Diagnosis and Management of Heart Failure in Elderly Patients from Hospital Admission to Discharge: Position Paper
Abstract
:1. Introduction
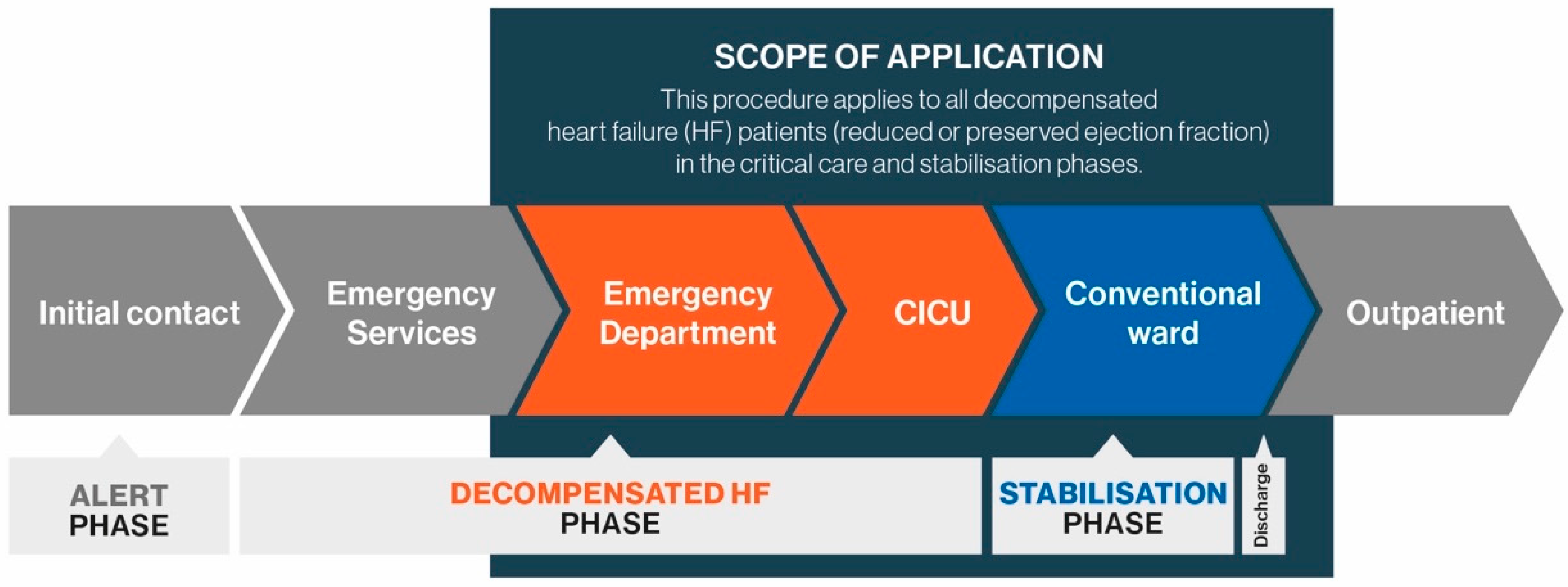
2. Patient Care Pathway
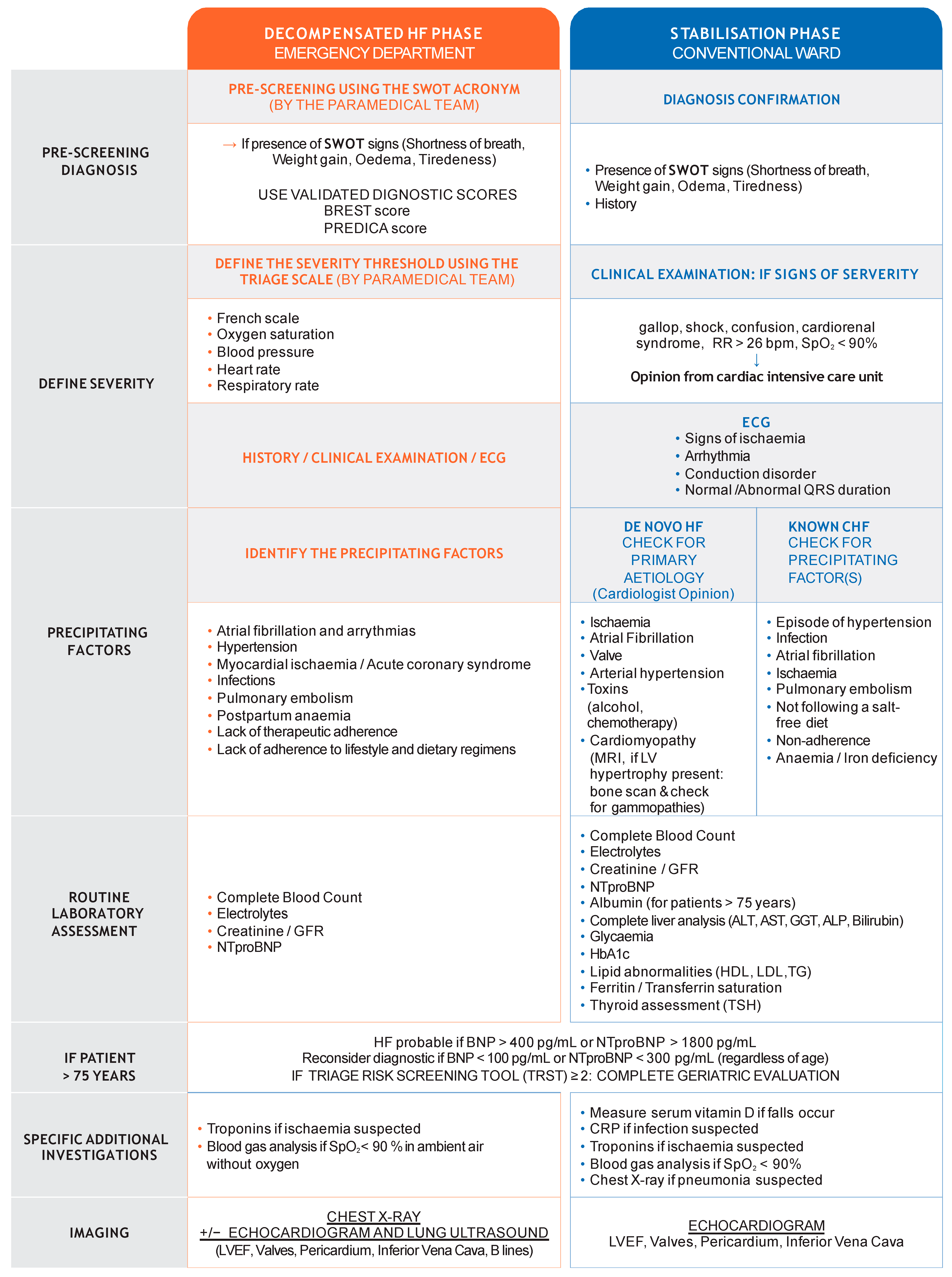
2.1. Pre-Screening and HF Diagnosis in the Emergency Department
2.2. Stratify Severity
- Dyspnoea severity: respiratory rate, intolerance of the supine position, breathing effort, and oxygen saturation.
