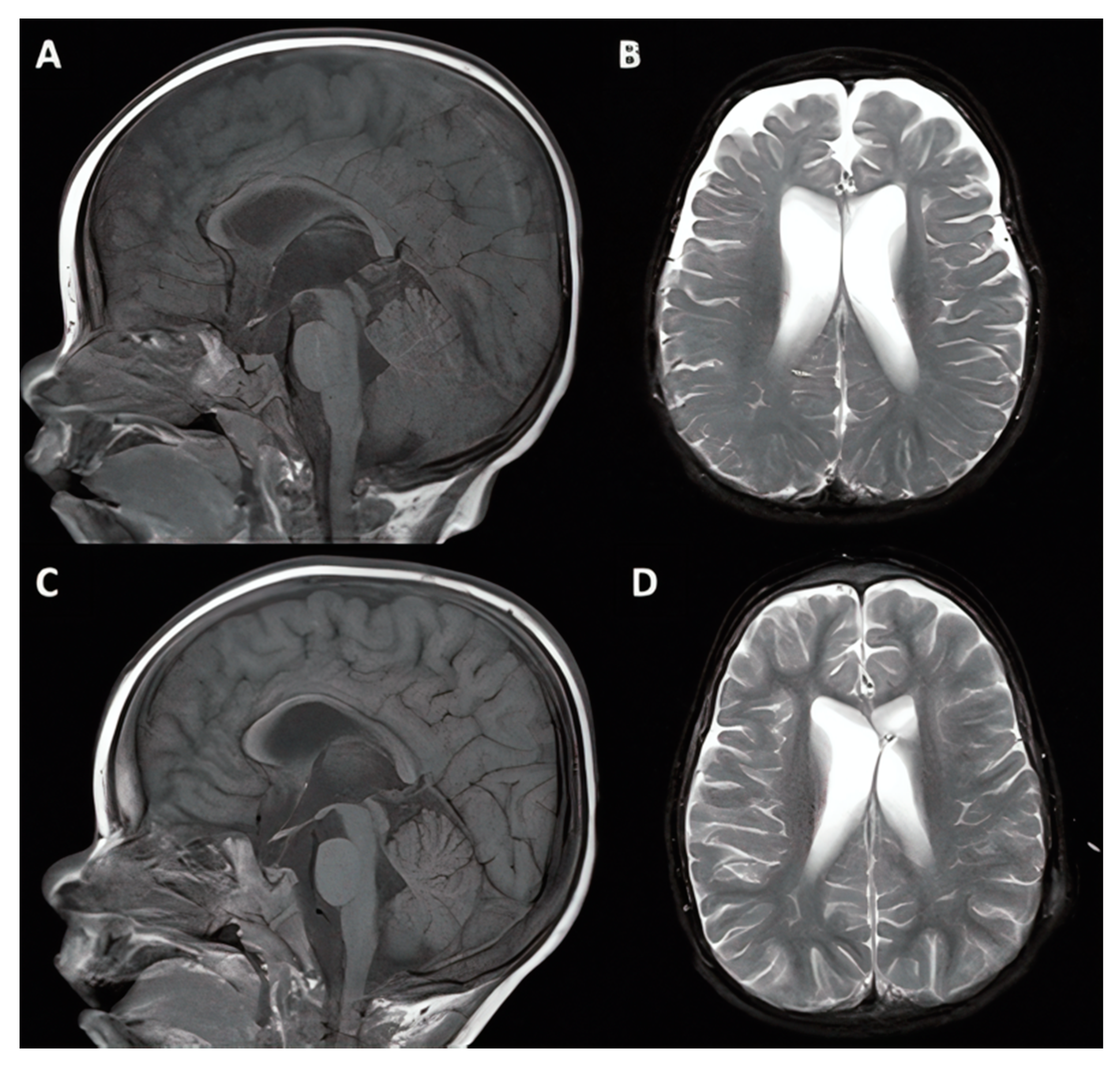
3.1.1. Case #1. Hydrocephalus with Chiari I Malformation
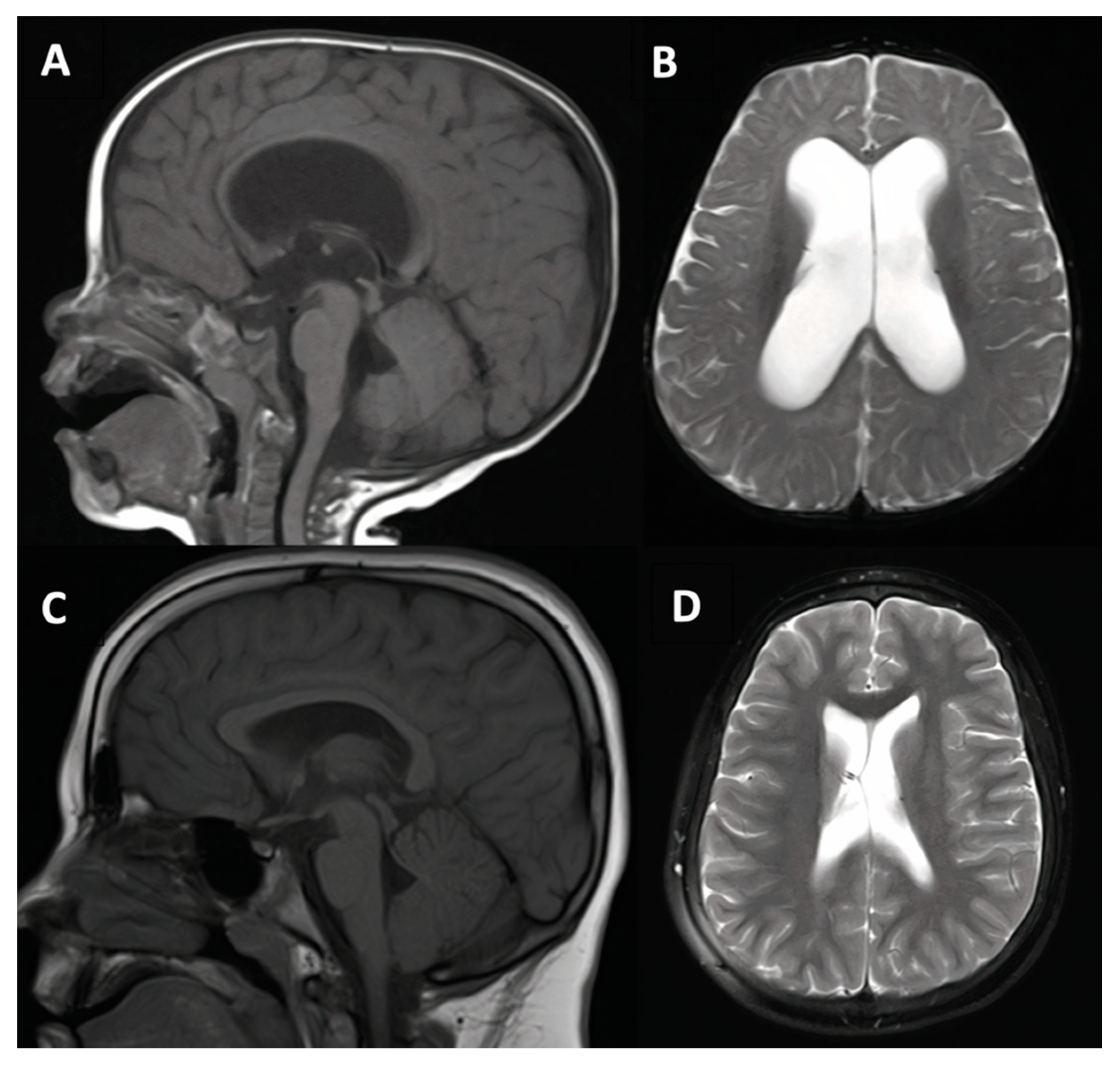
3.1.2. Case #2. Congenital Hydrocephalus with Cerebral Palsy
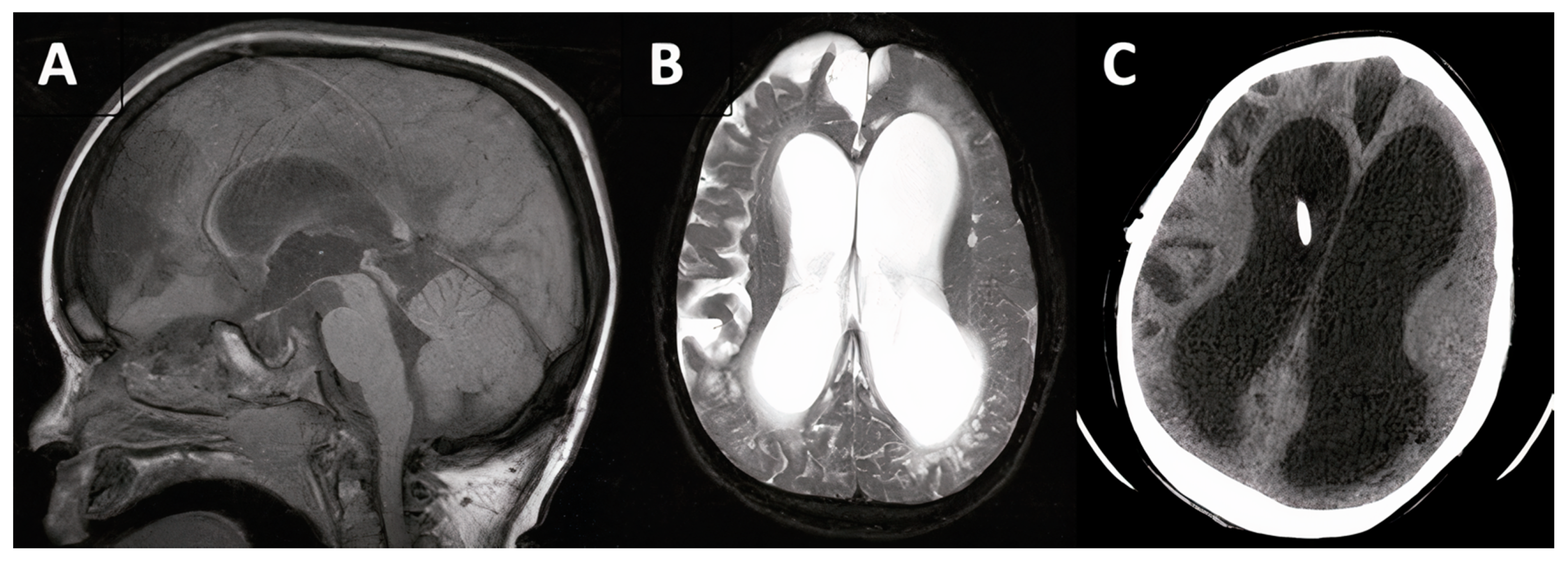
3.1.3. Case #4. Congenital Hydrocephalus with Prematurity
3.1.4. Cases #7–8. Hydrocephalus with Hunter Syndrome
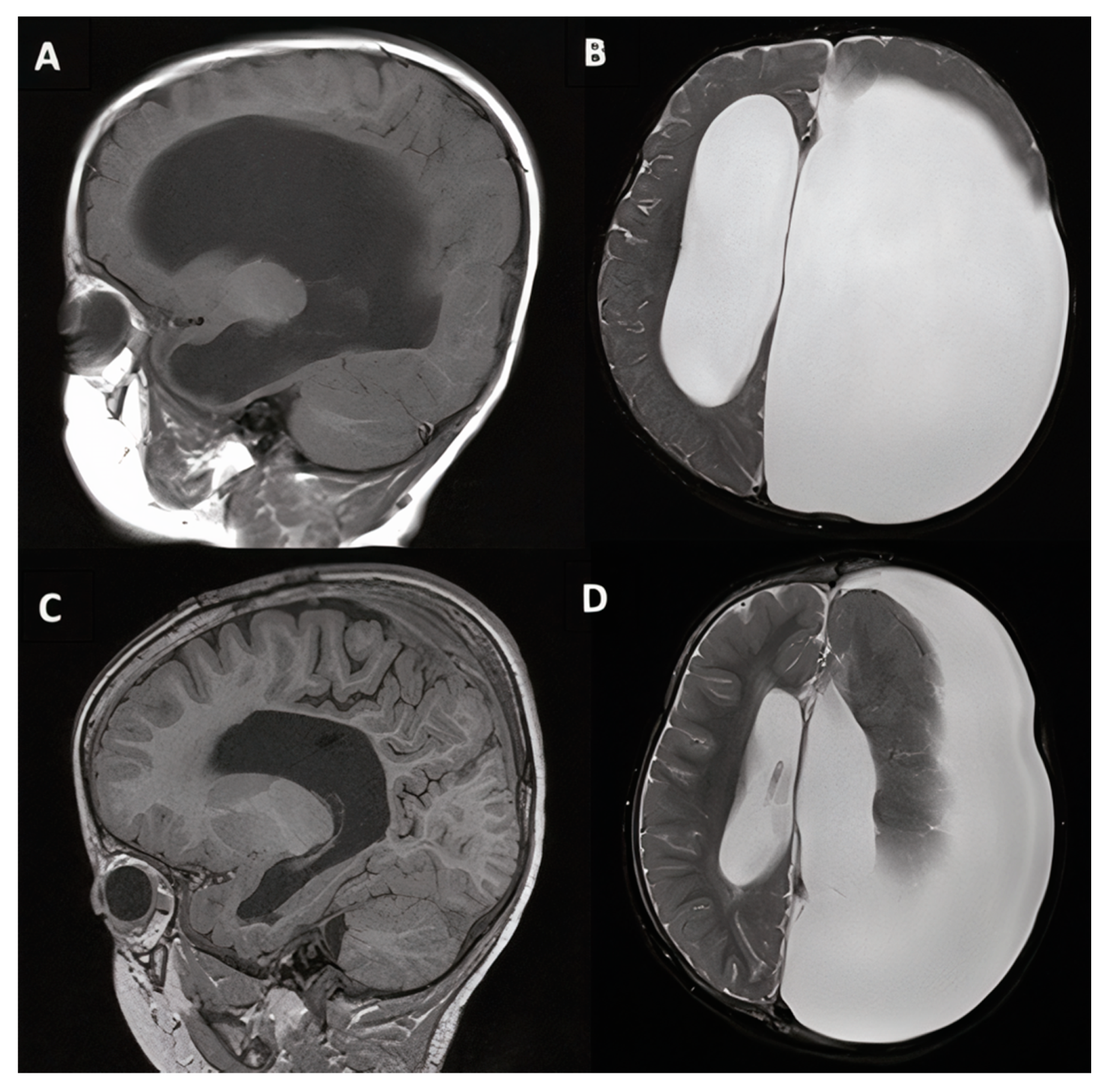
3.1.5. Case #9. Congenital Hydrocephalus Following Intrauterine Stroke
4. Discussion
4.1. Mechanisms of Pediatric NPH
4.2. Treatment and Outcome
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rocca, M.S.; Piatti, G.; Michelucci, A.; Guazzo, R.; Bertini, V.; Vinanzi, C.; Caligo, M.A.; Valetto, A.; Foresta, C. A novel genetic variant in DNAI2 detected by custom gene panel in a newborn with Primary Ciliary Dyskinesia: Case report. BMC Med. Genet. 2020, 21, 220. [Google Scholar] [CrossRef] [PubMed]
- Bateman, G.A.; Yap, S.L.; Subramanian, G.M.; Bateman, A.R. The incidence of significant venous sinus stenosis and cerebral hyperemia in childhood hydrocephalus: Prognostic value with regards to differentiating active from compensated disease. Fluids Barriers CNS 2020, 17, 33. [Google Scholar] [CrossRef]
- Kageyama, H.; Miyajima, M.; Ogino, I.; Nakajima, M.; Shimoji, K.; Fukai, R.; Miyake, N.; Nishiyama, K.; Matsumoto, N.; Arai, H. Panventriculomegaly with a wide foramen of Magendie and large cisterna magna. J. Neurosurg. 2016, 124, 1858–1866. [Google Scholar] [CrossRef] [PubMed]
- Bateman, G. Hyperemic hydrocephalus: A new form of childhood hydrocephalus analogous to hyperemic intracranial hypertension in adults. J. Neurosurg. Pediatr. 2010, 5, 20–26. [Google Scholar] [CrossRef]
- Bateman, G.A.; Smith, R.L.; Siddique, S.H. Idiopathic hydrocephalus in children and idiopathic intracranial hypertension in adults: Two manifestations of the same pathophysiological process? J. Neurosurg. 2007, 107, 439–444. [Google Scholar] [CrossRef]
- Crawford, T.S.; Olivero, W.C.; Hanigan, W.C. The prognosis of children with hydrocephalus and congenital heart disease. Pediatr. Neurosurg. 2000, 33, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Bret, P.; Chazal, J. Chronic (“normal pressure”) hydrocephalus in childhood and adolescence. A review of 16 cases and reappraisal of the syndrome. Childs Nerv. Syst. 1995, 11, 687–691. [Google Scholar] [CrossRef]
