Disease Manifestations in Mucopolysaccharidoses and Their Impact on Anaesthesia-Related Complications—A Retrospective Analysis of 99 Patients
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Sites and Patients
2.2. Data Acquisition
2.3. Statistics
3. Results
3.1. Data Acquisition and Quality
3.2. Patient Characteristics
3.3. Characteristics of Anaesthesia Procedures
3.4. Anaesthesia-Related Complication
3.5. Best Forecast Performance of Anaesthesia-Related Complications by a Model Based on Disease Manifestations, Disease-Specific Therapies and the Indication for Surgery
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galimberti, C.; Madeo, A.; Di Rocco, M.; Fiumara, A. Mucopolysaccharidoses: Early diagnostic signs in infants and children. Ital. J. Pediatr. 2018, 44, 133. [Google Scholar] [CrossRef]
- Berger, K.I.; Fagondes, S.C.; Giugliani, R.; Hardy, K.A.; Lee, K.S.; McArdle, C.; Scarpa, M.; Tobin, M.J.; Ward, S.A.; Rapoport, D.M. Respiratory and sleep disorders in mucopolysaccharidosis. J. Inherit. Metab. Dis. 2013, 36, 201–210. [Google Scholar] [CrossRef]
- De Ru, M.H.; Boelens, J.J.; Das, A.M.; Jones, S.A.; van der Lee, J.H.; Mahlaoui, N.; Mengel, E.; Offringa, M.; O’Meara, A.; Parini, R.; et al. Enzyme replacement therapy and/or hematopoietic stem cell transplantation at diagnosis in patients with mucopolysaccharidosis type I: Results of a European consensus procedure. Orphanet J. Rare Dis. 2011, 6, 55. [Google Scholar] [CrossRef] [Green Version]
- Arn, P.; Wraith, J.E.; Underhill, L. Characterization of surgical procedures in patients with mucopolysaccharidosis type I: Findings from the MPS I Registry. J. Pediatr. 2009, 154, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Moretto, A.; Bosatra, M.G.; Marchesini, L.; Tesoro, S. Anesthesiological risks in mucopolysaccharidoses. Ital. J. Pediatr. 2018, 44, 116. [Google Scholar] [CrossRef]
