Available Bleeding Scoring Systems Poorly Predict Major Bleeding in the Acute Phase of Pulmonary Embolism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Setting
2.2. Bleeding Definition
2.3. Bleeding Predicting Scores
2.4. Statistical Analysis
3. Results
3.1. Major Bleeding Events
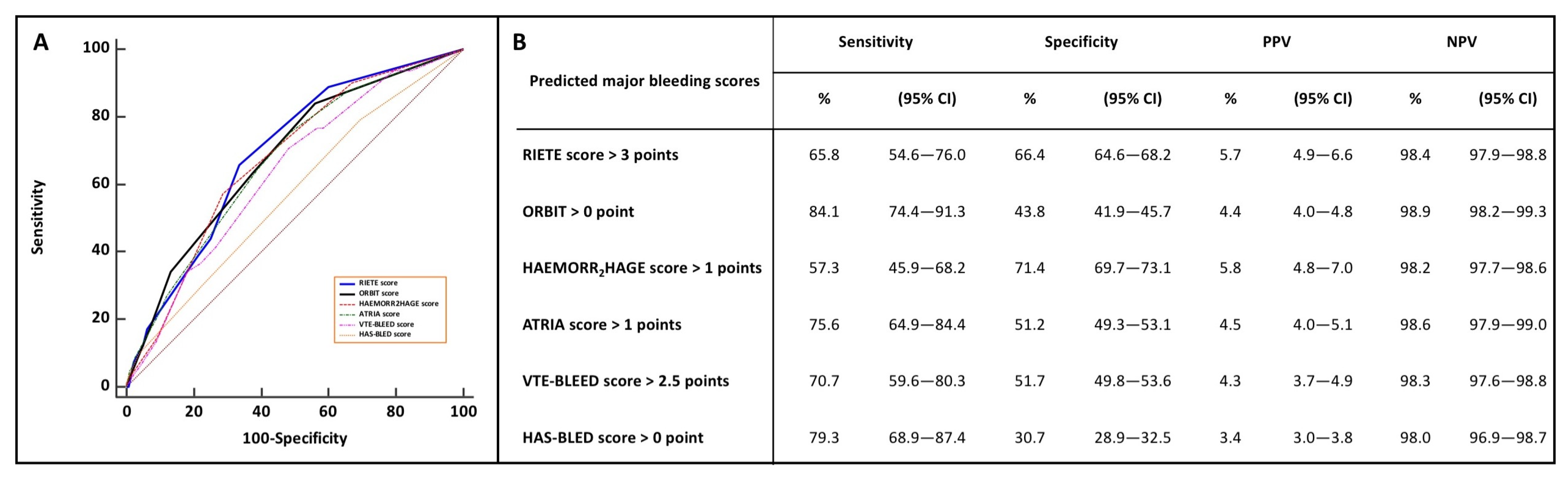
3.2. Bleeding Scores and Prediction of Early Major Bleeding
3.3. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Becattini, C.; Agnelli, G. Acute treatment of venous thromboembolism. Blood 2020, 135, 305–316. [Google Scholar] [CrossRef]
- Becattini, C.; Casazza, F.; Forgione, C.; Porro, F.; Fadin, B.M.; Stucchi, A.; Lignani, A.; Conte, L.; Imperadore, F.; Bongarzoni, A.; et al. Acute pulmonary embolism: External validation of an integrated risk stratification model. Chest 2013, 144, 1539–1545. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jimenez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef] [PubMed]
- Meyer, G.; Vicaut, E.; Danays, T.; Agnelli, G.; Becattini, C.; Beyer-Westendorf, J.; Bluhmki, E.; Bouvaist, H.; Brenner, B.; Couturaud, F.; et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N. Engl. J. Med. 2014, 370, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Lecumberri, R.; Alfonso, A.; Jimenez, D.; Fernandez Capitan, C.; Prandoni, P.; Wells, P.S.; Vidal, G.; Barillari, G.; Monreal, M.; RIETE investigators. Dynamics of case-fatalilty rates of recurrent thromboembolism and major bleeding in patients treated for venous thromboembolism. Thromb. Haemost. 2013, 110, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Kresoja, K.P.; Ebner, M.; Rogge, N.I.J.; Sentler, C.; Keller, K.; Hobohm, L.; Hasenfuss, G.; Konstantinides, S.V.; Pieske, B.; Lankeit, M. Prediction and prognostic importance of in-hospital major bleeding in a real-world cohort of patients with pulmonary embolism. Int. J. Cardiol. 2019, 290, 144–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prandoni, P.; Trujillo-Santos, J.; Sanchez-Cantalejo, E.; Dalla Valle, F.; Piovella, C.; Pesavento, R.; Nieto Rodriguez, J.A.; Monreal, M.; Group, R. Major bleeding as a predictor of mortality in patients with venous thromboembolism: Findings from the RIETE Registry. J. Thromb. Haemost. 2010, 8, 2575–2577. [Google Scholar] [CrossRef]
- Klok, F.A.; Kooiman, J.; Huisman, M.V.; Konstantinides, S.; Lankeit, M. Predicting anticoagulant-related bleeding in patients with venous thromboembolism: A clinically oriented review. Eur. Respir. J. 2015, 45, 201–210. [Google Scholar] [CrossRef] [Green Version]
- Chopard, R.; Serzian, G.; Humbert, S.; Falvo, N.; Morel-Aleton, M.; Bonnet, B.; Napporn, G.; Kalbacher, E.; Obert, L.; Degano, B.; et al. Non-recommended dosing of direct oral anticoagulants in the treatment of acute pulmonary embolism is related to an increased rate of adverse events. J. Thromb. Thrombolysis 2018, 46, 283–291. [Google Scholar] [CrossRef]
