Establishment of a Robust and Simple Corneal Organ Culture Model to Monitor Wound Healing
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Cultivation of Porcine Corneas
2.2. Determination of Corneal Wound Area
2.3. Lactate Dehydrogenase Assay for Cytotoxicity Estimation
2.4. Tissue Processing for Paraffin Sections
2.5. Haematoxylin and Eosin Staining of Corneal Sections
2.6. Evaluation of Apoptosis with TUNEL Assay
2.7. Statistical Analysis
3. Results
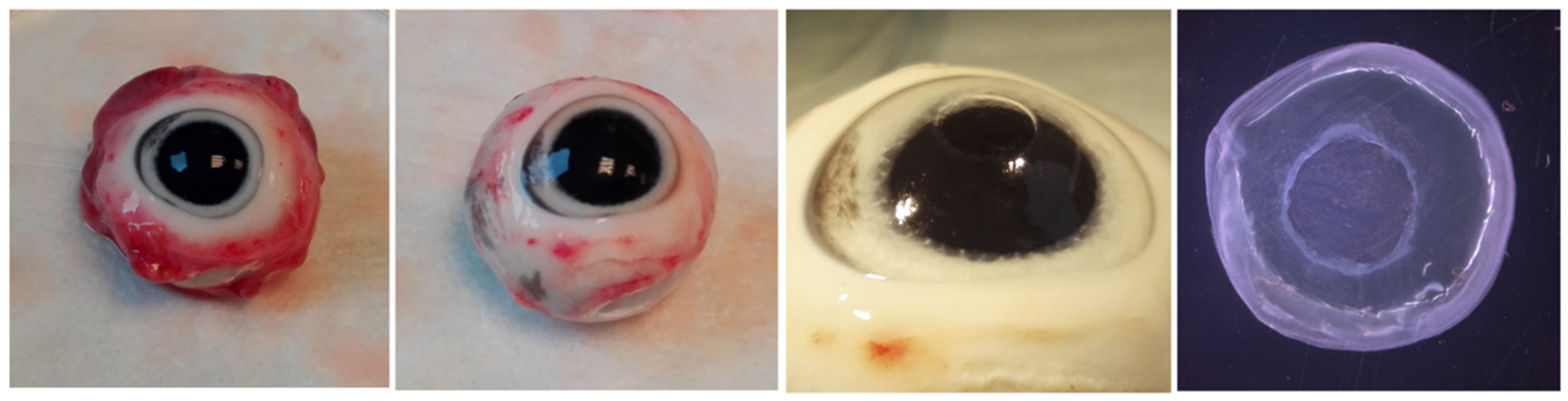
3.1. Tissue Preparation for Corneal Ex Vivo Culture
3.2. Optical Documentation of the Corneal Defect for 72 h
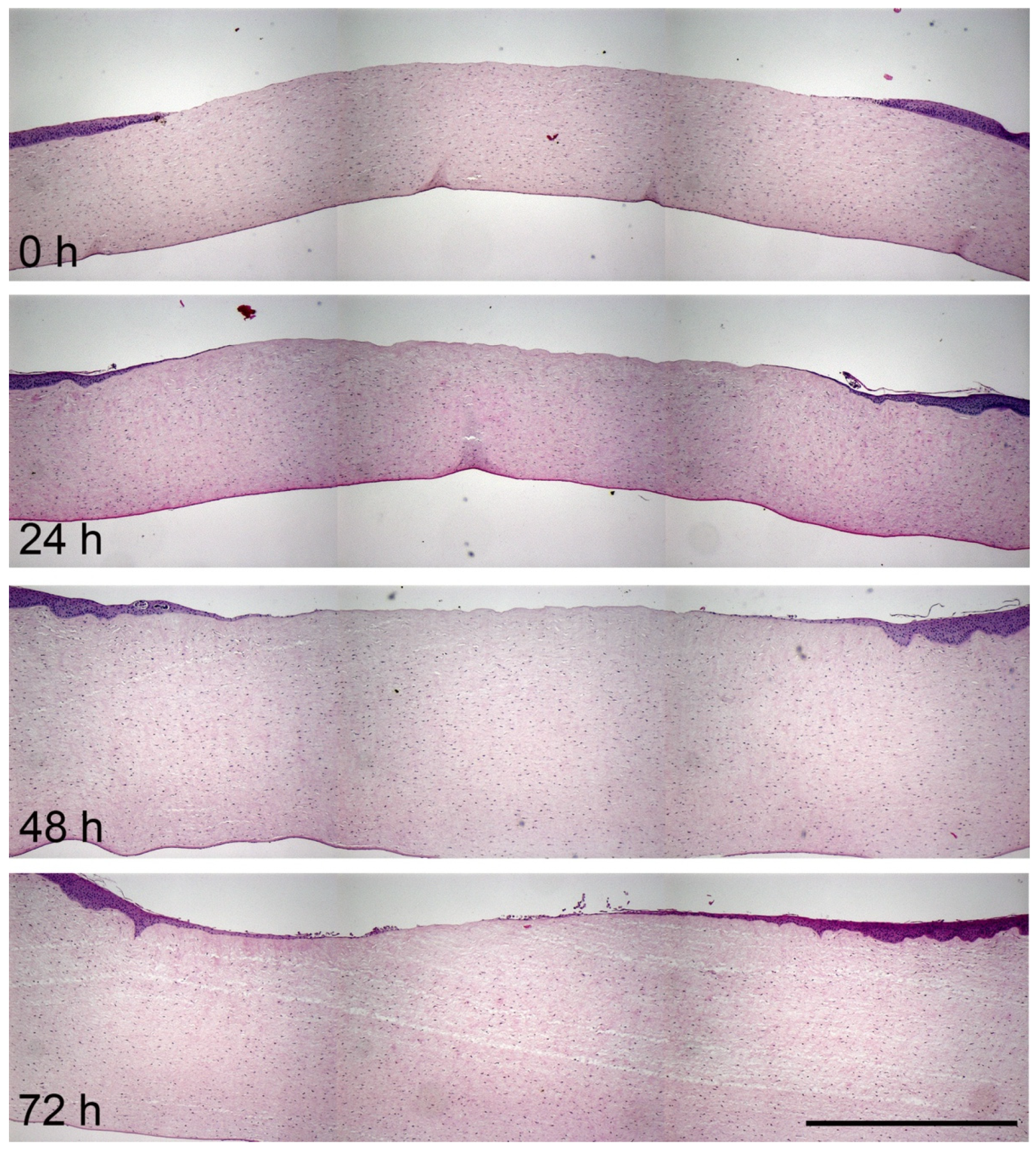
3.3. Histological Analysis of Corneal Wound Healing
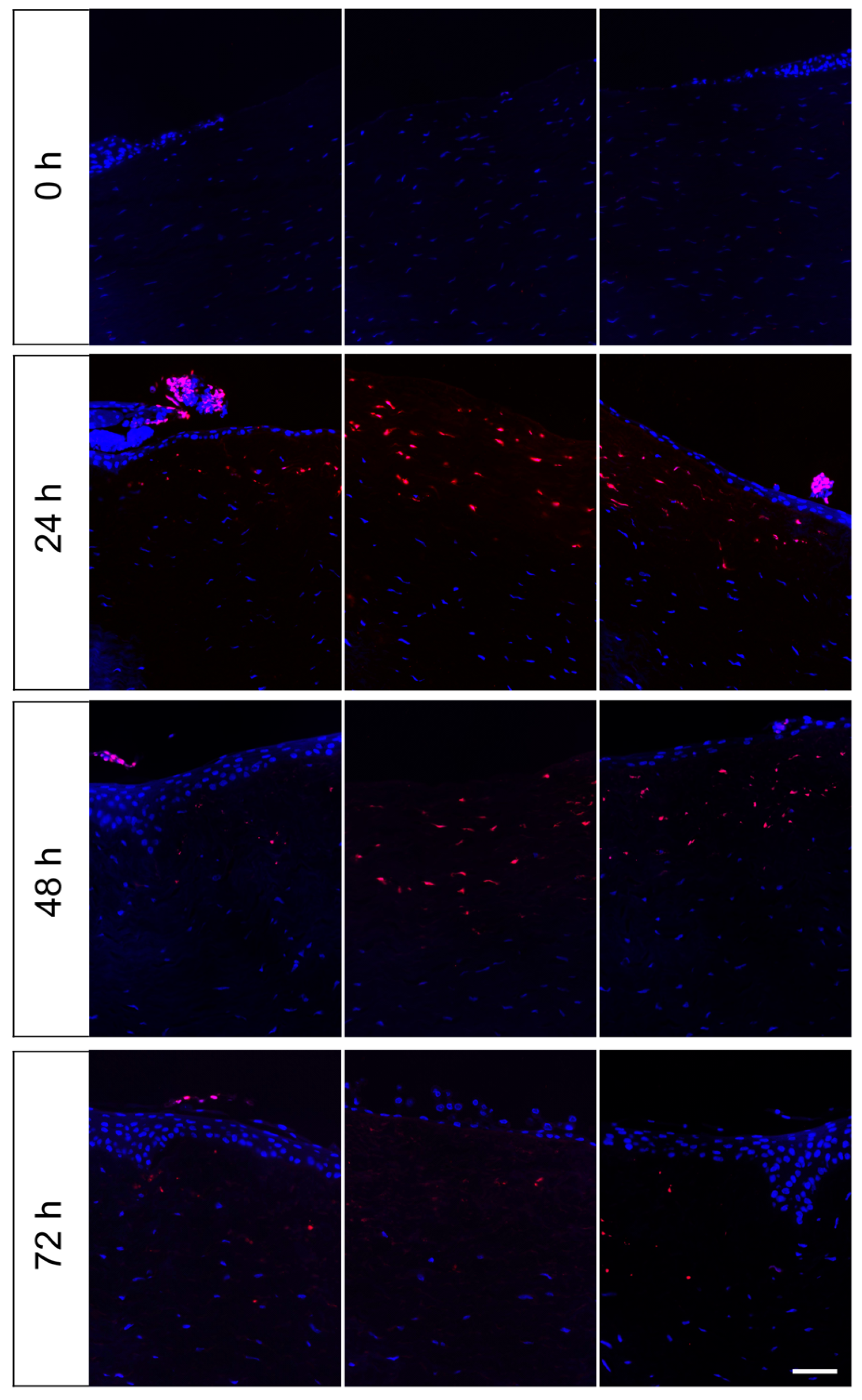
3.4. Evaluation of Apoptotic Cells in the Wound Area in the Course of Cornea Ex Vivo Cultivation
3.5. Influence of Defect Size and Presence of Limbus on Corneal Wound Healing
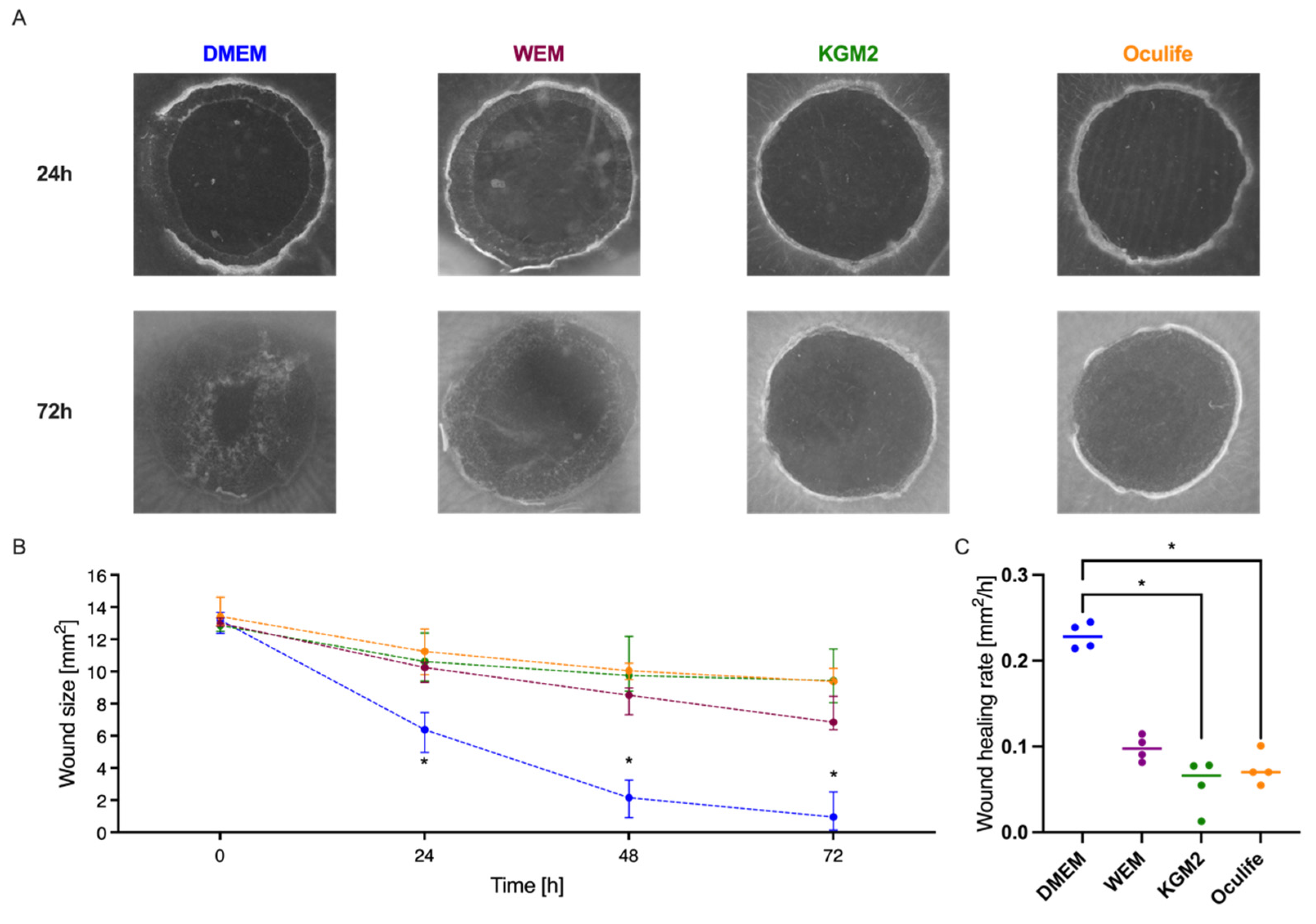
3.6. Influence of Culture Medium on Corneal Wound Healing
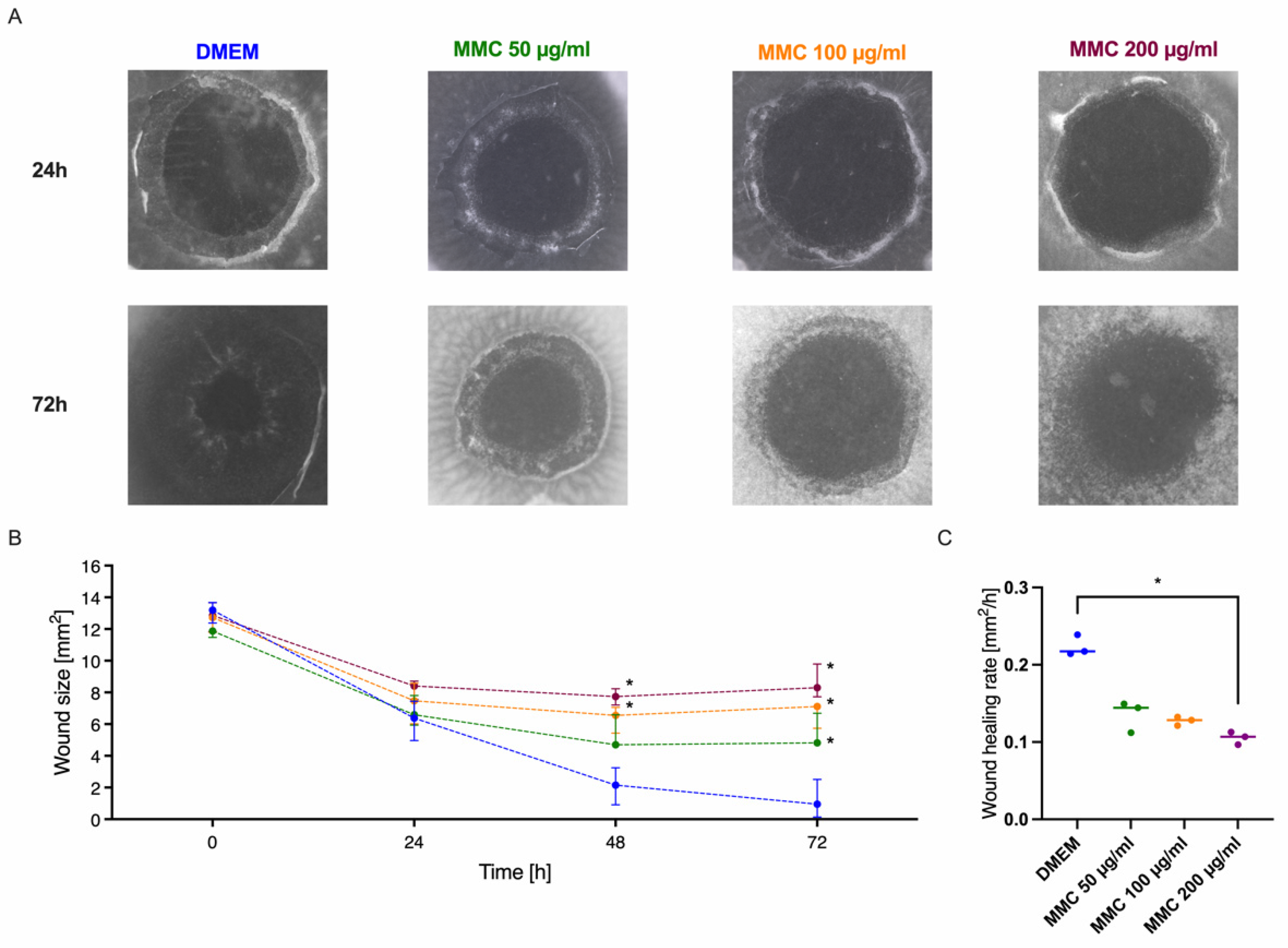
3.7. Modulation of the Cornea Model with the Wound Healing Inhibitor Mitomycin
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kinoshita, S.; Adachi, W.; Sotozono, C.; Nishida, K.; Yokoi, N.; Quantock, A.J.; Okubo, K. Characteristics of the Human Ocular Surface Epithelium. Prog. Retin. Eye Res. 2001, 20, 639–673. [Google Scholar] [CrossRef]