- Walker, R.; Belani, K.G.; Braunlin, E.A.; Bruce, I.A.; Hack, H.; Harmatz, P.R.; Jones, S.; Rowe, R.; Solanki, G.A.; Valdemarsson, B. Anaesthesia and airway management in mucopolysaccharidosis. J. Inherit. Metab. Dis. 2013, 36, 211–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scaravilli, V.; Zanella, A.; Ciceri, V.; Bosatra, M.; Flandoli, C.; La Bruna, A.; Sosio, S.; Parini, R.; Gasperini, S.; Pesenti, A.; et al. Safety of anesthesia for children with mucopolysaccharidoses: A retrospective analysis of 54 patients. Paediatr. Anaesth. 2018, 28, 436–442. [Google Scholar] [CrossRef]
- Kirkpatrick, K.; Ellwood, J.; Walker, R.W. Mucopolysaccharidosis type I (Hurler syndrome) and anesthesia: The impact of bone marrow transplantation, enzyme replacement therapy, and fiberoptic intubation on airway management. Paediatr. Anaesth. 2012, 22, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Arn, P.; Whitley, C.; Wraith, J.E.; Webb, H.W.; Underhill, L.; Rangachari, L.; Cox, G.F. High rate of postoperative mortality in patients with mucopolysaccharidosis I: Findings from the MPS I Registry. J. Pediatr. Surg. 2012, 47, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Jöhr, M.; Berger, T.M. Fiberoptic intubation through the laryngeal mask airway (LMA) as a standardized procedure. Paediatr. Anaesth. 2004, 14, 614. [Google Scholar] [CrossRef] [PubMed]
- Dohrmann, T.; Muschol, N.M.; Sehner, S.; Punke, M.A.; Haas, S.A.; Roeher, K.; Breyer, S.; Koehn, A.F.; Ullrich, K.; Zöllner, C.; et al. Airway management and perioperative adverse events in children with mucopolysaccharidoses and mucolipidoses: A retrospective cohort study. Paediatr. Anaesth. 2020, 30, 181–190. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, J.; Leung, W.T.; Wang, L. A basic understanding of mucopolysaccharidosis: Incidence, clinical features, diagnosis, and management. Intractable Rare Dis. Res. 2020, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Madoff, L.U.; Kordun, A.; Cravero, J.P. Airway management in patients with mucopolysaccharidoses: The progression toward difficult intubation. Paediatr. Anaesth. 2019, 29, 620–627. [Google Scholar] [CrossRef]