- Klok, F.A.; Hosel, V.; Clemens, A.; Yollo, W.D.; Tilke, C.; Schulman, S.; Lankeit, M.; Konstantinides, S.V. Prediction of bleeding events in patients with venous thromboembolism on stable anticoagulation treatment. Eur. Respir. J. 2016, 48, 1369–1376. [Google Scholar] [CrossRef]
- Ruiz-Gimenez, N.; Suarez, C.; Gonzalez, R.; Nieto, J.A.; Todoli, J.A.; Samperiz, A.L.; Monreal, M.; Riete Investigators. Predictive variables for major bleeding events in patients presenting with documented acute venous thromboembolism. Findings from the RIETE Registry. Thromb. Haemost. 2008, 100, 26–31. [Google Scholar] [CrossRef]
- Fang, M.C.; Go, A.S.; Chang, Y.; Borowsky, L.H.; Pomernacki, N.K.; Udaltsova, N.; Singer, D.E. A new risk scheme to predict warfarin-associated hemorrhage: The ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J. Am. Coll. Cardiol. 2011, 58, 395–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisters, R.; Lane, D.A.; Nieuwlaat, R.; de Vos, C.B.; Crijns, H.J.; Lip, G.Y. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest 2010, 138, 1093–1100. [Google Scholar] [CrossRef] [Green Version]
- Gage, B.F.; Yan, Y.; Milligan, P.E.; Waterman, A.D.; Culverhouse, R.; Rich, M.W.; Radford, M.J. Clinical classification schemes for predicting hemorrhage: Results from the National Registry of Atrial Fibrillation (NRAF). Am. Heart J. 2006, 151, 713–719. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, E.C.; Simon, D.N.; Thomas, L.E.; Hylek, E.M.; Gersh, B.J.; Ansell, J.E.; Kowey, P.R.; Mahaffey, K.W.; Chang, P.; Fonarow, G.C.; et al. The ORBIT bleeding score: A simple bedside score to assess bleeding risk in atrial fibrillation. Eur. Heart J. 2015, 36, 3258–3264. [Google Scholar] [CrossRef] [Green Version]
- Klok, F.A.; Niemann, C.; Dellas, C.; Hasenfuss, G.; Konstantinides, S.; Lankeit, M. Performance of five different bleeding-prediction scores in patients with acute pulmonary embolism. J. Thromb. Thrombolysis 2016, 41, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Meneveau, N.; Ider, O.; Seronde, M.F.; Chopard, R.; Davani, S.; Bernard, Y.; Schiele, F. Long-term prognostic value of residual pulmonary vascular obstruction at discharge in patients with intermediate- to high-risk pulmonary embolism. Eur. Heart J. 2013, 34, 693–701. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; Strobe Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Epidemiology 2007, 18, 800–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Remy-Jardin, M.; Remy, J.; Wattinne, L.; Giraud, F. Central pulmonary thromboembolism: Diagnosis with spiral volumetric CT with the single-breath-hold technique--comparison with pulmonary angiography. Radiology 1992, 185, 381–387. [Google Scholar] [CrossRef]