- Czosnyka, M.; Batorski, L.; Roszkowski, M.; Tomaszewski, J.; Wocjan, J.; Walencik, A.; Zabolotny, W. Cerebrospinal compensation in hydrocephalic children. Childs Nerv. Syst. 1993, 9, 17–22. [Google Scholar] [CrossRef]
- el Awad, M.E. Infantile hydrocephalus in the south-western region of Saudi Arabia. Ann. Trop. Pediatr. 1992, 12, 335–338. [Google Scholar] [CrossRef]
- Barnett, G.H.; Hahn, J.F.; Palmer, J. Normal pressure hydrocephalus in children and young adults. Neurosurgery 1987, 20, 904–907. [Google Scholar] [CrossRef]
- Torkelson, R.D.; Leibrock, L.G.; Gustavson, J.L.; Sundell, R.R. Neurological and neuropsychological effects of cerebral spinal fluid shunting in children with assumed arrested (“normal pressure”) hydrocephalus. J. Neurol. Neurosurg. Psychiatry 1985, 48, 799–806. [Google Scholar] [CrossRef]
- Hill, A.; Volpe, J.J. Normal pressure hydrocephalus in the newborn. Pediatrics 1981, 68, 623–629. [Google Scholar] [PubMed]
- Brumback, R.A.; Yoder, F.W.; Andrews, A.D.; Peck, G.L.; Robbins, J.H. Normal pressure hydrocephalus. Recognition and relationship to neurological abnormalities in Cockayne’s syndrome. Arch. Neurol. 1978, 35, 337–345. [Google Scholar] [CrossRef]
- Hammock, M.K.; Milhorat, T.H.; Baron, I.S. Normal pressure hydrocephalus in patients with myelomeningocele. Dev. Med. Child Neurol. Suppl. 1976. [Google Scholar] [CrossRef] [PubMed]
- Stein, B.M.; Fraser, R.A.; Tenner, M.S. Normal pressure hydrocephalus: Complication of posterior fossa surgery in children. Pediatrics 1972, 49, 50–58. [Google Scholar]
- Hakim, S.; Adams, R.D. The special clinical problem of symptomatic hydrocephalus with normal cerebrospinal fluid pressure. Observations on cerebrospinal fluid hydrodynamics. J. Neurol. Sci. 1965, 2, 307–327. [Google Scholar] [CrossRef]
- Relkin, N.; Marmarou, A.; Klinge, P.; Bergsneider, M.; Black, P.M. Diagnosing idiopathic normal-pressure hydrocephalus. Neurosurgery 2005, 57, S4–S16. [Google Scholar] [CrossRef]
- Bergsneider, M.; Black, P.M.; Klinge, P.; Marmarou, A.; Relkin, N. Surgical management of idiopathic normal-pressure hydrocephalus. Neurosurgery 2005, 57, S29–S39. [Google Scholar] [CrossRef] [PubMed]
- Daou, B.; Klinge, P.; Tjoumakaris, S.; Rosenwasser, R.H.; Jabbour, P. Revisiting secondary normal pressure hydrocephalus: Does it exist? A review. Neurosurg. Focus 2016, 41, E6. [Google Scholar] [CrossRef]
- Klinge, P.; Hellström, P.; Tans, J.; Wikkelsø, C. One-year outcome in the European multicentre study on iNPH. Acta Neurol. Scand. 2012, 126, 145–153. [Google Scholar] [CrossRef]