- Abnormal blood pressure (BP): systolic and diastolic blood pressure (within the context of the patient’s history).
- Abnormal heart rate (HR) and rhythm (within the context of the patient’s history).
- Other cardiopulmonary instability signs including body temperature and signs/symptoms of hypoperfusion (cool extremities, narrow pulse pressure, mental status) [9].
2.3. Specificity of Diagnosis in Older Patients
3. Initial Assessment and Differential Diagnoses
3.1. Laboratory and Clinical Assessments
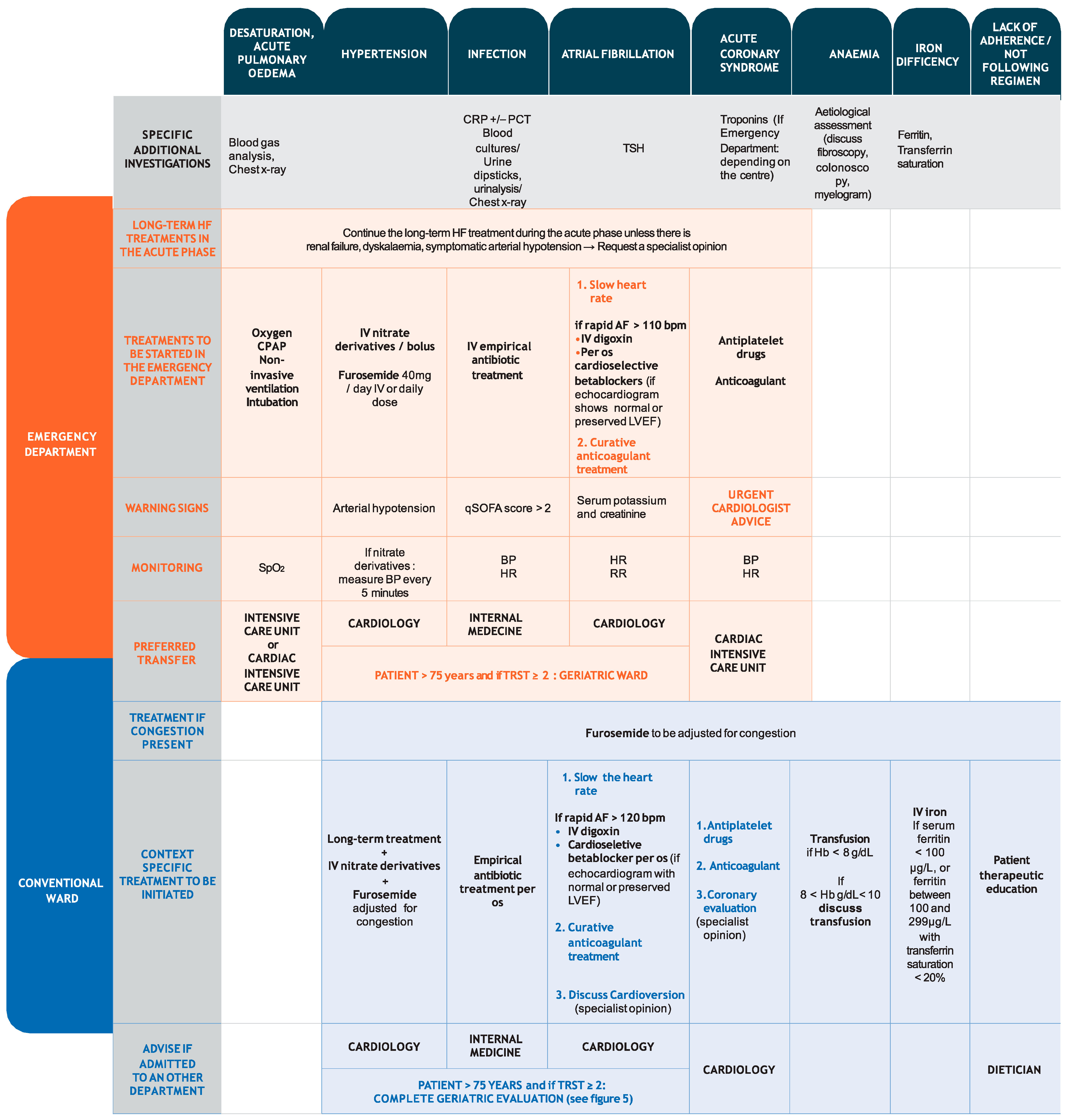
3.2. Therapeutic Management of HF and Precipitating Factors
3.2.1. Thromboembolism
3.2.2. Hypoxia
3.2.3. Hypertension
3.2.4. Infection
3.2.5. Atrial Fibrillation
3.2.6. Acute Coronary Syndrome
3.2.7. Cardiac Cachexia
4. Stabilisation and Management Phase
4.1. Congestion
4.2. Management of Precipitating Factors
4.2.1. Infection
4.2.2. Atrial Fibrillation and Acute Coronary Syndrome
4.2.3. Anaemia and Iron Deficiency
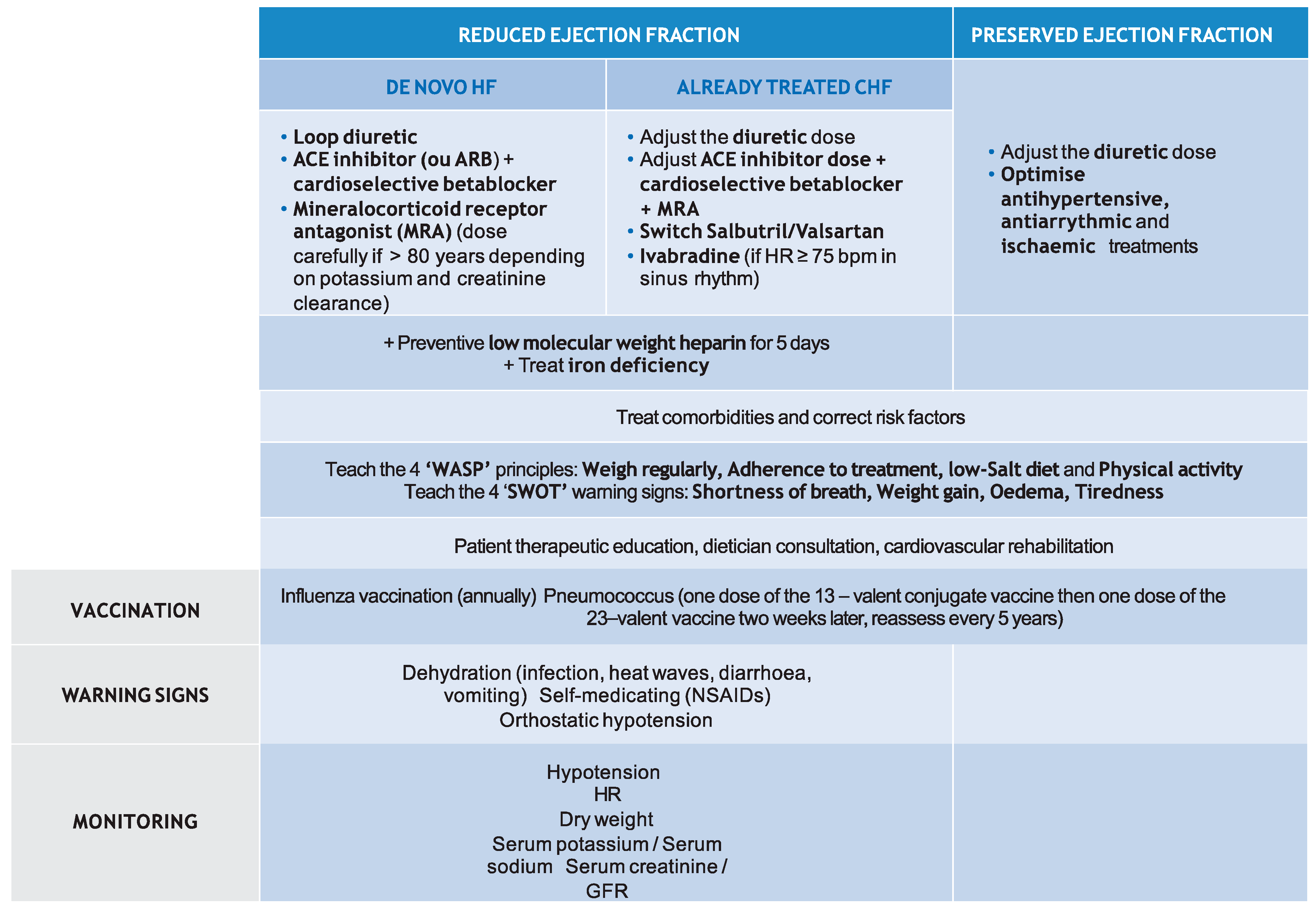
4.3. HF Management
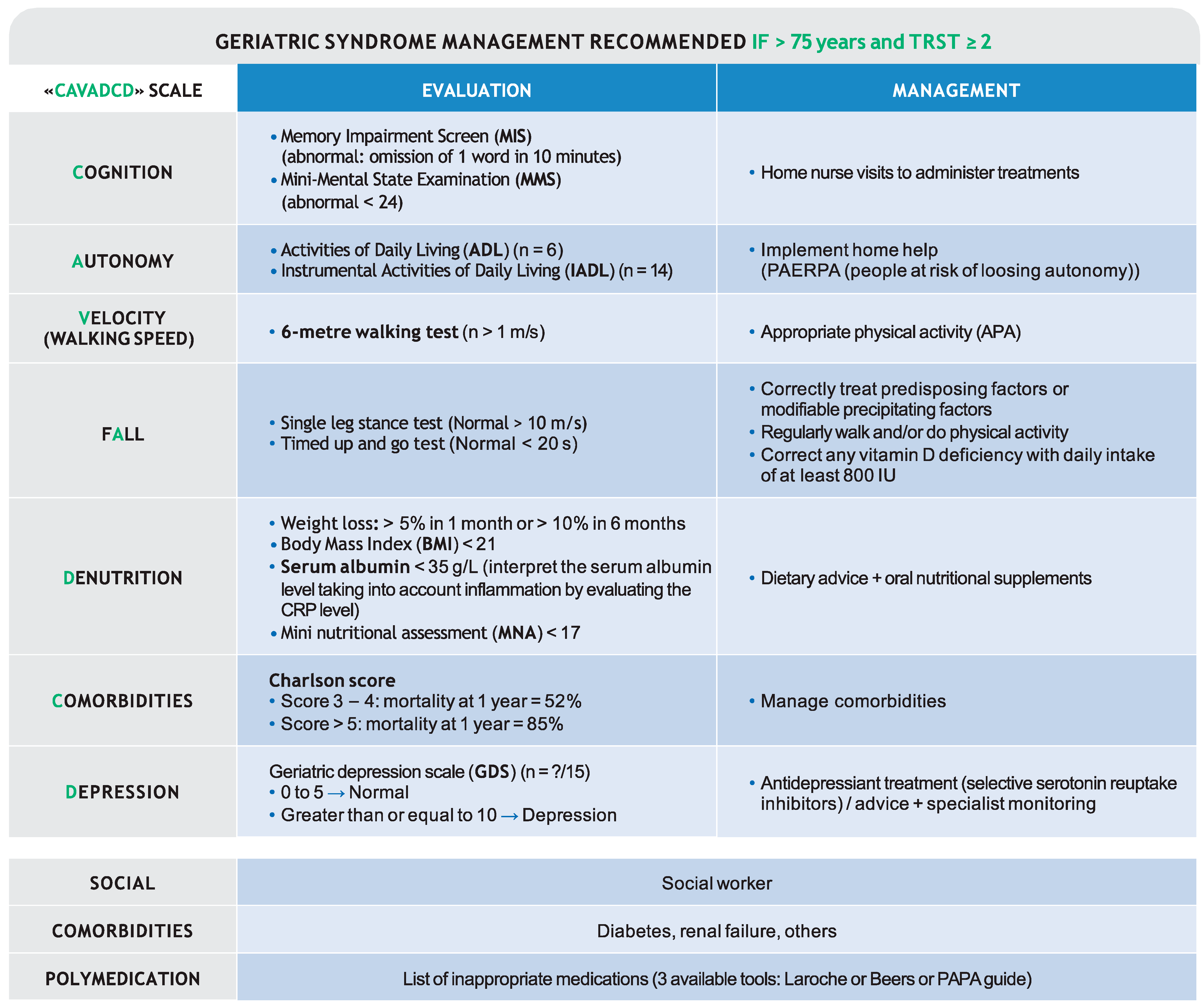
4.4. Geriatric Syndrome Management
4.5. Patient Discharge: A Transitional Care Intervention
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ameli.fr. Définition et Causes de L’insuffisance Cardiaque. Available online: https://www.ameli.fr/assure/sante/themes/insuffisance-cardiaque/definition-causes (accessed on 29 July 2020).
- DREES. Rapport Etat de Santé de la Population DREES 2017; DREES: Pays de la Loire, France, 2017. [Google Scholar]