- Pioped Investigators. Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). JAMA 1990, 263, 2753–2759. [Google Scholar] [CrossRef]
- Jaff, M.R.; McMurtry, M.S.; Archer, S.L.; Cushman, M.; Goldenberg, N.; Goldhaber, S.Z.; Jenkins, J.S.; Kline, J.A.; Michaels, A.D.; Thistlethwaite, P.; et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: A scientific statement from the American Heart Association. Circulation 2011, 123, 1788–1830. [Google Scholar] [CrossRef] [Green Version]
- Konstantinides, S.V. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. Eur. Heart J. 2014, 35, 3145–3146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulman, S.; Kearon, C.; the Subcommittee on Control of Anticoagulation of the scientific; Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 2005, 3, 692–694. [Google Scholar] [CrossRef] [PubMed]
- Barnard, J.; Meng, X.L. Applications of multiple imputation in medical studies: From AIDS to NHANES. Stat. Methods Med. Res. 1999, 8, 17–36. [Google Scholar] [CrossRef]
- Johnston, R.; Jones, K.; Manley, D. Confounding and collinearity in regression analysis: A cautionary tale and an alternative procedure, illustrated by studies of British voting behaviour. Qual. Quant. 2018, 52, 1957–1976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kearon, C.; Akl, E.A.; Ornelas, J.; Blaivas, A.; Jimenez, D.; Bounameaux, H.; Huisman, M.; King, C.S.; Morris, T.A.; Sood, N.; et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest 2016, 149, 315–352. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Chen, W.; Petrick, N.A.; Gallas, B.D. Comparing two correlated C indices with right-censored survival outcome: A one-shot nonparametric approach. Stat. Med. 2015, 34, 685–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, N.R.; Ridker, P.M. Advances in measuring the effect of individual predictors of cardiovascular risk: The role of reclassification measures. Ann. Intern. Med. 2009, 150, 795–802. [Google Scholar] [CrossRef]
- Nieto, J.A.; Solano, R.; Trapero Iglesias, N.; Ruiz-Gimenez, N.; Fernandez-Capitan, C.; Valero, B.; Tiberio, G.; Bura-Riviere, A.; Monreal, M.; RIETE Investigators. Validation of a score for predicting fatal bleeding in patients receiving anticoagulation for venous thromboembolism. Thromb. Res. 2013, 132, 175–179. [Google Scholar] [CrossRef]
- Bassand, J.P.; Accetta, G.; Camm, A.J.; Cools, F.; Fitzmaurice, D.A.; Fox, K.A.; Goldhaber, S.Z.; Goto, S.; Haas, S.; Hacke, W.; et al. Two-year outcomes of patients with newly diagnosed atrial fibrillation: Results from GARFIELD-AF. Eur. Heart J. 2016, 37, 2882–2889. [Google Scholar] [CrossRef]
- Ageno, W.; Haas, S.; Weitz, J.I.; Goldhaber, S.Z.; Turpie, A.G.G.; Goto, S.; Angchaisuksiri, P.; Nielsen, J.D.; Kayani, G.; Pieper, K.S.; et al. Characteristics and Management of Patients with Venous Thromboembolism: The GARFIELD-VTE Registry. Thromb. Haemost. 2019, 119, 319–327. [Google Scholar] [CrossRef] [Green Version]