- Klinge, P.; Marmarou, A.; Bergsneider, M.; Relkin, N.; Black, P.M. Outcome of shunting in idiopathic normal-pressure hydrocephalus and the value of outcome assessment in shunted patients. Neurosurgery 2005, 57, S40–S52. [Google Scholar] [CrossRef]
- Hill, A. Ventricular dilation following intraventricular hemorrhage in the premature infant. Can. J. Neurol. Sci. 1983, 10, 81–85. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Taghdiri, F.; Gumus, M.; Algarni, M.; Fasano, A.; Tang-Wai, D.; Tartaglia, M.C. Association Between Cerebrospinal Fluid Biomarkers and Age-related Brain Changes in Patients with Normal Pressure Hydrocephalus. Sci. Rep. 2020, 10, 9106. [Google Scholar] [CrossRef] [PubMed]
- Niermeyer, M.; Gaudet, C.; Malloy, P.; Piryatinsky, I.; Salloway, S.; Klinge, P.; Lee, A. Frontal Behavior Syndromes in Idiopathic Normal Pressure Hydrocephalus as a Function of Alzheimer’s Disease Biomarker Status. J. Int. Neuropsychol. Soc. 2020, 26, 883–893. [Google Scholar] [CrossRef]
- Fasano, A.; Espay, A.J.; Tang-Wai, D.F.; Wikkelsö, C.; Krauss, J.K. Gaps, Controversies, and Proposed Roadmap for Research in Normal Pressure Hydrocephalus. Mov. Disord. 2020, 35, 1945–1954. [Google Scholar] [CrossRef] [PubMed]
- Marano, M.; Pompucci, A.; Motolese, F.; Rossi, M.; Coletta, E.; Di Lazzaro, V.; Fasano, A.; Petrella, G. Normal Pressure Hydrocephalus in Down Syndrome: The Report of Two Cases. J. Alzheimers Dis. 2020, 77, 979–984. [Google Scholar] [CrossRef]
- Bradley, W.G., Jr. CSF Flow in the Brain in the Context of Normal Pressure Hydrocephalus. AJNR Am. J. Neuroradiol. 2015, 36, 831–838. [Google Scholar] [CrossRef]
- Bateman, G.A. Magnetic resonance imaging quantification of compliance and collateral flow in late-onset idiopathic aqueductal stenosis: Venous pathophysiology revisited. J. Neurosurg. 2007, 107, 951–958. [Google Scholar] [CrossRef]
- Atkins, D.; Best, D.; Briss, P.A.; Eccles, M.; Falck-Ytter, Y.; Flottorp, S.; Guyatt, G.H.; Harbour, R.T.; Haugh, M.C.; Henry, D.; et al. Grading quality of evidence and strength of recommendations. BMJ 2004, 328, 1490. [Google Scholar] [CrossRef]
- West, S.; King, V.; Carey, T.S.; Lohr, K.N.; McKoy, N.; Sutton, S.F.; Lux, L. Systems to rate the strength of scientific evidence. Evid. Rep. Technol. Assess 2002, 47, 1–11. [Google Scholar]
- Wright, J.G.; Swiontkowski, M.F.; Heckman, J.D. Introducing levels of evidence to the journal. J. Bone Jt. Surg. Am. 2003, 85, 1–3. [Google Scholar] [CrossRef]