- Carrier, P.; Deschambeault, A.; Talbot, M.; Giasson, C.J.; Auger, F.A.; Guérin, S.L.; Germain, L. Characterization of Wound Reepithelialization Using a New Human Tissue-Engineered Corneal Wound Healing Model. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1376–1385. [Google Scholar] [CrossRef] [PubMed]
- Proietto, L.R.; Whitley, R.D.; Brooks, D.E.; Schultz, G.E.; Gibson, D.J.; Berkowski, W.M., Jr.; Salute, M.E.; Plummer, C.E. Development and Assessment of a Novel Canine Ex Vivo Corneal Model. Curr. Eye Res. 2017, 42, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Dua, H.S.; Gomes, J.A.; Singh, A. Corneal Epithelial Wound Healing. Br. J. Ophthalmol. 1994, 78, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Bukowiecki, A.; Hos, D.; Cursiefen, C.; Eming, S.A. Wound-Healing Studies in Cornea and Skin: Parallels, Differences and Opportunities. Int. J. Mol. Sci. 2017, 18, 1257. [Google Scholar] [CrossRef] [PubMed]
- Holzhey, A.; Sonntag, S.; Rendenbach, J.; Ernesti, J.S.; Kakkassery, V.; Grisanti, S.; Reinholz, F.; Freidank, S.; Vogel, A.; Ranjbar, M. Development of a Noninvasive, Laser-Assisted Experimental Model of Corneal Endothelial Cell Loss. J. Vis. Exp. JoVE 2020, 158, e60542. [Google Scholar] [CrossRef]
- Richard, N.R.; Anderson, J.A.; Weiss, J.L.; Binder, P.S. Air/Liquid Corneal Organ Culture: A Light Microscopic Study. Curr. Eye Res. 1991, 10, 739–749. [Google Scholar] [CrossRef]