- Robertson, L.; Jones, L.E. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for the initial treatment of venous thromboembolism. Cochrane. Database Syst. Rev. 2017, 2, CD001100. [Google Scholar] [CrossRef]
- Van Es, N.; Coppens, M.; Schulman, S.; Middeldorp, S.; Buller, H.R. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: Evidence from phase 3 trials. Blood 2014, 124, 1968–1975. [Google Scholar] [CrossRef]
- Marti, C.; John, G.; Konstantinides, S.; Combescure, C.; Sanchez, O.; Lankeit, M.; Meyer, G.; Perrier, A. Systemic thrombolytic therapy for acute pulmonary embolism: A systematic review and meta-analysis. Eur. Heart J. 2015, 36, 605–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalra, R.; Bajaj, N.S.; Arora, P.; Arora, G.; Crosland, W.A.; McGiffin, D.C.; Ahmed, M.I. Surgical Embolectomy for Acute Pulmonary Embolism: Systematic Review and Comprehensive Meta-Analyses. Ann. Thorac. Surg. 2017, 103, 982–990. [Google Scholar] [CrossRef] [Green Version]
- Kaymaz, C.; Akbal, O.Y.; Tanboga, I.H.; Hakgor, A.; Yilmaz, F.; Ozturk, S.; Poci, N.; Turkday, S.; Ozdemir, N.; Konstantinides, S. Ultrasound-Assisted Catheter-Directed Thrombolysis in High-Risk and Intermediate-High-Risk Pulmonary Embolism: A Meta-Analysis. Curr. Vasc. Pharmacol. 2018, 16, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, M.; Bay, C.; Skrocki, L.; Rahimi, F.; Mehdipour, M.; Investigators, M. Moderate pulmonary embolism treated with thrombolysis (from the “MOPETT” Trial). Am. J. Cardiol. 2013, 111, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Bikdeli, B.; Chatterjee, S.; Desai, N.R.; Kirtane, A.J.; Desai, M.M.; Bracken, M.B.; Spencer, F.A.; Monreal, M.; Goldhaber, S.Z.; Krumholz, H.M. Inferior Vena Cava Filters to Prevent Pulmonary Embolism: Systematic Review and Meta-Analysis. J. Am. Coll. Cardiol. 2017, 70, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Huisman, M.V. How I assess and manage the risk of bleeding in patients treated for venous thromboembolism. Blood 2020, 135, 724–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chopard, R.; Piazza, G.; Falvo, N.; Ecarnot, F.; Besutti, M.; Capellier, G.; Schiele, F.; Badoz, M.; Meneveau, N. An original risk score to predict early major bleeding in acute pulmonary embolism:The Syncope, Anemia, Renal Dysfunction (PE-SARD) bleeding score. Chest 2021. [Google Scholar] [CrossRef] [PubMed]