- Yang, H.W.; Lee, S.; Yang, D.; Dai, H.; Zhang, Y.; Han, L.; Zhao, S.; Zhang, S.; Ma, Y.; Johnson, M.F.; et al. Deletions in CWH43 cause idiopathic normal pressure hydrocephalus. EMBO Mol. Med. 2021, 13, e13249. [Google Scholar] [CrossRef] [PubMed]
- Botfield, H.; Gonzalez, A.M.; Abdullah, O.; Skjolding, A.D.; Berry, M.; McAllister, J.P., 2nd; Logan, A. Decorin prevents the development of juvenile communicating hydrocephalus. Brain 2013, 136, 2842–2858. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Wang, X.; Wang, Y.; Wang, C.; Tang, Z.; Zhang, Z.; Liu, J.; Xiao, G. The Pathogenesis Based on the Glymphatic System, Diagnosis, and Treatment of Idiopathic Normal Pressure Hydrocephalus. Clin. Interv. Aging 2021, 16, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, Y.; Yokoyama, R.; Takashima, H.; Kawata, Y.; Arihara, M.; Chiba, R.; Kimura, Y.; Mikami, T.; Mikuni, N. Accumulation of Macromolecules in Idiopathic Normal Pressure Hydrocephalus. Neurol. Med. Chir. 2021, 61, 211–218. [Google Scholar] [CrossRef]
- Migliorati, K.; Panciani, P.P.; Pertichetti, M.; Borroni, B.; Archetti, S.; Rozzini, L.; Padovani, A.; Terzi, L.; Bruscella, S.; Fontanella, M.M. P-Tau as prognostic marker in long term follow up for patients with shunted iNPH. Neurol. Res. 2021, 43, 78–85. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Mestre, H.; Nedergaard, M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018, 17, 1016–1024. [Google Scholar] [CrossRef]
- Silverberg, G.D.; Miller, M.C.; Machan, J.T.; Johanson, C.E.; Caralopoulos, I.N.; Pascale, C.L.; Heile, A.; Klinge, P.M. Amyloid and Tau accumulate in the brains of aged hydrocephalic rats. Brain Res. 2010, 1317, 286–296. [Google Scholar] [CrossRef]
- Deren, K.E.; Forsyth, J.; Abdullah, O.; Hsu, E.W.; Klinge, P.M.; Silverberg, G.D.; Johanson, C.E.; McAllister, J.P., 2nd. Low levels of amyloid-beta and its transporters in neonatal rats with and without hydrocephalus. Cerebrospinal Fluid Res. 2009, 6, 4. [Google Scholar] [CrossRef]
- Golomb, J.; Wisoff, J.; Miller, D.C.; Boksay, I.; Kluger, A.; Weiner, H.; Salton, J.; Graves, W. Alzheimer’s disease comorbidity in normal pressure hydrocephalus: Prevalence and shunt response. J. Neurol. Neurosurg. Psychiatry 2000, 68, 778–781. [Google Scholar] [CrossRef]