- Meyer, A.; Kossow, K.; Gal, A.; Mühlhausen, C.; Ullrich, K.; Braulke, T.; Muschol, N. Scoring evaluation of the natural course of mucopolysaccharidosis type IIIA (Sanfilippo syndrome type A). Pediatrics 2007, 120, e1255–e1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groll, A.; Trutz, G. Variable selection for generalized linear mixed models by L1-penalized estimation. Stat. Comput. 2014, 24, 137–154. [Google Scholar] [CrossRef] [Green Version]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Arn, P.; Bruce, I.A.; Wraith, J.E.; Travers, H.; Fallet, S. Airway-related symptoms and surgeries in patients with mucopolysaccharidosis I. Ann. Otol. Rhinol. Laryngol. 2015, 124, 198–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck, M.; Arn, P.; Giugliani, R.; Muenzer, J.; Okuyama, T.; Taylor, J.; Fallet, S. The natural history of MPS I: Global perspectives from the MPS I Registry. Genet. Med. Off. J. Am. Coll. Med. Genet. 2014, 16, 759–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montaño, A.M.; Tomatsu, S.; Gottesman, G.S.; Smith, M.; Orii, T. International Morquio A Registry: Clinical manifestation and natural course of Morquio A disease. J. Inherit. Metab. Dis. 2007, 30, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, E.G.; Jones, S.A.; Escolar, M.L. Developmental and behavioral aspects of mucopolysaccharidoses with brain manifestations—Neurological signs and symptoms. Mol. Genet. Metab. 2017, 122, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, E.G.; Escolar, M.L.; Delaney, K.A.; Mitchell, J.J. Assessments of neurocognitive and behavioral function in the mucopolysaccharidoses. Mol. Genet. Metab. 2017, 122, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Remondino, R.G.; Tello, C.A.; Noel, M.; Wilson, A.F.; Galaretto, E.; Bersusky, E.; Piantoni, L. Clinical Manifestations and Surgical Management of Spinal Lesions in Patients With Mucopolysaccharidosis: A Report of 52 Cases. Spine Deform. 2019, 7, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Theroux, M.C.; Nerker, T.; Ditro, C.; Mackenzie, W.G. Anesthetic care and perioperative complications of children with Morquio syndrome. Paediatr. Anaesth. 2012, 22, 901–907. [Google Scholar] [CrossRef]
- Averill, L.W.; Kecskemethy, H.H.; Theroux, M.C.; Mackenzie, W.G.; Pizarro, C.; Bober, M.B.; Ditro, C.P.; Tomatsu, S. Tracheal narrowing in children and adults with mucopolysaccharidosis type IVA: Evaluation with computed tomography angiography. Pediatr. Radiol. 2021, 51, 1202–1213. [Google Scholar] [CrossRef] [PubMed]