- Mehran, R.; Rao, S.V.; Bhatt, D.L.; Gibson, C.M.; Caixeta, A.; Eikelboom, J.; Kaul, S.; Wiviott, S.D.; Menon, V.; Nikolsky, E.; et al. Standardized bleeding definitions for cardiovascular clinical trials: A consensus report from the Bleeding Academic Research Consortium. Circulation 2011, 123, 2736–2747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subherwal, S.; Bach, R.G.; Chen, A.Y.; Gage, B.F.; Rao, S.V.; Newby, L.K.; Wang, T.Y.; Gibler, W.B.; Ohman, E.M.; Roe, M.T.; et al. Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: The CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) Bleeding Score. Circulation 2009, 119, 1873–1882. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| All Study Patients (n = 2754) | Missing Values (%) | Patients without Major Bleeding (n = 2672) | Patients with Major Bleeding (n = 82) | p Value | |
|---|---|---|---|---|---|
| Age, year | 67.3 ± 17.4 | 0 | 67.2 ± 17.4 | 70.2 ± 14.4 | 0.12 |
| Female sex (%) | 1414 (51.3) | 0 | 1362 (50.1) | 52 (63.4) | 0.03 |
| BMI, kg/m2 | 27.4 ± 5.9 | 0.2 | 27.4 ± 5.9 | 27.8 ± 7.4 | 0.59 |
| Comorbidities (%) | |||||
| Hypertension | 1300 (47.2) | 0.1 | 1258 (47.1) | 42 (51.2) | 0.45 |
| Diabetes mellitus | 366 (13.3) | 0.2 | 354 (13.2) | 12 (14.6) | 0.71 |
| Chronic pulmonary disease | 236 (8.6) | 0.3 | 23 (8.6) | 5 (6.1) | 0.41 |
| Coronary artery disease | 378 (13.7) | 0.2 | 365 (13.7) | 13 (15.5) | 0.56 |
| Active cancer a | 507 (18.4) | 0.1 | 490 (18.3) | 17 (20.7) | 0.58 |
| Prior stroke | 166 (6.0) | 0.1 | 157 (5.9) | 9 (11.0) | 0.09 |
| Prior VTE | 654 (23.8) | 0 | 631 (23.6) | 23 (3.5) | 0.35 |
| Prior bleeding | 42 (1.5) | 0.3 | 38 (1.4) | 4 (4.9) | 0.07 |
| Recent surgery b | 192 (7.0) | 0.2 | 181 (6.8) | 11 (13.4) | 0.02 |
| Concomitant medication usage predisposing to bleeding c | 112 (4.1) | 0.1 | 102 (3.8) | 10 (12.2) | 0.008 |
| Antiplatelet therapy | 93 (3.4) | - | 17 (0.6) | 3 (3.7) | - |
| Anticoagulant | 20 (0.7) | - | 86 (3.2) | 7 (8.5) | - |
| Low-risk for long-term recurrence | 702 (25.6) | 0.3 | 678 (25.4) | 24 (29.3) | 0.42 |
| Associated DVT | 1120 (40.7) | 1.1 | 1082 (40.5) | 38 (46.3) | 0.30 |
| Clinical characteristics | |||||
| HR at admission, bpm | 89.9 ± 19.1 | 0.6 | 89.8 ± 19.1 | 94.1 ± 9.8 | 0.04 |
| SBP at admission, mmHg | 137.7 ± 23.6 | 0.1 | 138.0 ± 23.4 | 131.6 ± 24.0 | 0.01 |
| SaO2 at admission, % | 93.4 ± 5.6 | 1.1 | 93.5 ± 5.3 | 90.4 ± 9.9 | <0.001 |
| Biological data | |||||
| Hemoglobin (g/dL) | 13.3 ± 2.9 | 1.0 | 13.4 ± 2.8 | 11.1 ± 2.8 | <0.001 |
| eGRFCKD-EPI, mmol/L | 75.9 ± 24.9 | 0.9 | 76.3 ± 24.9 | 62.2 ± 24.9 | <0.001 |
| Positive troponin | 981 (35.6) | 0.9 | 938 (35.1) | 43 (52.4) | 0.002 |
| Echo data | |||||
| RV dysfunction | 911 (33.1) | 1.1 | 871 (32.6) | 40 (48.8) | 0.003 |
| sPESI (median, Q1–Q3) | 2 (1–3) | 0.9 | 2 (1–3) | 3 (2–3) | 0.009 |
| ESC-defined risk PE category (%) | <0.001 | ||||
| Low-risk | 443 (16.1) | - | 438 (16.4) | 5 (6.1) | |
| Intermediate-low risk | 1594 (57.9) | - | 1550 (58.0) | 44 (53.7) | |
| Intermediate-high risk | 584 (21.2) | - | 563 (21.1) | 21 (25.6) | |
| High-risk | 133 (4.8) | - | 121 (4.5) | 12 (14.6) | |
| Bleeding scores (median, Q1–Q3) | |||||