- Eklund, A.; Agren-Wilsson, A.; Andersson, N.; Bergenheim, A.T.; Koskinen, L.O.; Malm, J. Two computerized methods used to analyze intracranial pressure B waves: Comparison with traditional visual interpretation. J. Neurosurg. 2001, 94, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Raftopoulos, C.; Chaskis, C.; Delecluse, F.; Cantraine, F.; Bidaut, L.; Brotchi, J. Morphological quantitative analysis of intracranial pressure waves in normal pressure hydrocephalus. Neurol. Res. 1992, 14, 389–396. [Google Scholar] [CrossRef]
- Lundberg, N. Continuous recording and control of ventricular fluid pressure in neurosurgical practice. Acta Psychiatr. Scand. Suppl. 1960, 36, 1–193. [Google Scholar] [CrossRef]
- Iborra, J.; Pagès, E.; Cuxart, A.; Poca, A.; Sahuquillo, J. Increased intracranial pressure in myelomeningocele (MMC) patients never shunted: Results of a prospective preliminary study. Spinal Cord. 2000, 38, 495–497. [Google Scholar] [CrossRef][Green Version]
- Mataró, M.; Poca, M.A.; Sahuquillo, J.; Cuxart, A.; Iborra, J.; de la Calzada, M.D.; Junqué, C. Cognitive changes after cerebrospinal fluid shunting in young adults with spina bifida and assumed arrested hydrocephalus. J. Neurol. Neurosurg. Psychiatry 2000, 68, 615–621. [Google Scholar] [CrossRef] [PubMed]


| Reference | Study Design and Population | # of Patients | Key Findings, NPH Diagnostic Criteria, and Possible or Suggested Etiology | Evidence Level |
|---|---|---|---|---|
| Rocca et al., 2020 [1] |
| 1 | Findings: A specific rare mutation was found in a patient with PCD who also presented with NPH, suggesting a new potential genetic risk factor Diagnosis: Ventriculomegaly on magnetic resonance imaging (MRI) without obstructive etiology Etiology: Mutation of Dynein Axonemal Intermediate Chain 2 (DNAI2), which is involved in cilia motility | 4 |
| Bateman et al., 2020 [2] |
| 36 | Findings: 19/36 patients with communicating hydrocephalus had significant venous sinus stenosis; venous sinus stenosis > 65% appears to be a marker of active (vs. compensated) hydrocephalus Diagnosis: 36 patients with communicating hydrocephalus as identified based on imaging studies indicating ventriculomegaly with at least some aqueductal flow Etiology: Heterogeneous etiologies (Table 2 in [2]) including infectious, congenital, and idiopathic cases | 3 |
| Kageyama et al., 2016 [3] |
| 5 | Findings: PaVM is associated with an NPH-like presentation seen in both adults and children and DNAH14 mutations, and can be successfully treated with endoscopic third ventriculostomy when symptomatic Diagnosis: Ventriculomegaly with patent aqueduct, foramen of Magendie, and large cisterna magna as well as flow void Etiology: Mutation of dynein heavy chain protein gene DNAH14, which is functionally associated with cilia motility | 4 |