- Foreman, D.M.; Pancholi, S.; Jarvis-Evans, J.; McLeod, D.; Boulton, M.E. A Simple Organ Culture Model for Assessing the Effects of Growth Factors on Corneal Re-Epithelialization. Exp. Eye Res. 1996, 62, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Chuck, R.S.; Behrens, A.; Wellik, S.; Liaw, L.L.; Dolorico, A.M.; Sweet, P.; Chao, L.C.; Osann, K.E.; McDonnell, P.J.; Berns, M.W. Re-Epithelialization in Cornea Organ Culture after Chemical Burns and Excimer Laser Treatment. Arch. Ophthalmol. 2001, 119, 1637–1642. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Cooper, L.J.; Brahma, A.; MacNeil, S.; Rimmer, S.; Fullwood, N.J. Development of a Three-Dimensional Organ Culture Model for Corneal Wound Healing and Corneal Transplantation. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2840–2846. [Google Scholar] [CrossRef]
- Castro-Combs, J.; Noguera, G.; Cano, M.; Yew, M.; Gehlbach, P.L.; Palmer, J.; Behrens, A. Corneal Wound Healing Is Modulated by Topical Application of Amniotic Fluid in an Ex Vivo Organ Culture Model. Exp. Eye Res. 2008, 87, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, P.; Ortega, Í.; Sefat, F.; Sangwan, V.S.; Green, N.; Claeyssens, F.; MacNeil, S. Rocking Media over Ex Vivo Corneas Improves This Model and Allows the Study of the Effect of Proinflammatory Cytokines on Wound Healing. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1553–1561. [Google Scholar] [CrossRef][Green Version]
- Castro, N.; Gillespie, S.R.; Bernstein, A.M. Ex Vivo Corneal Organ Culture Model for Wound Healing Studies. J. Vis. Exp. JoVE 2019, 144, e58562. [Google Scholar] [CrossRef]