| VTE-BLEED score | 2.5 (1.5–3.5) | - | 2.5 1 (1.5–3.5) | 3.0 (2.5–4.5) | <0.001 |
| RIETE score | 3 (2–4) | - | 1 (0–2) | 3.5 (3–4.5) | <0.001 |
| ORBIT score | 1 (0–2) | - | 1 (0–2) | 2 (1–3) | <0.001 |
| HAEMORR2HAGES score | 1 (0–1) | - | 1 (0–2) | 2 (1–2) | <0.001 |
| ATRIA score | 1 (0–3) | - | 1 (0–3) | 3 (2–5) | |
| HAS-BLED score | 1 (0–1) | - | 1 (0–1) | 1 (1–2) | 0.01 |
| In-hospital treatments (%) | |||||
| Anticoagulation | |||||
| UFH | 603 (21.9) | - | 559 (20.9) | 44 (53.7) | <0.001 |
| LMWH/fondaparinux | 1538 (42.3) | - | 1501(56.2) | 39 (44.0) | <0.001 |
| DOAC | 613 (22.2) | - | 612 (22.9) | 1 (1.2) | <0.001 |
| Reperfusion therapy | |||||
| Thrombolysis | 107 (3.9) | - | 98 (3.7) | 9 (11.0) | <0.001 |
| Surgical embolectomy | 13 (0.5) | - | 7 (0.3) | 6 (7.3) | <0.001 |
| ECMO | 17 (0.6) | - | 9 (0.3) | 8 (9.8) | <0.001 |
| Inferior vena cava filter | 9 (0.3) | - | 7 (0.3) | 2 (2.4) | <0.001 |
| Variable | OR (95% CI) | p Value | OR (95% CI) | p Value |
|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||
| Major Bleeding | ||||
| Female sex | 1.5 (1.04–2.1) | 0.03 | - | - |
| Age > 80 (years) | 1.6 (1.04–2.5) | 0.03 | - | - |
| Weight < 60 (kg) | 1.6 (1.01–2.6) | 0.04 | ||
| Recent surgery a | 2.1 (1.1–4.1) | 0.02 | - | - |
| Concomitant medication usage predisposing to bleeding b | 2.8 (1.4–5.4) | 0.004 | 2.3 (1.1–4.7) | 0.02 |
| Syncope | 3.5 (2.0–6.1) | <0.001 | 2.4 (1.3–4.3) | 0.004 |
| Heart rate > 80 (b.p.m) | 1.9 (1.1–3.2) | 0.02 | 1.8 (1.1–3.1) | 0.03 |
| Arterial oxyhemoglobin saturation < 90 (%) | 1.5 (1.0–2.4) | 0.05 | - | - |
| Positive troponin c | 2.0 (1.3–3.2) | 0.001 | - | - |
| Platelet count < 150,000/mm3) | 2.0 (1.3–3.3) | 0.007 | - | - |
| GFR CKD-EPI d < 60 (mL/min) | 2.0 (1.2–3.3) | 0.007 | 1.8 (1.1–2.9) | 0.03 |
| Hemoglobin < 12 (g/dL) | 4.6 (2.9–7.2) | <0.001 | 3.7 (2.3–6.1) | <0.001 |
| RV dysfunction e | 1.9 (1.3–3.1) | 0.002 | - | - |
| Systemic thrombolysis during in-hospital phase | 3.2 (1.6–6.7) | 0.001 | - | - |
| Length of stay | ||||
| Age > 80 years | 2.3 (1.9–2.6) | <0.001 | 1.9 (1.6–2.2) | <0.001 |
| Female sex | 1.3 (1.1–1.5) | <0.001 | ||
| Hypertension | 1.4 (1.2–1.6) | <0.001 | ||
| Diabetes mellitus | 1.4 (1.2–1.7) | <0.001 | ||
| Chronic pulmonary disease | 2.0 (1.6–2.5) | <0.001 | 2.0 (1.6–2.6) | <0.001 |
| Coronary artery disease | 1.4 (1.2–1.7) | <0.001 | ||
| Prior stroke | 2.0 (1.6–2.7) | <0.001 | ||
| Cognitive disorders | 1.6 (1.1–2.3) | 0.009 | ||
| Concomitant medication usage predisposing to bleeding b | 1.6 (1.1–2.3) | 0.008 | ||
| Associated DVT | 1.4 (1.3–1.6) | <0.001 | 1.4 (1.2–1.6) | <0.001 |
| Syncope | 1.7 (1.3–2.1) | <0.001 | ||
| PAS < 90 mmHg at admission | 1.6 (1.2–2.2) | 0.002 | ||
| Heart rate > 110 b.p.m | 1.7 (1.4–1.9) | <0.001 | 1.6 (1.3–1.9) | <0.001 |
| RV dysfunction at admission d | 1.6 (1.4–1.9) | <0.001 | ||