- Charrow, J.; Alden, T.D.; Breathnach, C.A.; Frawley, G.P.; Hendriksz, C.J.; Link, B.; Mackenzie, W.G.; Manara, R.; Offiah, A.C.; Solano, M.L.; et al. Diagnostic evaluation, monitoring, and perioperative management of spinal cord compression in patients with Morquio syndrome. Mol. Genet. Metab. 2015, 114, 11–18. [Google Scholar] [CrossRef]
- Tomatsu, S.; Mackenzie, W.G.; Theroux, M.C.; Mason, R.W.; Thacker, M.M.; Shaffer, T.H.; Montaño, A.M.; Rowan, D.; Sly, W.; Alméciga-Díaz, C.J.; et al. Current and emerging treatments and surgical interventions for Morquio A syndrome: A review. Res. Rep. Endocr. Disord. 2012, 2012, 65–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habre, W.; Disma, N.; Virag, K.; Becke, K.; Hansen, T.G.; Jöhr, M.; Leva, B.; Morton, N.S.; Vermeulen, P.M.; Zielinska, M.; et al. Incidence of severe critical events in paediatric anaesthesia (APRICOT): A prospective multicentre observational study in 261 hospitals in Europe. Lancet Respir. Med. 2017, 5, 412–425. [Google Scholar] [CrossRef]
- Kurth, C.D.; Tyler, D.; Heitmiller, E.; Tosone, S.R.; Martin, L.; Deshpande, J.K. National pediatric anesthesia safety quality improvement program in the United States. Anesth. Analg. 2014, 119, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Frawley, G.; Fuenzalida, D.; Donath, S.; Yaplito-Lee, J.; Peters, H. A retrospective audit of anesthetic techniques and complications in children with mucopolysaccharidoses. Paediatr. Anaesth. 2012, 22, 737–744. [Google Scholar] [CrossRef]

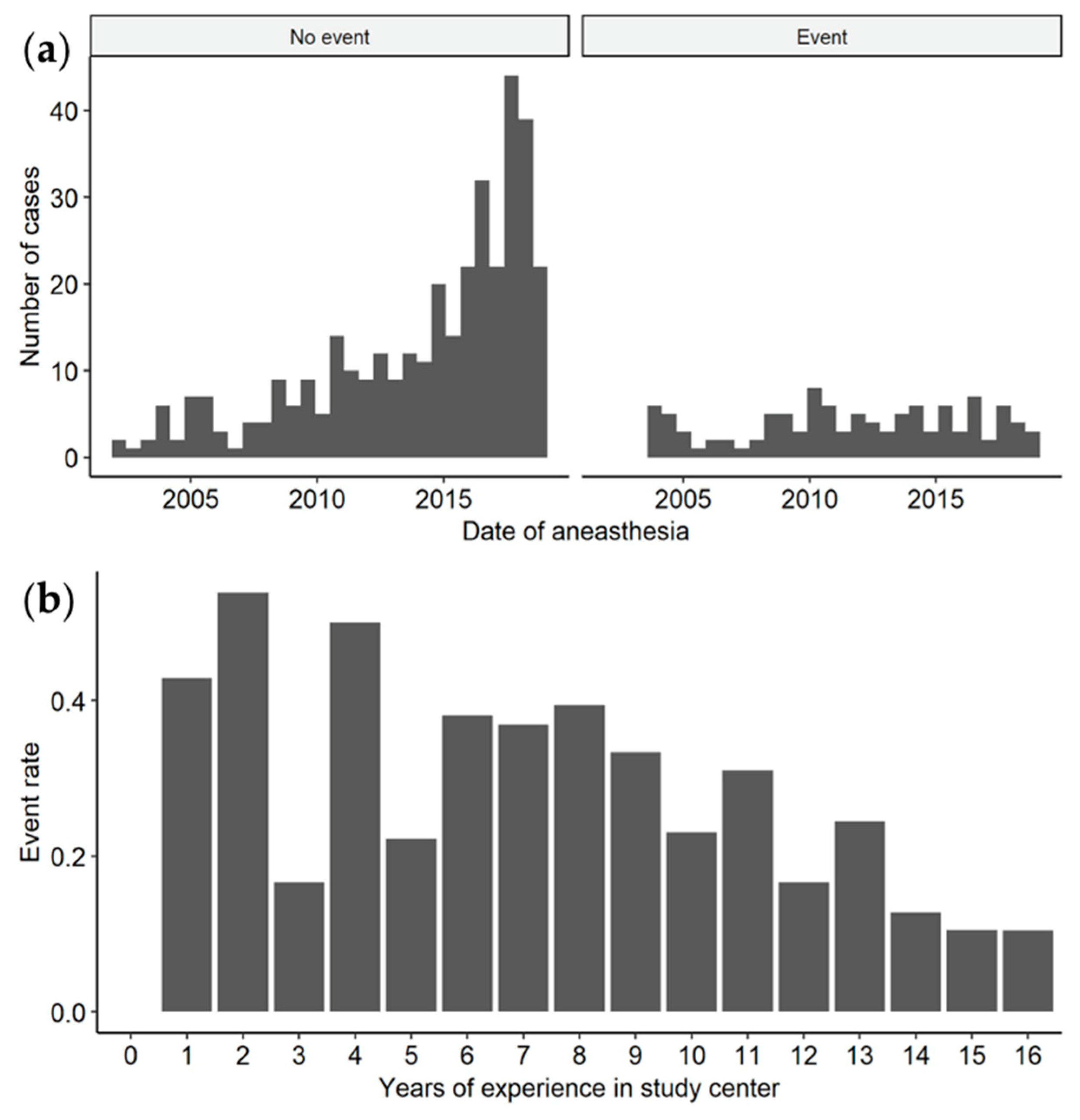
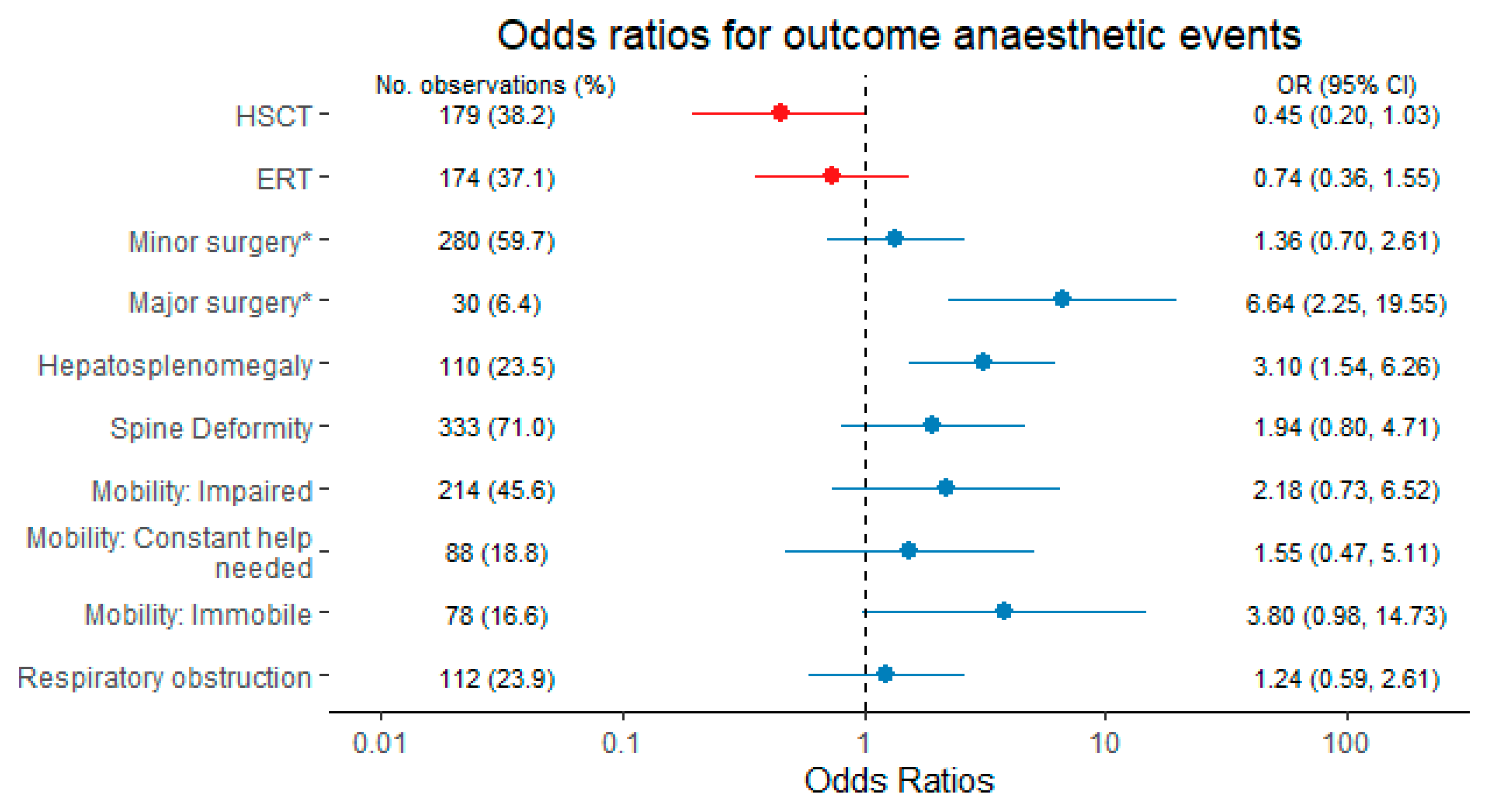
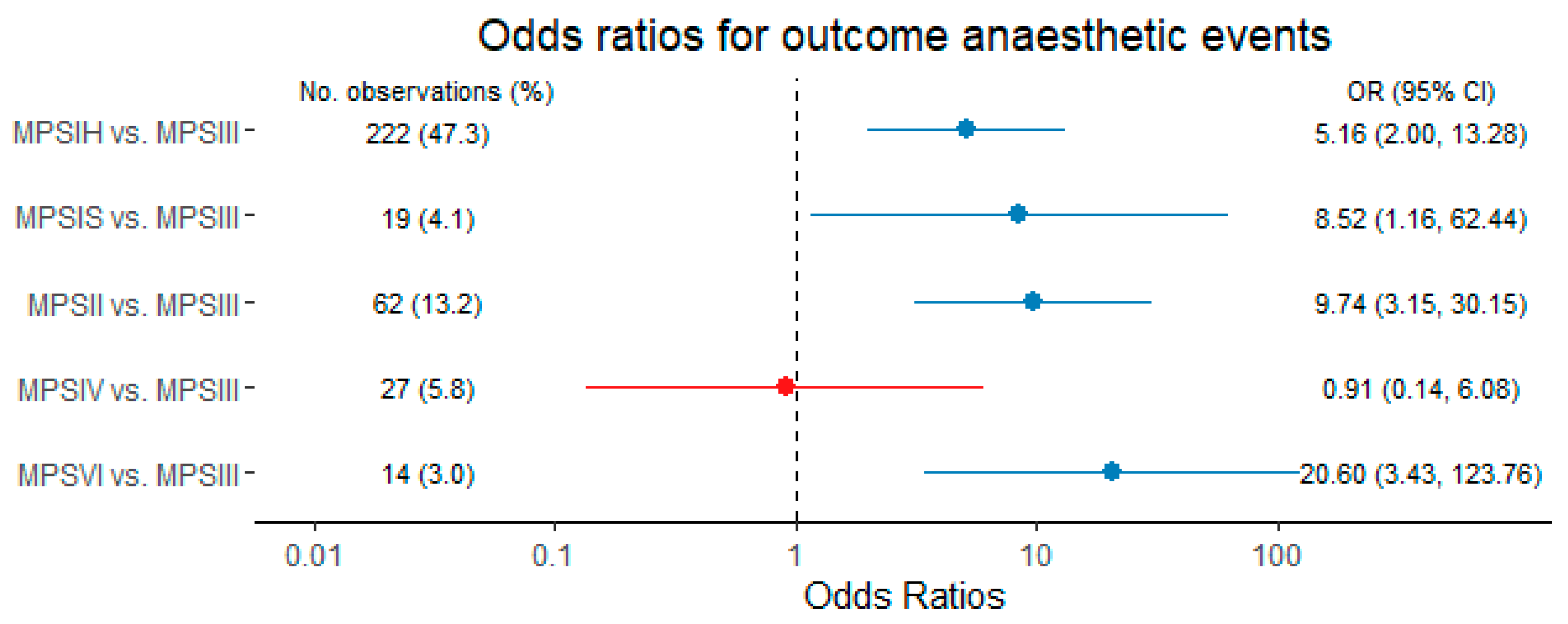
| Characteristics | Overall N = 99 | MPSIH N = 32 | MPSIS N = 3 | MPSII N = 16 | MPSIII N = 37 | MPSIV N = 7 | MPSVI N = 4 |
|---|---|---|---|---|---|---|---|
| Demographic characteristics | |||||||
| Sex, male, n (%) | 71 (71.7) | 20 (62.5) | 1 (33.3) | 16 (100.0) | 27 (73.10) | 4 (57.1) | 3 (75.0) |