- Janin-Manificat, H.; Rovère, M.-R.; Galiacy, S.D.; Malecaze, F.; Hulmes, D.J.S.; Moali, C.; Damour, O. Development of Ex Vivo Organ Culture Models to Mimic Human Corneal Scarring. Mol. Vis. 2012, 18, 2896–2908. [Google Scholar]
- Piehl, M.; Gilotti, A.; Donovan, A.; DeGeorge, G.; Cerven, D. Novel Cultured Porcine Corneal Irritancy Assay with Reversibility Endpoint. Toxicol. Vitr. 2010, 24, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Dohlman, C.H.; Gasset, A.R.; Rose, J. The Effect of the Absence of Corneal Epithelium or Endothelium on the Stromal Keratocytes. Investig. Ophthalmol. 1968, 7, 520–534. [Google Scholar]
- Xu, K.P.; Li, X.F.; Yu, F.S. Corneal Organ Culture Model for Assessing Epithelial Responses to Surfactants. Toxicol. Sci. Off. J. Soc. Toxicol. 2000, 58, 306–314. [Google Scholar] [CrossRef]
- Sriram, S.; Gibson, D.J.; Robinson, P.; Pi, L.; Tuli, S.; Lewin, A.S.; Schultz, G. Assessment of Anti-Scarring Therapies in Ex Vivo Organ Cultured Rabbit Corneas. Exp. Eye Res. 2014, 125, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Redbrake, C.; Salla, S.; Nilius, R.; Becker, J.; Reim, M. A Histochemical Study of the Distribution of Dextran 500 in Human Corneas during Organ Culture. Curr. Eye Res. 1997, 16, 405–411. [Google Scholar] [CrossRef]
- Borderie, V.M.; Baudrimont, M.; Lopez, M.; Carvajal, S.; Laroche, L. Evaluation of the Deswelling Period in Dextran-Containing Medium after Corneal Organ Culture. Cornea 1997, 16, 215–223. [Google Scholar] [CrossRef]