| Positive troponin b | 2.4 (2.1–2.7) | <0.001 | 2.0 (1.7–2.3) | <0.001 |
| GFR CKD-EPI < 60 mL/min c | 1.9 (1.7–2.2) | <0.001 | ||
| Hemoglobin at admission < 12 g/dL | 1.4 (1.2–1.6) | <0.001 | ||
| In-hospital major bleeding | 4.8 (3.2–7.1) | <0.001 | 4.2 (2.9–5.8) | <0.001 |
| In-hospital death | ||||
| Age > 80 years | 1.8 (1.2–2.7) | 0.002 | - | - |
| BMI (per kg−1) | 1.1 (1.02–1.2) | 0.003 | - | - |
| Coronary artery disease | 2.3 (1.5–3.6) | <0.001 | 2.1 (1.2–3.7) | 0.01 |
| Active cancer | 4.7 (3.2–6.8) | <0.001 | 6.9 (4.0–11.9) | <0.001 |
| Prior VTE | 0.5 (0.3–0.8) | 0.009 | - | - |
| Associated DVT | 0.6 (0.3–0.8) | 0.002 | - | - |
| Syncope | 2.6 (1.5–4.2) | <0.001 | - | - |
| PAS < 90 mmHg at admission | 6.7 (4.1–10.7) | <0.001 | 4.0 (1.8–3.7) | <0.001 |
| Heart rate > 110 b.p.m | 1.9 (1.2–2.9) | 0.003 | - | - |
| RV dysfunction at admission d | 1.7 (1.2–2.4) | 0.005 | - | - |
| Positive troponin b | 2.7 (1.9–4.0) | <0.001 | 2.9 (1.7–4.9) | <0.001 |
| GFR CKD-EPI < 60 mL/min c | 3.2 (2.2–4.7) | <0.001 | - | - |
| Hemoglobin at admission < 12 g/dL | 3.9 (2.7–5.7) | <0.001 | - | - |
| In-hospital major bleeding | 9.1 (5.3–15.6) | <0.001 | 8.4 (4.0–17.6) | <0.001 |
| RIETE Score | ORBIT Score | HAEMORR2HAGES Score | ATRIA Score | VTE-BLEED Score | HAS-BLED Score | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3-level categories | Number of major bleeding/number of patients (%, 95% CI) | |||||||||||
| Low risk | - | - | 54/2371 | 2.3%; (1.7–3.0) | 35/1943 | 1.8%; (1.2–2.5) | 56/2314 | 2.4%; (1.8–3.1) | - | - | 17/837 | 2.0%; (1.2–3.2) |
| Intermediate risk | 50/2150 | 2.3%; (1.71–3.0) | 13/169 | 7.7%; (4.1–12.8) | 44/764 | 5.8%; (4.2–7.7) | 4/100 | 4.0%; (1.1–9.3) | - | - | 57/1805 | 3.2%; (2.4–4.1) |
| High risk | 32/604 | 5.3%; (3.6–7.4) | 15/199 | 7.0%; (4.0–11.3) | 3/47 | 6.4%; (1.3–17.6) | 22/318 | 6.5%; (4.1–9.7) | - | - | 8/112 | 7.1%; (3.1–13.4) |
| 2-level risk categories * | Number of major bleeding/number of patients (%, 95% CI) | |||||||||||
| Low risk | 28/1803 | 1.5%; (1.0–2.2) | 13/1183 | 1.1%; (0.6–1.9) | 35/1943 | 1.8%; (1.3–2.5) | 20/1389 | 1.5%; (0.8–2.1) | 24/1405 | 1.7%; (1.1–2.5) | 17/837 | 2.0%; (1.2–3.2) |
| High risk | 54/951 | 5.7%; (4.3–7.4) | 69/1571 | 4.4%; (3.4–5.5) | 47/811 | 5.8%; (4.3–7.6) | 62/1365 | 4.5%; (3.5–7.4) | 58/1349 | 4.3%; (3.2–5.5) | 65/1917 | 3.4%; (2.2–4.3) |
| Score | VTE-BLEED | RIETE | ORBIT | HAEMORR2HAGES | ATRIA | HAS-BLED |
|---|---|---|---|---|---|---|
| Overall model fit | ||||||
| BIC | 817.4 | 721.2 | 722.1 | 728.2 | 726.4 | 746.7 |
| AIC | 734.5 | 709.3 | 710.3 | 716.4 | 714.6 | 734.8 |
| Nagelkerke’s R2 (%) | 4.81 | 4.89 | 4.85 | 3.91 | 4.19 | 1.08 |
| Discrimination | ||||||
| Harrell’s c index | 0.633 | 0.692 | 0.681 | 0.674 | 0.669 | 0.570 |
| Score Comparison | ∆C-Index (%) | p Value | IDI (%) (95% CI) | p Value | NRI (%) (95% CI) | p Value |