| Min. age at anaesthesia (years) * | 4.8 (0.7–38.3) | 1.9 (0.8–20.8) | 13.9 (7.1–29.1) | 7.8 (0.7–29.7) | 5.8 (1.1–38.3) | 8.6 (4.1–18.6) | 10.4 (7.5–24.2) |
| Max. age at anaesthesia (years) * | 9.7 (0.8–38.7) | 8.6 (0.8–23.8) | 24.0 (7.1–29.1) | 12.2 (2.5–32.1) | 8.9 (3.5–38.7) | 15.7 (6.1–18.6) | 21.6 (8.5–38.2) |
| Medical history | |||||||
| Age at diagnosis (years) * | 3.1 (0.0–29.0) | 1.2 (0.0–8.0) | 6.6 (3.8–29.0) | 4.5 (0.1–15.8) | 3.7 (0.5–16.8) | 4.3 (0.9–8.6) | 4.4 (1.6–11.5) |
| Total no. of anaesthesias | 3.0 (1.0–19.0) | 5.0 (1.0–19.0) | 1.0 (1.0–18.0) | 3.0 (1.0–10.0) | 2.5 (1.0–12.0) | 3.0 (1.0–9.0) | 5.5 (1.0–11.0) |
| HSCT, n (%) | 28 (28.3) | 22 (68.8) | - | 3 (18.8) | 3 (8.1) | - | - |
| Age at HSCT (years) * | 1.3 (0.4–1.8) | 1.4 (1.1–1.8) | N/A | 0.4 (0.4–0.4) | 4.7 (3.0–5.7) | N/A | N/A |
| ERT, n (%) | 36 (36.4) | 15 (46.9) | 3 (100.0) | 11 (68.8) | - | 5 (71.4) | 3 (75.0) |
| Age at ERT-start (years) * | 6.2 (0.2–29.0) | 1.0 (0.7–6.4) | 7.0 (6.7–29.0) | 8.2 (0.2–22.1) | N/A | 12.0 (4.6–14.5) | 11.5 (11.5–11.5) |
| Craniofacial pathologies | |||||||
| Facial dysmorphism, n (%) | 91 (91.9) | 30 (93.8) | 2 (66.7) | 15 (93.8) | 35 (94.6) | 5 (71.4) | 4 (100.0) |
| Macroglossia, n (%) | 51 (51.5) | 20 (62.5) | 1 (33.3) | 11 (68.8) | 16 (43.2) | 1 (14.3) | 2 (50.0) |
| Tonsil hyperplasia, n (%) | 45 (45.5) | 17 (53.1) | - | 6 (37.5) | 15 (40.5) | 4 (57.1) | 3 (75.0) |
| Short neck, n (%) | 84 (84.8) | 27 (84.4) | 2 (66.7) | 16 (100.0) | 29 (78.4) | 6 (85.7) | 4 (100.0) |
| Respiratory manifestations | |||||||
| Lung function, n (%) | |||||||
| Normal | 49 (49.5) | 13 (40.6) | 2 (66.7) | 4 (25.0) | 25 (67.6) | 4 (57.1) | 1 (25.0) |
| Obstruction | 23 (23.2) | 6 (18.8) | - | 4 (25.0) | 12 (32.4) | 1 (14.3) | - |
| Restriction | 15 (15.2) | 12 (37.5) | - | 1 (6.2) | - | 1 (14.3) | 1 (25.0) |
| Both | 12 (12.1) | 1 (3.1) | 1 (33.3) | 7 (43.8) | - | 1 (14.3) | 2 (50.0) |
| Sleep apnoea, n (%) | |||||||
| No | 60 (60.6) | 18 (56.2) | 1 (33.3) | 7 (43.8) | 30 (81.1) | 4 (57.1) | - |
| Suspected | 17 (17.2) | 5 (15.6) | - | 4 (25.0) | 5 (13.5) | 1 (14.3) | 2 (50.0) |
| Diagnosis | 11 (11.1) | 5 (15.6) | 1 (33.3) | 3 (18.8) | 1 (2.7) | - | 1 (25.0) |