- Thuret, G.; Manissolle, C.; Campos-Guyotat, L.; Guyotat, D.; Gain, P. Animal Compound-Free Medium and Poloxamer for Human Corneal Organ Culture and Deswelling. Investig. Ophthalmol. Vis. Sci. 2005, 46, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-Y.; Green, C.R.; McGhee, C.N.J.; Sherwin, T. Acute Wound Healing in the Human Central Corneal Epithelium Appears to Be Independent of Limbal Stem Cell Influence. Investig. Ophthalmol. Vis. Sci. 2008, 49, 5279–5286. [Google Scholar] [CrossRef] [PubMed]
- Crosson, C.E.; Klyce, S.D.; Beuerman, R.W. Epithelial Wound Closure in the Rabbit Cornea. A Biphasic Process. Investig. Ophthalmol. Vis. Sci. 1986, 27, 464–473. [Google Scholar]
- Müller, L.J.; Pels, E.; Vrensen, G.F. The effects of organ-culture on the density of keratocytes and collagen fibers in human corneas. Cornea 2001, 20, 86–95. [Google Scholar] [CrossRef]
- Robertson, D.M.; Li, L.; Fisher, S.; Pearce, V.P.; Shay, J.W.; Wright, W.E.; Cavanagh, H.D.; Jester, J.V. Characterization of growth and differentiation in a telomerase-immortalized human corneal epithelial cell line. Investig. Ophthalmol. Vis. Sci. 2005, 46, 470–478. [Google Scholar] [CrossRef]
- Fukuda, T.; Gouko, R.; Eitsuka, T.; Suzuki, R.; Takahashi, K.; Nakagawa, K.; Sugano, E.; Tomita, H.; Kiyono, T. Human-Derived Corneal Epithelial Cells Expressing Cell Cycle Regulators as a New Resource for in vitro Ocular Toxicity Testing. Front. Genet. 2019, 10, 587. [Google Scholar] [CrossRef]