- Gorlicki, J.; Boubaya, M.; Cottin, Y.; Angoulvant, D.; Soulat, L.; Guinemer, S.; Bloch-Queyrat, C.; Deltour, S.; Lambert, Y.; Juilliere, Y.; et al. Patient care pathways in acute heart failure and their impact on in-hospital mortality, a French national prospective survey. Int. J. Cardiol. Heart Vasc. 2020, 26, 100448. [Google Scholar] [CrossRef]
- Komajda, M.; Hanon, O.; Hochadel, M.; Follath, F.; Swedberg, K.; Gitt, A.; Cleland, J.G. Management of octogenarians hospitalized for heart failure in Euro Heart Failure Survey I. Eur. Heart J. 2007, 28, 1310–1318. [Google Scholar] [CrossRef] [Green Version]
- Logeart, D.; Isnard, R.; Resche-Rigon, M.; Seronde, M.F.; de Groote, P.; Jondeau, G.; Galinier, M.; Mulak, G.; Donal, E.; Delahaye, F.; et al. Current aspects of the spectrum of acute heart failure syndromes in a real-life setting: The OFICA study. Eur. J. Heart Fail. 2013, 15, 465–476. [Google Scholar] [CrossRef] [Green Version]
- Seferovic, P.M.; Ponikowski, P.; Anker, S.D.; Bauersachs, J.; Chioncel, O.; Cleland, J.G.F.; de Boer, R.A.; Drexel, H.; Ben Gal, T.; Hill, L.; et al. Clinical practice update on heart failure 2019: Pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 1169–1186. [Google Scholar] [CrossRef]
- HAS. Insuffisence Cardiac recommandations HAS.pdf. 2014. Available online: https://www.has-sante.fr/upload/docs/application/pdf/2012-04/guide_parcours_de_soins_ic_web.pdf (accessed on 29 July 2020).