| CPAP | 11 (11.1) | 4 (12.5) | 1 (33.3) | 2 (12.5) | 1 (2.7) | 2 (28.6) | 1 (25.0) |
| Thorax deformity, n (%) | 26 (26.3) | 11 (34.4) | - | 4 (25.0) | 3 (8.1) | 6 (85.7) | 2 (50.0) |
| Cardiac manifestations | |||||||
| Cardiac pathology, n (%) | |||||||
| No | 29 (29.3) | 5 (15.6) | - | 3 (18.8) | 19 (51.4) | 2 (28.6) | - |
| Mild | 38 (38.4) | 13 (40.6) | 2 (66.7) | 5 (31.2) | 12 (32.4) | 4 (57.1) | 2 (50.0) |
| Moderate | 15 (15.2) | 4 (12.5) | 1 (33.3) | 5 (31.2) | 3 (8.1) | 1 (14.3) | 1 (25.0) |
| Severe | 17 (17.2) | 10 (31.2) | - | 3 (18.8) | 3 (8.1) | - | 1 (25.0) |
| Gastrointestinal manifestations | |||||||
| Organomegaly, n (%) | |||||||
| No | 40 (40.4) | 11 (34.4) | 2 (66.7) | 5 (31.2) | 13 (35.1) | 7 (100.0) | 2 (50.0) |
| Any | 23 (23.2) | 8 (25.0) | - | 1 (6.2) | 13 (35.1) | - | 1 (25.0) |
| Hepatosplenomegaly | 36 (36.4) | 13 (40.6) | 1 (33.3) | 10 (62.5) | 11 (29.7) | - | 1 (25.0) |
| Dysphagia, n (%) | 29 (29.3) | 4 (12.5) | 1 (33.3) | 5 (31.2) | 19 (51.4) | - | - |
| Spine disease | |||||||
| Cervical spine stability, n (%) | |||||||
| Stable | 75 (75.8) | 18 (56.2) | 3 (100.0) | 13 (81.2) | 37 (100) | 3 (42.9) | 1 (25.0) |
| Instable | 19 (19.2) | 11 (34.4) | - | 3 (18.8) | - | 3 (42.9) | 2 (50.0) |
| Surgical Fusion | 5 (5.1) | 3 (9.4) | - | - | - | 1 (14.3) | 1 (25.0) |
| Cervical spine stenosis, n (%) | |||||||
| No | 57 (57.6) | 11 (34.4) | 1 (33.3) | 11 (68.8) | 33 (89.2) | 1 (14.3) | - |
| Stenosis | 25 (25.3) | 12 (37.5) | - | 3 (18.8) | 4 (10.8) | 4 (57.1) | 2 (50.0) |
| Stenosis with myelopathy | 6 (6.1) | 3 (9.4) | 1 (33.3) | 2 (12.5) | - | - | - |
| Decompression surgery | 11 (11.1) | 6 (18.8) | 1 (33.3) | - | - | 2 (28.6) | 2 (50.0) |
| Spine deformity, n (%) | |||||||
| No | 35 (35.4) | 1 (3.1) | 1 (33.3) | 8 (50.0) | 25 (67.6) | - | - |
| Scoliosis | 10 (10.1) | 2 (6.2) | - | 5 (31.2) | 2 (5.4) | - | 1 (25.0) |
| Kyphosis | 26 (26.3) | 13 (40.6) | - | 1 (6.2) | 7 (18.9) | 3 (42.9) | 2 (50.0) |
| Both | 28 (28.3) | 16 (50.0) | 2 (66.7) | 2 (12.5) | 3 (8.1) | 4 (57.1) | 1 (25.0) |
| Neurological manifestations | |||||||
| Seizures, n (%) | 19 (19.2) | 3 (9.4) | - | 2 (12.5) | 14 (37.8) | - | - |