- Meier, N.T.; Haslam, I.S.; Pattwell, D.M.; Zhang, G.-Y.; Emelianov, V.; Paredes, R.; Debus, S.; Augustin, M.; Funk, W.; Amaya, E.; et al. Thyrotropin-releasing hormone (TRH) promotes wound re-epithelialisation in frog and human skin. PLoS ONE 2013, 8, e73596. [Google Scholar] [CrossRef]
- Liao, T.; Lehmann, J.; Sternstein, S.; Yay, A.; Zhang, G.; Matthießen, A.E.; Schumann, S.; Siemers, F.; Kruse, C.; Hundt, J.E.; et al. Nestin+ progenitor cells isolated from adult human sweat gland stroma promote reepithelialisation and may stimulate angiogenesis in wounded human skin ex vivo. Arch Dermatol. Res. 2019, 311, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Van den Berghe, C.; Guillet, M.C.; Compan, D. Performance of Porcine Corneal Opacity and Permeability Assay to Predict Eye Irritation for Water-Soluble Cosmetic Ingredients. Toxicol. Vitr. 2005, 19, 823–830. [Google Scholar] [CrossRef]
- Hafezi, F.; Gatzioufas, Z.; Angunawela, R.; Ittner, L.M. Absence of IL-6 Prevents Corneal Wound Healing after Deep Excimer Laser Ablation in Vivo. Eye 2018, 32, 156–157. [Google Scholar] [CrossRef] [PubMed]
- Napoli, P.E.; Nioi, M.; Gabiati, L.; Laurenzo, M.; De-Giorgio, F.; Scorcia, V.; Grassi, S.; d’Aloja, E.; Fossarello, M. Repeatability and Reproducibility of Post-Mortem Central Corneal Thickness Measurements Using a Portable Optical Coherence Tomography System in Humans: A Prospective Multicenter Study. Sci. Rep. 2020, 10, 14508. [Google Scholar] [CrossRef] [PubMed]


| Medium | Ingredients | Distributor |
|---|---|---|
| DMEM | Dulbeccos Modified Eagle Medium, high Glucose, w/L-Glutamin | Thermo Fisher Scientific GmbH, Waltham, MA, USA |