- Boully, C.; Vidal, J.S.; Guibert, E.; Ghazali, F.N.; Pesce, A.; Beauplet, B.; Roger, J.D.; Carrière, I.; Timbely, B.; Idiri, H.; et al. National survey on the management of heart failure in individuals over 80 years of age in French geriatric care units. BMC Geriatr. 2019, 19, 204. [Google Scholar] [CrossRef] [Green Version]
- Mebazaa, A.; Yilmaz, M.B.; Levy, P.; Ponikowski, P.; Peacock, W.F.; Laribi, S.; Ristic, A.D.; Lambrinou, E.; Masip, J.; Riley, J.P.; et al. Recommendations on pre-hospital and early hospital management of acute heart failure: A consensus paper from the Heart Failure Association of the European Society of Cardiology, the European Society of Emergency Medicine and the Society of Academic Emergency Medicine--short version. Eur. Heart J. 2015, 36, 1958–1966. [Google Scholar] [CrossRef] [Green Version]
- Tuppin, P.; Cuerq, A.; de Peretti, C.; Fagot-Campagna, A.; Danchin, N.; Juillière, Y.; Alla, F.; Allemand, H.; Bauters, C.; Drici, M.D.; et al. Two-year outcome of patients after a first hospitalization for heart failure: A national observational study. Arch. Cardiovasc. Dis. 2014, 107, 158–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greene, S.J.; Fonarow, G.C.; Vaduganathan, M.; Khan, S.S.; Butler, J.; Gheorghiade, M. The vulnerable phase after hospitalization for heart failure. Nat. Rev. Cardiol. 2015, 12, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Améliorer la Qualité du Système de Santé et Maîtriser Les Dépenses. Available online: https://www.ameli.fr/sites/default/files/rapport-charges-et-produits-2020.pdf (accessed on 29 July 2020).