| Bateman, 2010 [4] |
| 9 | Findings: Cerebral arterial flow was elevated, but sinus outflow was similar, in pediatric idiopathic hydrocephalus relative to age-matched controls; Increase in sinus pressure may lead to communicating hydrocephalus Diagnosis: Symptomatic idiopathic ventriculomegaly on imaging with (n = 3) or without (n = 6) aqueductal occlusion Etiology: Elevated cerebral sinus pressure leading to communicating hydrocephalus and possible increased, compensatory collateral venous outflow | 3 |
| Bateman et al., 2007 [5] |
| 14 | Findings: Cerebral arterial flow was similar, but sinus outflow was reduced, in pediatric idiopathic hydrocephalus relative to age-matched controls. Diagnosis: Symptomatic idiopathic ventriculomegaly on imaging (n = 14) Etiology: Similar to adult patients with idiopathic intracranial hypertension, children with idiopathic hydrocephalus show significant elevation in collateral venous flow; ventricular dilation may depend on brain compliance in the presence of these altered flow dynamics | 3 |
| Crawford et al., 2000 [6] |
| 3 | Findings: Of 11 children with hydrocephalus and congenital heart defects, 3 had idiopathic hydrocephalus. The population was characterized by developmental disabilities and high mortality rate (9/11 overall) Diagnosis: Symptomatic ventriculomegaly (without aqueductal stenosis or other obstructive cause, in idiopathic cases) Etiology: Not provided/evidenced; “idiopathic” | 4 |
| Bret et al., 1995 [7] |
| 16 | Findings: Among 16 patients < 20 years in age presenting with NPH, most displayed elements of the diagnostic triad, and intracranial pressure (ICP) was found to be within normal limits for 6/6 patients tested; shunting produced symptomatic improvement in 12/16 Diagnosis: ICP pressure measurement (6/6); adapted symptomatic triad, including psychomotor delay, gait abnormality, and urinary incontinence Etiology: Variable, including idiopathic, neonatal intraventricular hemorrhage, prior radiotherapy, aqueductal stenosis | 4 |
| Czosnyka et al., 1993 [8] |