| Penicillin/Streptomycin solution 1× | Merck KGaA, Darmstadt, Germany | |
| 2.5 µg/mL Amphotericin B | Merck KGaA, Darmstadt, Germany | |
| 6% Dextran-500 | Carl Roth GmbH + Co. KG, Karlsruhe, Germany | |
| KGM2 | Keratinocyte Growth Medium 2 | PromoCell, Heidelberg, Germany |
| Supplement Mix including Bovine Pituitary Extract, Epidermal Growth Factor, Insulin, Hydrocortisone, Epinephrine, Transferrin, CaCl2 | PromoCell, Heidelberg, Germany | |
| Penicillin/Streptomycin solution 1× | Merck KGaA, Darmstadt, Germany | |
| 2.5 µg/mL Amphotericin B | Merck KGaA, Darmstadt, Germany | |
| 6% Dextran-500 | Carl Roth GmbH + Co. KG, Karlsruhe, Germany | |
| OcuLife | OcuLife ™ Epithelial Basal Medium | CellSystems GmbH, Troisdorf, Germany |
| OcuLife Supplements (“Life Factors”) including Insulin, Glutamin, Epinephrin, Apo-Transferrin, Extract PTM, Hydrocortisone, OcuFactorTM | CellSystems GmbH, Troisdorf, Germany | |
| Penicillin/Streptomycin solution 1× | Merck KGaA, Darmstadt, Germany | |
| 2.5 µg/mL Amphotericin B | Merck KGaA, Darmstadt, Germany | |
| 6% Dextran-500 | Carl Roth GmbH + Co. KG, Karlsruhe, Germany | |
| WEM | William’s Medium E | Merck KGaA, Darmstadt, Germany |
| 10 µg/mL Insulin | Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany | |
| 0.1 µg/mL Hydrocortisone | Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany | |
| 2 mM L-Glutamine | PAA Laboratories GmbH, Cölbe, Germany | |