- Ma Sante 2022: Un Engagement Collectif. Available online: https://solidarites-sante.gouv.fr/systeme-de-sante-et-medico-social/masante2022/ (accessed on 29 July 2020).
- GICC. Les Symptômes de L’insuffisance Cardiaque (EPOF). Available online: https://giccardio.fr/patient/linsuffisance-cardiaque/les-symptomes-de-linsuffisance-cardiaque (accessed on 29 July 2020).
- Roncalli, J.; Picard, F.; Delarche, N.; Faure, I.; Pradeau, C.; Thicoipe, M.; Galinier, M.; Charpentier, S. Predictive criteria for acute heart failure in emergency department patients with acute dyspnoea: The PREDICA study. Eur. J. Emerg. Med. 2019, 26, 400–404. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- Basset, A.; Nowak, E.; Castellant, P.; Gut-Gobert, C.; Le Gal, G.; L’Her, E. Development of a clinical prediction score for congestive heart failure diagnosis in the emergency care setting: The Brest score. Am. J. Emerg. Med. 2016, 34, 2277–2283. [Google Scholar] [CrossRef]
- McMurray, J.J. Clinical practice. Systolic heart failure. N. Engl. J. Med. 2010, 362, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Normand, S.L.; Wang, Y.; Krumholz, H.M. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998–2008. JAMA 2011, 306, 1669–1678. [Google Scholar] [CrossRef] [Green Version]
- Dunlay, S.M.; Redfield, M.M.; Weston, S.A.; Therneau, T.M.; Hall Long, K.; Shah, N.D.; Roger, V.L. Hospitalizations after heart failure diagnosis a community perspective. J. Am. Coll. Cardiol. 2009, 54, 1695–1702. [Google Scholar] [CrossRef] [Green Version]
- Taboulet, P.; Maillard-Acker, C.; Ranchon, G.; Goddet, S.; Dufau, R.; Vincent-Cassy, C.; Yordanov, Y.; El Khoury, C. Triage des patients à l’accueil d’une structure d’urgences. Présentation de l’échelle de tri élaborée par la Société française de médecine d’urgence: La FRench Emergency Nurses Classification in Hospital (FRENCH). Ann. Françaises Médecine D’urgence 2019, 9, 51–59. [Google Scholar] [CrossRef]
- Hanon, O.; Belmin, J.; Benetos, A.; Chassagne, P.; De Decker, L.; Jeandel, C.; Krolak-Salmon, P.; Nourhashemi, F.; Paccalin, M. Consensus of experts from the French Society of Geriatrics and Gerontology on the management of heart failure in very old subjects. Arch. Cardiovasc. Dis. 2021. [Google Scholar] [CrossRef]
- Mojoli, F.; Bouhemad, B.; Mongodi, S.; Lichtenstein, D. Lung Ultrasound for Critically Ill Patients. Am. J. Respir. Crit. Care Med. 2019, 199, 701–714. [Google Scholar] [CrossRef] [PubMed]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019, 140, e125–e151. [Google Scholar] [CrossRef] [PubMed]
- HAS. Actes et Prestations—Affection de Longue Durée-Insuffisance Cardiac Systolic. Available online: Liste_ald_insuf_card_systolique.pdf (accessed on 29 July 2020).
- HAS. Chutes Personnes Agées 2009 synthese. 2009. Available online: https://www.has-sante.fr/upload/docs/application/pdf/2009-06/chutes_personnes_agees_synthese.pdf (accessed on 29 July 2020).
- HAS. Chutes Repetees Personnes Agées-Recommandations. 2009. Available online: https://www.has-sante.fr/upload/docs/application/pdf/2009-06/chutes_repetees_personnes_agees_-_recommandations.pdf (accessed on 29 July 2020).
- Jourdain, P.; Lefevre, G.; Oddoze, C.; Sapin, V.; Dievart, F.; Jondeau, G.; Meune, C.; Galinier, M. NT-proBNP in practice: From chemistry to medicine. Ann. Biol. Clin. 2009, 67, 255–271. [Google Scholar] [CrossRef]
- Roffi, M.; Patrono, C.; Collet, J.P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; Bax, J.J.; Borger, M.A.; Brotons, C.; Chew, D.P.; et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 267–315. [Google Scholar] [CrossRef]
- Steg, P.G.; James, S.K.; Atar, D.; Badano, L.P.; Blomstrom-Lundqvist, C.; Borger, M.A.; Di Mario, C.; Dickstein, K.; Ducrocq, G. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2012, 33, 2569–2619. [Google Scholar] [CrossRef]
- HAS. Guide Parcours de Soin IC-Recommandations. 2015. Available online: https://has-sante.fr/jcms/c_2906058/en/insuffisance-cardiaque-parcours-de-soins (accessed on 29 July 2020).
- Steffel, J.; Verhamme, P.; Potpara, T.S.; Albaladejo, P.; Antz, M.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur. Heart J. 2018, 39, 1330–1393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- HAS. Fiche Parcours Rehospitalisations Evitables.pdf. 2013. Available online: https://webzine.has-sante.fr/upload/docs/application/pdf/2013-06/fiche_parcours_rehospitalisations_evitables_vf.pdf (accessed on 29 July 2020).