| 18 | Findings: Among 115 cases of pediatric ‘arrested hydrocephalus’ described as hydrocephalus with non-progressing symptoms, 18 were classified as having NPH based on low resting cerebrospinal fluid (CSF) pressure and increased resistance to CSF outflow on lumbar infusion test; others could not be classified using these variables alone Diagnosis: Symptoms and computerized lumbar infusion test results Etiology: Factors frequently linked to the NPH group included birth trauma, head injury, infection, and congenital malformation (not further specified) | 3 |
| El Awad, 1992 [9] |
| 3 (19) | Findings: Among 62 cases of infantile hydrocephalus identified in southwestern Saudi Arabia in a 3 year period, 3 had congenital idiopathic hydrocephalus, though 16 additional cases of “non-idiopathic” hydrocephalus were secondary to either cerebral hemorrhage or meningitis Diagnosis: retrospective epidemiological study (available hospital records) Etiology: Congenital idiopathic; 16 additional cases linked to pre- or postnatal hemorrhage or infection consistent with NPH-like etiology, but insufficient detail to be certain | 4 |
| Barnett et al., 1987 [10] |
| 4 | Findings: 4 cases in which NPH was identified in children and young adults underwent VPS with improvement in symptoms Diagnosis: Normal measured CSF pressure with ventriculomegaly, chronic or progressive neurologic deficits, and functional deterioration at presentation Etiology: Intraventricular hemorrhage, pre-mature birth w/cerebral palsy, meningitis, progressive cerebral atrophy | 4 |
| Torkelson et al., 1985 [11] |
| 4 | Findings: 4 cases in which pediatric hydrocephalus was not actively progressing and findings of sometimes subtle cognitive impairment on neuropsychology testing benefitted from shunting, suggesting sometimes subtle presentation of NPH in children Diagnosis: Ventriculomegaly with normal CSF pressure, neuropsychology testing consistent with cognitive impairment, and variable functional impairment and gait disturbances across the four patients Etiology: Variable, including idiopathic, meningitis, ventricular hemorrhage, and unknown due to unavailable early history | 4 |
| Hill and Volpe, 1981 [12] |
| 20 | Findings: Of 87 patients born prematurely with intraventricular hemorrhage, 20 were found to develop NPH, highlighting these factors as potentially etiological in the development of NPH in children. Early recognition and suspicion can prevent harmful complications. Some cases may progress while others do not Diagnosis: Ventriculomegaly with serial imaging and correlation with symptoms Etiology: Prematurity with IVH | 4 |
| Brumback et al., 1978 [13] |