|---|---|---|---|---|---|---|
| HAEMORR2HAGES vs. RIETE | 0.2 (−5.2–5.6) | 0.94 | −0.1 (−0.5–0.3) | 0.57 | 1.4 (−19.2–22.1) | 0.90 |
| ORBIT vs. RIETE | 1.7 (−0.1–0.3) | 0.73 | 1.2 (−0.1–0.3) | 0.36 | −3.7 (−15.2–7.8) | 0.74 |
| ATRIA vs. RIETE | 2.3 (−3.3–5.6) | 0.62 | 0.2 (−0.9–0.5) | 0.18 | 7.1 (−8.9–23.1) | 0.63 |
| VTE-BLEED vs. RIETE | 3.3 (−1.3–7.9) | 0.15 | 0.4 (0.2–0.7) | <0.001 | 58.1 (44.2–72.2) | <0.001 |
| HAS-BLED vs. RIETE | 9.6 (5.1–134.0) | <0.001 | 0.8 (0.6–1.2) | <0.001 | 58.2 (44.2–72.2) | <0.001 |
| HAEMORR2HAGES vs. ORBIT | −0.3 (−0.5–0.5) | 0.88 | −0.2 (−0.6–0.1) | 0.28 | −2.6 (−22.6–17.4) | 0.81 |
| ATRIA vs. ORBIT | 1.6 (−2.5–3.6) | 0.72 | 0.8 (−0.1–0.3) | 0.37 | 2.1 (−10.0–14.4) | 0.19 |
| VTE-BLEED vs. ORBIT | 2.7 (−2.0–7.6) | 0.26 | 0.3 (0.06–0.6) | 0.001 | 55.8 (39.6–72.1) | <0.001 |
| HAS-BLED vs. ORBIT | 8.9 (3.8–14.1) | <0.001 | 0.8 (0.5–1.0) | <0.001 | 55.8 (39.6–72.1) | <0.001 |
| ATRIA vs. HAEMORR2HAGES | 0.9 (−4.8–6.6) | 0.74 | 0.3 (−0.1–0.7) | 0.14 | −6.5 (−19.5–18.2) | 0.95 |
| VTE-BLEED vs. HAEMORR2HAGES | 3.1 (−2.0–8.3) | 0.22 | 0.5 (0.2–0.9) | 0.002 | 9.8 (–7.2–26.8) | 0.87 |
| HAS-BLED vs. HAEMORR2HAGES | 9.3 (3.6–15.1) | 0.001 | 1.0 (0.6–1.4) | <0.001 | 57.4 (35.7–79.1) | <0.001 |
| VTE-BLEED vs. ATRIA | 2.2 (−3.7–8.1) | 0.46 | 0.2 (−0.09–0.5) | 0.14 | 53.7 (34.7–72.7) | <0.001 |
| HAS-BLED vs. ATRIA | 2.9 (2.6–14.3) | 0.004 | 0.7 (0.4–1.0) | <0.001 | 53.7 (34.7–72.6) | <0.001 |
| HAS-BLED vs. VTE-BLEED | 6.2 (0.4–12.0) | 0.03 | 0.4 (0.2–0.7) | <0.001 | 44.8 (24.7–64.9) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathonier, C.; Meneveau, N.; Besutti, M.; Ecarnot, F.; Falvo, N.; Guillon, B.; Schiele, F.; Chopard, R. Available Bleeding Scoring Systems Poorly Predict Major Bleeding in the Acute Phase of Pulmonary Embolism. J. Clin. Med. 2021, 10, 3615. https://doi.org/10.3390/jcm10163615
Mathonier C, Meneveau N, Besutti M, Ecarnot F, Falvo N, Guillon B, Schiele F, Chopard R. Available Bleeding Scoring Systems Poorly Predict Major Bleeding in the Acute Phase of Pulmonary Embolism. Journal of Clinical Medicine. 2021; 10(16):3615. https://doi.org/10.3390/jcm10163615
Chicago/Turabian StyleMathonier, Camille, Nicolas Meneveau, Matthieu Besutti, Fiona Ecarnot, Nicolas Falvo, Benoit Guillon, François Schiele, and Romain Chopard. 2021. "Available Bleeding Scoring Systems Poorly Predict Major Bleeding in the Acute Phase of Pulmonary Embolism" Journal of Clinical Medicine 10, no. 16: 3615. https://doi.org/10.3390/jcm10163615
APA StyleMathonier, C., Meneveau, N., Besutti, M., Ecarnot, F., Falvo, N., Guillon, B., Schiele, F., & Chopard, R. (2021). Available Bleeding Scoring Systems Poorly Predict Major Bleeding in the Acute Phase of Pulmonary Embolism. Journal of Clinical Medicine, 10(16), 3615. https://doi.org/10.3390/jcm10163615