| Penicillin/Streptomycin solution 1× | Merck KGaA, Darmstadt, Germany | |
| 2.5 µg/mL Amphotericin B | Merck KGaA, Darmstadt, Germany | |
| 6% Dextran-500 | Carl Roth GmbH + Co. KG, Karlsruhe, Germany |
| Influence on Test Model | Parameters |
|---|---|
| Defect size | 4 mm vs. 8 mm |
| Role of limbus | With vs. without limbus |
| Organ culture medium | DMEM vs. KGM2 vs. Oculife vs. WEM |
| Mitomycin C as cytostatic, antiproliferative agent | 50 µg/mL vs. 100 µg/mL vs. 200 µg/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schumann, S.; Dietrich, E.; Kruse, C.; Grisanti, S.; Ranjbar, M. Establishment of a Robust and Simple Corneal Organ Culture Model to Monitor Wound Healing. J. Clin. Med. 2021, 10, 3486. https://doi.org/10.3390/jcm10163486
Schumann S, Dietrich E, Kruse C, Grisanti S, Ranjbar M. Establishment of a Robust and Simple Corneal Organ Culture Model to Monitor Wound Healing. Journal of Clinical Medicine. 2021; 10(16):3486. https://doi.org/10.3390/jcm10163486
Chicago/Turabian StyleSchumann, Sandra, Eva Dietrich, Charli Kruse, Salvatore Grisanti, and Mahdy Ranjbar. 2021. "Establishment of a Robust and Simple Corneal Organ Culture Model to Monitor Wound Healing" Journal of Clinical Medicine 10, no. 16: 3486. https://doi.org/10.3390/jcm10163486
APA StyleSchumann, S., Dietrich, E., Kruse, C., Grisanti, S., & Ranjbar, M. (2021). Establishment of a Robust and Simple Corneal Organ Culture Model to Monitor Wound Healing. Journal of Clinical Medicine, 10(16), 3486. https://doi.org/10.3390/jcm10163486