| Shunted hydrocephalus, n (%) | 5 (5.1) | 3 (9.4) | 1 (33.3) | - | - | - | 1 (25.0) |
| Cognitive function, n (%) | |||||||
| Normal | 25 (25.3) | 11 (34.4) | 2 (66.7) | 5 (31.2) | - | 5 (71.4) | 2 (50.0) |
| Impaired | 46 (46.5) | 18 (56.2) | 1 (33.3) | 7 (43.8) | 16 (34.2) | 2 (28.6) | 2 (50.0) |
| Regression | 15 (15.2) | 2 (6.2) | - | 4 (25.0) | 9 (24.3) | - | - |
| Unresponsiveness | 13 (13.1) | 1 (3.1) | - | - | 12 (32.4) | - | - |
| Motor function, n (%) | |||||||
| No impairment | 14 (14.1) | 1 (3.1) | - | 5 (31.2) | 8 (21.6) | - | - |
| Impaired | 34 (34.3) | 16 (50.0) | 1 (33.3) | 6 (37.5) | 7 (18.9) | 2 (28.6) | 2 (50.0) |
| Constant help needed | 23 (23.2) | 7 (21.9) | 1 (33.3) | 1 (6.2) | 11 (29.7) | 3 (42.9) | - |
| Immobile | 28 (28.3) | 8 (25.0) | 1 (33.3) | 4 (25.0) | 11 (29.7) | 2 (28.6) | 2 (50.0) |
| Characteristics | Overall N = 484 | MPSIH N = 224 | MPSIS N = 20 | MPSII N = 62 | MPSIII N = 126 | MPSIV N = 29 | MPSVI N = 23 |
|---|---|---|---|---|---|---|---|
| Respiratory events, n (%) | 86 (17.8) | 38 (17.0) | 6 (30.0) | 25 (40.3) | 7 (5.6) | 2 (6.9) | 8 (34.8) |
| Respiratory insufficiency | 23 (4.8) | 8 (3.6) | 2 (10.0) | 7 (11.3) | 2 (1.6) | 1 (3.4) | 3 (13.0) |
| Hypoxemia | 17 (3.5) | 11 (4.9) | 1 (5.0) | 2 (3.2) | 2 (1.6) | - | 1 (5.0) |
| Airway obstruction | 19 (3.9) | 6 (2.7) | 2 (10.0) | 8 (12.9) | 1 (0.8) | - | 2 (10.0) |
| Increased ventilation pressure | 8 (1.7) | 2 (0.9) | - | 4 (6.5) | - | 1 (3.4) | 1 (5.0) |
| Hypercapnia | 7 (1.4) | 7 (3.1) | - | - | - | - | - |
| Pneumonia | 6 (1.2) | 2 (0.9) | 1 (5.0) | 2 (3.2) | - | - | 1 (5.0) |
| Atelectasis | 4 (0.8) | 2 (0.9) | - | - | 2 (1.6) | - | - |
| Pneumothorax | 1 (0.2) | - | - | 1 (1.6) | - | - | - |
| Cardiocirculatory events, n (%) | 12 (2.5) | 3 (1.3) | - | 1 (1.6) | 4 (3.2) | - | 4 (17.4) |
| Bradycardia/tachycardia | 6 (1.2) | 2 (0.9) | - | 1 (1.6) | 3 (2.4) | - | - |
| Hypotension | 3 (0.6) | 1 (0.4) | - | - | - | - | 2 (8.7) |
| Heart failure | 3 (0.6) | - | - | - | 1 (0.8) | - | 2 (8.7) |
| Difficult airway management, n (%) | 60 (12.4) | 37 (16.5) | 5 (25.0) | 13 (21.0) | 2 (1.6) | - | 3 (13.0) |