- Freund, Y.; Gorlicki, J.; Cachanado, M.; Salhi, S.; Lemaitre, V.; Simon, T.; Mebazaa, A. Early and comprehensive care bundle in the elderly for acute heart failure in the emergency department: Study protocol of the ELISABETH stepped-wedge cluster randomized trial. Trials 2019, 20, 95. [Google Scholar] [CrossRef]
- Matsue, Y.; Damman, K.; Voors, A.A.; Kagiyama, N.; Yamaguchi, T.; Kuroda, S.; Okumura, T.; Kida, K.; Mizuno, A.; Oishi, S.; et al. Time-to-Furosemide Treatment and Mortality in Patients Hospitalized With Acute Heart Failure. J. Am. Coll. Cardiol. 2017, 69, 3042–3051. [Google Scholar] [CrossRef] [PubMed]
- Seymour, C.W.; Liu, V.X.; Iwashyna, T.J.; Brunkhorst, F.M.; Rea, T.D.; Scherag, A.; Rubenfeld, G.; Kahn, J.M.; Shankar-Hari, M.; Singer, M.; et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 762–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cilloniz, C.; Dominedo, C.; Pericas, J.M.; Rodriguez-Hurtado, D.; Torres, A. Community-acquired pneumonia in critically ill very old patients: A growing problem. Eur. Respir. Rev. 2020, 29. [Google Scholar] [CrossRef] [Green Version]
- Arnold, F.W. How Antibiotics Should be Prescribed to Hospitalized Elderly Patients with Community-Acquired Pneumonia. Drugs Aging 2017, 34, 13–20. [Google Scholar] [CrossRef]
- Mullens, W.; Damman, K.; Harjola, V.P.; Mebazaa, A.; Brunner-La Rocca, H.P.; Martens, P.; Testani, J.M.; Tang, W.H.W.; Orso, F.; Rossignol, P.; et al. The use of diuretics in heart failure with congestion-a position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Uranga, A.; Espana, P.P.; Bilbao, A.; Quintana, J.M.; Arriaga, I.; Intxausti, M.; Lobo, J.L.; Tomas, L.; Camino, J.; Nunez, J.; et al. Duration of Antibiotic Treatment in Community-Acquired Pneumonia: A Multicenter Randomized Clinical Trial. JAMA Intern. Med. 2016, 176, 1257–1265. [Google Scholar] [CrossRef] [Green Version]
- Cook, J.C.; Tran, R.H.; Patterson, J.H.; Rodgers, J.E. Evolving therapies for the management of chronic and acute decompensated heart failure. Am. J. Health Syst. Pharm. 2016, 73, 1745–1754. [Google Scholar] [CrossRef]
- Tran, P.; Banerjee, P. Iatrogenic Decompensated Heart Failure. Curr. Heart Fail. Rep. 2020, 17, 21–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunha, G.J.L.; Rocha, B.M.L.; Menezes Falcao, L. Iron deficiency in chronic and acute heart failure: A contemporary review on intertwined conditions. Eur. J. Intern. Med. 2018, 52, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.P.; Doehner, W.; Comin-Colet, J.; Group, I.C. Iron deficiency in chronic heart failure: Case-based practical guidance. ESC Heart Fail. 2018, 5, 764–771. [Google Scholar] [CrossRef] [Green Version]
- Duc, S.; Fernandez, C.; Moheb, B.; Dang, V.M.; Bloch, F.; Floccia, M.; Videau, M.N.; Tournier Louvel, S.; Ducastaing, L.; Couturier, P.; et al. Triage risk screening tool (TRST) in screening elderly patients requiring the intervention of a mobile geriatric team: Results of a pilot study. Geriatr. Psychol. Neuropsychiatr. Vieil. 2015, 13, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.P.; Mielniczuk, L.M.; Whittingham, H.A.; Boseley, M.E.; Schramm, D.R.; Storrow, A.B. The use of noninvasive ventilation in emergency department patients with acute cardiogenic pulmonary edema: A systematic review. Ann. Emerg. Med. 2006, 48, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.T.; Yealy, D.M.; Filbin, M.R.; Brown, A.M.; Chang, C.H.; Doi, Y.; Donnino, M.W.; Fine, J.; Fine, M.J.; Fischer, M.A.; et al. Procalcitonin-Guided Use of Antibiotics for Lower Respiratory Tract Infection. N. Engl. J. Med. 2018, 379, 236–249. [Google Scholar] [CrossRef] [Green Version]
- Siniorakis, E.E.; Arapi, S.M.; Panta, S.G.; Pyrgakis, V.N.; Ntanos, I.T.; Limberi, S.J. Emergency department triage of acute heart failure triggered by pneumonia; when an intensive care unit is needed? Int. J. Cardiol. 2016, 220, 479–482. [Google Scholar] [CrossRef]
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. 200. Am. J. Respir. Critical Care Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- SPIF. Antibiothérapie Par Voie Générale Dans Les Infections Respiratoires Basses de L’adulte. Available online: https://www.infectiologie.com/UserFiles/File/medias/_documents/consensus/2010-infVRB-spilf-afssaps.pdf (accessed on 29 July 2020).
- Pieske, B.; Tschöpe, C.; de Boer, R.A.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.P.; et al. How to diagnose heart failure with preserved ejection fraction: The HFA-PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. J. Heart Fail. 2020, 22, 391–412. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Girerd, N.; Seronde, M.F.; Coiro, S.; Chouihed, T.; Bilbault, P.; Braun, F.; Kenizou, D.; Maillier, B.; Nazeyrollas, P.; Roul, G.; et al. Integrative Assessment of Congestion in Heart Failure Throughout the Patient Journey. JACC Heart Fail. 2018, 6, 273–285. [Google Scholar] [CrossRef]
- Clark, A.L.; Kalra, P.R.; Petrie, M.C.; Mark, P.B.; Tomlinson, L.A.; Tomson, C.R. Change in renal function associated with drug treatment in heart failure: National guidance. Heart 2019, 105, 904–910. [Google Scholar] [CrossRef]
- Bozkurt, B.; Coats, A.J.S.; Tsutsui, H.; Abdelhamid, C.M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Böhm, M.; Butler, J.; et al. Universal definition and classification of heart failure: A report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur. J. Heart Fail. 2021, 23, 352–380. [Google Scholar] [CrossRef] [PubMed]