| 4 | Findings: 4 cases of children presenting with Cockayne’s syndrome also present with symptomatic NPH, suggesting possible comorbidity between these disorders Diagnosis: Ventriculomegaly with developmental delay during childhood and gait disturbance, urinary incontinence, and/or psychomotor delay; normal CSF pressure in patients who were tested (2/4) Etiology: Premature birth with progressive neurologic anomalies classic to Cockayne’s syndrome | 4 |
| Hammock et al., 1976 [14] |
| 8 | Findings: NPH-like presentation was identified in 8 patients with ventricular enlargement and known myelomeningocele, suggesting possible comorbidity related to flow dynamics; improvement after shunting Diagnosis: Ventriculomegaly with failure to keep up with developmental milestones on detailed neuropsychological testing (verbal outpacing overall performance), and improvement after shunting Etiology: Possibly related to flow dynamics in the setting of underlying myelomeningocele | 4 |
| Stein et al., 1972 [15] |
| 3 | Findings: NPH as a subtle complication of posterior fossa surgery is a recognizable post-operative event which can be successfully treated with shunting Diagnosis: Symptomatic, progressive post-operative ventriculomegaly with triad of symptoms similar to adult; normal CSF pressure Etiology: Possibly related to altered flow dynamics secondary to posterior fossa surgery | 4 |
| Case # | Age and Sex | History and NPH-Like Presentation | MRI Findings | Outcome and Clinical Course Following VPS Placement |
|---|---|---|---|---|
| 1 | 6 y, F |
|
|
|
| 2 | 17 m, M |
|
|
|
| 3 | 20 m, M |
|
|
|
| 4 | 18 m, F |
|
|
|
| 5 | 12 m, F |
|
|
|
| 6 | 3 y, F |
|
|
|
| 7 | 12 y, M |
|
|
|
| 8 | 10 y, M |
|
|
|
| 9 | 18 m, M |
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leary, O.P.; Svokos, K.A.; Klinge, P.M. Reappraisal of Pediatric Normal-Pressure Hydrocephalus. J. Clin. Med. 2021, 10, 2026. https://doi.org/10.3390/jcm10092026
Leary OP, Svokos KA, Klinge PM. Reappraisal of Pediatric Normal-Pressure Hydrocephalus. Journal of Clinical Medicine. 2021; 10(9):2026. https://doi.org/10.3390/jcm10092026
Chicago/Turabian StyleLeary, Owen P., Konstantina A. Svokos, and Petra M. Klinge. 2021. "Reappraisal of Pediatric Normal-Pressure Hydrocephalus" Journal of Clinical Medicine 10, no. 9: 2026. https://doi.org/10.3390/jcm10092026
APA StyleLeary, O. P., Svokos, K. A., & Klinge, P. M. (2021). Reappraisal of Pediatric Normal-Pressure Hydrocephalus. Journal of Clinical Medicine, 10(9), 2026. https://doi.org/10.3390/jcm10092026