| Technique changes necessary | 31 (6.4) | 22 (9.8) | 1 (5) | 5 (8.1) | 1 (0.8) | - | 2 (8.7) |
| Blind intubation | 12 (2.5) | 7 (3.1) | 1 (5) | 3 (4.8) | 1 (0.8) | - | - |
| Primary technique difficulty | 11 (2.3) | 7 (3.1) | 2 (10) | 2 (8.1) | - | - | - |
| Airway could not be secured | 4 (0.8) | - | 1 (5) | 2 (8.1) | - | - | 1 (4.3) |
| Prolonged ventilation due to difficult airway | 2 (0.4) | 1 (0.4) | - | 1 (1.6) | - | - | - |
| Other Events, n (%) | 13 (2.7) | 5 (2.2) | 1 (5.0) | 4 (6.5) | 2 (1.6) | - | 1 (4.3) |
| Seizures | 5 (1.0) | 1 (0.4) | - | 2 (3.2) | 2 (1.6) | - | - |
| Fever | 4 (0.8) | 3 (1.3) | - | - | - | - | 1 (4.3) |
| Delirium | 2 (0.4) | - | 1 (5.0) | 1 (1.6) | - | - | - |
| Neurological residues | 2 (0.4) | 1 (0.4) | - | 1 (1.6) | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ammer, L.S.; Dohrmann, T.; Muschol, N.M.; Lang, A.; Breyer, S.R.; Ozga, A.-K.; Petzoldt, M. Disease Manifestations in Mucopolysaccharidoses and Their Impact on Anaesthesia-Related Complications—A Retrospective Analysis of 99 Patients. J. Clin. Med. 2021, 10, 3518. https://doi.org/10.3390/jcm10163518
Ammer LS, Dohrmann T, Muschol NM, Lang A, Breyer SR, Ozga A-K, Petzoldt M. Disease Manifestations in Mucopolysaccharidoses and Their Impact on Anaesthesia-Related Complications—A Retrospective Analysis of 99 Patients. Journal of Clinical Medicine. 2021; 10(16):3518. https://doi.org/10.3390/jcm10163518
Chicago/Turabian StyleAmmer, Luise Sophie, Thorsten Dohrmann, Nicole Maria Muschol, Annika Lang, Sandra Rafaela Breyer, Ann-Kathrin Ozga, and Martin Petzoldt. 2021. "Disease Manifestations in Mucopolysaccharidoses and Their Impact on Anaesthesia-Related Complications—A Retrospective Analysis of 99 Patients" Journal of Clinical Medicine 10, no. 16: 3518. https://doi.org/10.3390/jcm10163518
APA StyleAmmer, L. S., Dohrmann, T., Muschol, N. M., Lang, A., Breyer, S. R., Ozga, A.-K., & Petzoldt, M. (2021). Disease Manifestations in Mucopolysaccharidoses and Their Impact on Anaesthesia-Related Complications—A Retrospective Analysis of 99 Patients. Journal of Clinical Medicine, 10(16), 3518. https://doi.org/10.3390/jcm10163518