- Damy, T.; Kallvikbacka-Bennett, A.; Zhang, J.; Goode, K.; Buga, L.; Hobkirk, J.; Yassin, A.; Dubois-Randé, J.L.; Hittinger, L.; Cleland, J.G.; et al. Does the physical examination still have a role in patients with suspected heart failure? Eur. J. Heart Fail. 2011, 13, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Pellicori, P.; Carubelli, V.; Zhang, J.; Castiello, T.; Sherwi, N.; Clark, A.L.; Cleland, J.G. IVC diameter in patients with chronic heart failure: Relationships and prognostic significance. JACC Cardiovasc. Imaging 2013, 6, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Rae, N.; Finch, S.; Chalmers, J.D. Cardiovascular disease as a complication of community-acquired pneumonia. Curr. Opin. Pulm. Med. 2016, 22, 212–218. [Google Scholar] [CrossRef]
- Karamichalakis, N.; Letsas, K.P.; Vlachos, K.; Georgopoulos, S.; Bakalakos, A.; Efremidis, M.; Sideris, A. Managing atrial fibrillation in the very elderly patient: Challenges and solutions. Vasc. Health Risk Manag. 2015, 11, 555–562. [Google Scholar] [CrossRef] [Green Version]
- Ponikowski, P.; Kirwan, B.A.; Anker, S.D.; McDonagh, T.; Dorobantu, M.; Drozdz, J.; Fabien, V.; Filippatos, G.; Gohring, U.M.; Keren, A.; et al. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: A multicentre, double-blind, randomised, controlled trial. Lancet 2020, 396, 1895–1904. [Google Scholar] [CrossRef]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef] [Green Version]
- Bavishi, C.; Ahmed, M.; Trivedi, V.; Khan, A.R.; Gongora, C.; Bangalore, S.; Messerli, F.H. Meta-Analysis of Randomized Trials on the Efficacy and Safety of Angiotensin-Converting Enzyme Inhibitors in Patients ≥65 Years of Age. Am. J. Cardiol. 2016, 118, 1427–1436. [Google Scholar] [CrossRef]
- Kotecha, D.; Manzano, L.; Krum, H.; Rosano, G.; Holmes, J.; Altman, D.G.; Collins, P.D.; Packer, M.; Wikstrand, J.; Coats, A.J.; et al. Effect of age and sex on efficacy and tolerability of β blockers in patients with heart failure with reduced ejection fraction: Individual patient data meta-analysis. BMJ 2016, 353, i1855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, J.P.; Rossello, X.; Eschalier, R.; McMurray, J.J.V.; Pocock, S.; Girerd, N.; Rossignol, P.; Pitt, B.; Zannad, F. MRAs in Elderly HF Patients: Individual Patient-Data Meta-Analysis of RALES, EMPHASIS-HF, and TOPCAT. JACC Heart Fail. 2019, 7, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Jhund, P.S.; Fu, M.; Bayram, E.; Chen, C.-H.; Negrusz-Kawecka, M.; Rosenthal, A.; Desai, A.S.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; et al. Efficacy and safety of LCZ696 (sacubitril-valsartan) according to age: Insights from PARADIGM-HF. Eur. Heart J. 2015, 36, 2576–2584. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Claggett, B.; Lefkowitz, M.P.; McMurray, J.J.V.; Rouleau, J.L.; Solomon, S.D.; Zile, M.R. Effect of neprilysin inhibition on renal function in patients with type 2 diabetes and chronic heart failure who are receiving target doses of inhibitors of the renin-angiotensin system: A secondary analysis of the PARADIGM-HF trial. Lancet Diabetes Endocrinol. 2018, 6, 547–554. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Kober, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Belohlavek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [Green Version]
- Mewton, N.; Girerd, N.; Boffa, J.J.; Courivaud, C.; Isnard, R.; Juillard, L.; Lamblin, N.; Legrand, M.; Logeart, D.; Mariat, C.; et al. Practical management of worsening renal function in outpatients with heart failure and reduced ejection fraction: Statement from a panel of multidisciplinary experts and the Heart Failure Working Group of the French Society of Cardiology. Arch. Cardiovasc. Dis. 2020. [Google Scholar] [CrossRef]
- Gonzalez-Lopez, E.; Gallego-Delgado, M.; Guzzo-Merello, G.; de Haro-Del Moral, F.J.; Cobo-Marcos, M.; Robles, C.; Bornstein, B.; Salas, C.; Lara-Pezzi, E.; Alonso-Pulpon, L.; et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur. Heart J. 2015, 36, 2585–2594. [Google Scholar] [CrossRef] [Green Version]
- Castano, A.; Narotsky, D.L.; Hamid, N.; Khalique, O.K.; Morgenstern, R.; DeLuca, A.; Rubin, J.; Chiuzan, C.; Nazif, T.; Vahl, T.; et al. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur. Heart J. 2017, 38, 2879–2887. [Google Scholar] [CrossRef] [Green Version]
- Maurer, M.S.; Schwartz, J.H.; Gundapaneni, B.; Elliott, P.M.; Merlini, G.; Waddington-Cruz, M.; Kristen, A.V.; Grogan, M.; Witteles, R.; Damy, T.; et al. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N. Engl. J. Med. 2018, 379, 1007–1016. [Google Scholar] [CrossRef]
- Jaarsma, T.; Hill, L.; Bayes-Genis, A.; La Rocca, H.B.; Castiello, T.; Čelutkienė, J.; Marques-Sule, E.; Plymen, C.M.; Piper, S.E.; Riegel, B.; et al. Self-care of heart failure patients: Practical management recommendations from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2021, 23, 157–174. [Google Scholar] [CrossRef]
- Cautela, J.; Tartiere, J.M.; Cohen-Solal, A.; Bellemain-Appaix, A.; Theron, A.; Tibi, T.; Januzzi, J.L., Jr.; Roubille, F.; Girerd, N. Management of low blood pressure in ambulatory heart failure with reduced ejection fraction patients. Eur. J. Heart Fail. 2020, 10.1002/ejhf.1835. [Google Scholar] [CrossRef]
- GICC. EPON: Une Règle de Vie. Available online: https://giccardio.fr/patient/des-traitements-pour-mieux-vivre/epon-une-regle-de-vie (accessed on 29 July 2020).
- CNSA. Cahier Pédagogique MAIA.pdf. 2014. Available online: https://www.cnsa.fr/documentation/CNSA_CahierPe_dagogique_MAIA_HD.pdf (accessed on 29 July 2020).
- HAS. Mode d’emploi du Plan Personnalisé de Santé (PPS) Pour les Personnes à Risque de Perte D’autonomie (PAERPA). 2014. Available online: https://www.has-sante.fr/jcms/c_1638463/fr/plan-personnalise-de-sante-pps-paerpa (accessed on 29 July 2020).
- HAS. Synthèse du mode d’emploi du Plan Personnalisé de Santé (PPS). Available online: https://www.has-sante.fr/jcms/c_1638463/fr/plan-personnalise-de-sante-pps-paerpa (accessed on 29 July 2020).
- Santé, M.D.S.E.D.L. Le Dispositif PAERPA. 2018. Available online: https://solidarites-sante.gouv.fr/systeme-de-sante-et-medico-social/parcours-des-patients-et-des-usagers/le-parcours-sante-des-aines-paerpa/article/le-dispositif-paerpa (accessed on 29 July 2020).
- HAS. Stratégie de Prise en Charge en Cas de Dénutrition Protéino-Energétique Chez la Personne Agée-Synthèse des Recommandations Professionnelles. 2007. Available online: https://www.has-sante.fr/jcms/c_546549/fr/strategie-de-prise-en-charge-en-cas-de-denutrition-proteino-energetique-chez-la-personne-agee (accessed on 29 July 2020).
- HAS. Arbre Décisionnel “Dépression Caracterisée”. 2014. Available online: https://www.has-sante.fr/upload/docs/application/pdf/2008-10/arbre_decisionnel__depression_caracterisee_.pdf (accessed on 29 July 2020).
- HAS. Dépression Characterisé. Available online: https://www.has-sante.fr/jcms/c_937773/fr/depression (accessed on 29 July 2020).
- By the American Geriatrics Society Beers Criteria Update Expert, P. American Geriatrics Society 2019 Updated AGS Beers Criteria(R) for Potentially Inappropriate Medication Use in Older Adults. J. Am. Geriatr. Soc. 2019, 67, 674–694. [Google Scholar] [CrossRef]
- HAS. Comment Prendre en Charge les Personnes Agées Fragiles en Ambulatoire? 2013. Available online: https://www.has-sante.fr/jcms/c_1718248/fr/comment-prendre-en-charge-les-personnes-agees-fragiles-en-ambulatoire. (accessed on 29 July 2020).
- SFGG. Prescriptions Médicamenteuses Adaptées Aux Personnes Agées (Guide PAPA). Available online: https://sfgg.org/actualites/guide-papa-coupon-pour-obtenir-le-guide/ (accessed on 29 July 2020).
- Laroche, M.L.; Bouthier, F.; Merle, L.; Charmes, J.P. Potentially inappropriate medications in the elderly: A list adapted to French medical practice. Rev. Med. Interne 2009, 30, 592–601. [Google Scholar] [CrossRef]
- Piffer, I.; Goetz, C.; Zevering, Y.; André, E.; Bourouis, Z.; Blettner, N. Ability of Emergency Department Physicians Using a Functional Autonomy-Assessing Version of the Triage Risk Screening Tool to Detect Frail Older Patients Who Require Mobile Geriatric Team Consultation. J. Nutr. Health Aging 2020, 24, 634–641. [Google Scholar] [CrossRef]
- de Rotrou, J.; Battal-Merlet, L.; Wenisch, E.; Chausson, C.; Bizet, E.; Dray, F.; Lenoir, H.; Rigaud, A.S.; Hanon, O. Relevance of 10-min delayed recall in dementia screening. Eur. J. Neurol. 2007, 14, 144–149. [Google Scholar] [CrossRef]
- Charlson, M.; Pompei, P.; Ales, K.; MacKenzie, R. A new method of classifying prognostic comorbidity in longditudinal studies: Development and validation. J. Chron. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, C.; Li, Y.; Li, H.; Cao, Q.; Li, F. Prognostic Value of Frailty for Older Patients with Heart Failure: A Systematic Review and Meta-Analysis of Prospective Studies. BioMed Res. Int. 2018, 2018, 8739058. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.R.; Hrobjartsson, A.; Pottegard, A.; Damkier, P.; Larsen, K.S.; Madsen, K.G.; dePont Christensen, R.; Kristensen, M.E.L.; Christensen, P.M.; Hallas, J. Postponement of Death by Statin Use: A Systematic Review and Meta-analysis of Randomized Clinical Trials. J. Gen. Intern. Med. 2019, 34, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Vedel, I.; Khanassov, V. Transitional Care for Patients with Congestive Heart Failure: A Systematic Review and Meta-Analysis. Ann. Fam. Med. 2015, 13, 562–571. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damy, T.; Chouihed, T.; Delarche, N.; Berrut, G.; Cacoub, P.; Henry, P.; Lamblin, N.; Andrès, E.; Hanon, O. Diagnosis and Management of Heart Failure in Elderly Patients from Hospital Admission to Discharge: Position Paper. J. Clin. Med. 2021, 10, 3519. https://doi.org/10.3390/jcm10163519
Damy T, Chouihed T, Delarche N, Berrut G, Cacoub P, Henry P, Lamblin N, Andrès E, Hanon O. Diagnosis and Management of Heart Failure in Elderly Patients from Hospital Admission to Discharge: Position Paper. Journal of Clinical Medicine. 2021; 10(16):3519. https://doi.org/10.3390/jcm10163519
Chicago/Turabian StyleDamy, Thibaud, Tahar Chouihed, Nicholas Delarche, Gilles Berrut, Patrice Cacoub, Patrick Henry, Nicholas Lamblin, Emmanuel Andrès, and Olivier Hanon. 2021. "Diagnosis and Management of Heart Failure in Elderly Patients from Hospital Admission to Discharge: Position Paper" Journal of Clinical Medicine 10, no. 16: 3519. https://doi.org/10.3390/jcm10163519
APA StyleDamy, T., Chouihed, T., Delarche, N., Berrut, G., Cacoub, P., Henry, P., Lamblin, N., Andrès, E., & Hanon, O. (2021). Diagnosis and Management of Heart Failure in Elderly Patients from Hospital Admission to Discharge: Position Paper. Journal of Clinical Medicine, 10(16), 3519. https://doi.org/10.3390/jcm10163519