Visual Acuity and Number of Amniotic Membrane Layers as Indicators of Efficacy in Amniotic Membrane Transplantation for Corneal Ulcers: A Multicenter Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Surgery and Follow-Up
2.3. Statistical Analysis
3. Results
3.1. Sex and Age
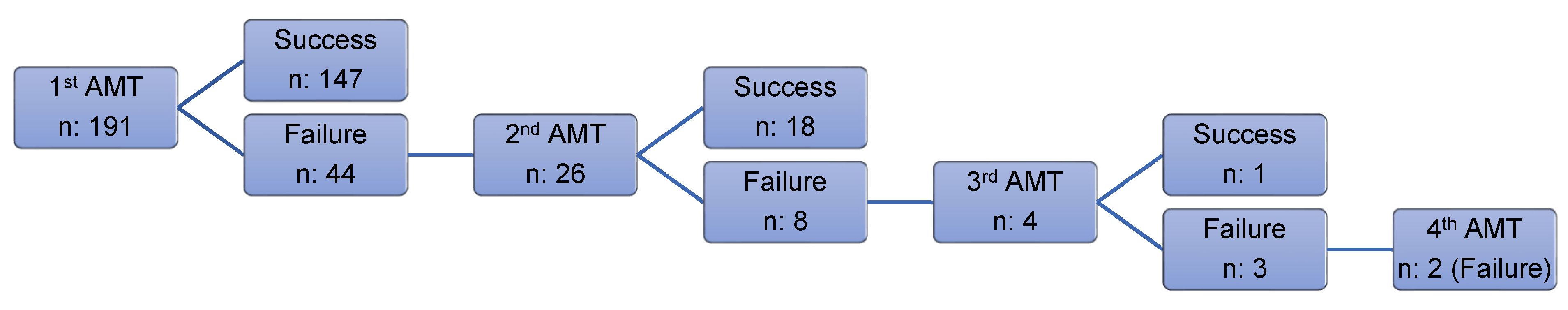
3.2. Success and Failure
3.3. Corneal Opacification
3.4. Visual Acuity
3.5. Efficacy of Monolayer Versus Multilayer AMT
3.6. Correlation of Monolayer/Multilayer AMT and VA Gain
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mead, O.; Tighe, S.; Tseng, S.C.G. Amniotic membrane transplantation for managing dry eye and neurotrophic keratitis. Taiwan J. Ophthalmol. 2020, 10, 13–21. [Google Scholar] [CrossRef]
- Napoli, P.E.; Nioi, M.; D’Aloja, E.; Loy, F.; Fossarello, M. The architecture of corneal stromal striae on optical coherence Tomography and histology in an animal model and in humans. Sci. Rep. 2020, 10, 19861. [Google Scholar] [CrossRef] [PubMed]
- Napoli, P.E.; Nioi, M.; D’Aloja, E.; Fossarello, M. The bull’s eye pattern of the tear film in humans during visual fixation on en-face optical coherence tomography. Sci. Rep. 2019, 9, 1413. [Google Scholar] [CrossRef]
- Nioi, M.; Napoli, P.E.; Demontis, R.; Locci, E.; Fossarello, M.; D’Aloja, E. Morphological analysis of corneal findings modifications after death: A preliminary OCT study on an animal model. Exp. Eye Res. 2018, 169, 20–27. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Y.; Jia, Y.; Liu, D.; Li, S.; Shi, W.; Gao, H. Active pedicle epithelial flap transposition combined with amniotic membrane transplantation for treatment of nonhealing corneal ulcers. J. Ophthalmol. 2016, 2016, 5742346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuerch, K.; Baeriswyl, A.; Frueh, B.E.; Tappeiner, C. Efficacy of amniotic membrane transplantation for the treatment of corneal ulcers. Cornea 2020, 39, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Lacorzana, J. Amniotic membrane, clinical applications and tissue engineering. Review of its ophthalmic use. Arch. Soc. Esp. Oftalmol. 2020, 95, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Meller, D.; Pauklin, M.; Thomasen, H.; Westekemper, H.; Steuhl, K.-P. Amniotic membrane transplantation in the human eye. Dtsch. Aerztebl. Int. 2011, 108, 243–248. [Google Scholar] [CrossRef]
- Alemañy González, J.; Camacho Ruaigip, F. Usos de la membrana amniótica humana en oftalmología. Rev. Cuba Oftalmol. 2006, 19, 1–7. [Google Scholar]
- Kobayashi, M.; Yakuwa, T.; Sasaki, K.; Sato, K.; Kikuchi, A.; Kamo, I.; Yokoyama, Y.; Sakuragawa, N. Multilineage potential of side population cells from human amnion mesenchymal layer. Cell Transplant. 2008, 17, 291–301. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, M.; Fujisawa, H.; Hayashi, Y.; Yamashita, J. Autologous amnion graft for repair of myelomeningocele: Technical note and clinical implication. J. Clin. Neurosci. 2004, 11, 408–411. [Google Scholar] [CrossRef]
- Favaron, P.; Carvalho, R.C.; Borghesi, J.; Anunciação, A.; Miglino, M. The amniotic membrane: Development and potential applications—A review. Reprod. Domest. Anim. 2015, 50, 881–892. [Google Scholar] [CrossRef] [PubMed]
- van Herendael, B.; Oberti, C.; Brosens, I. Microanatomy of the human amniotic membranes. Am. J. Obstet. Gynecol. 1978, 131, 872–880. [Google Scholar] [CrossRef]
- Lacorzana, J.; García-Serrano, J.; Prieto-Moreno, C.G.; Castillo-Rodríguez, S.; Lucena-Martín, J.; Pozo-Jiménez, I. Amniotic membrane, review of its ophthalmic use and results in the last five years (2013–2017) in Granada. Preliminary study. Actual Med. 2018, 103, 82–86. [Google Scholar] [CrossRef]
- Tseng, S.C.; Espana, E.M.; Kawakita, T.; Di Pascuale, M.A.; Li, W.; He, H.; Liu, T.-S.; Cho, T.-H.; Gao, Y.-Y.; Yeh, L.-K.; et al. How does amniotic membrane work? Ocul. Surf. 2004, 2, 177–187. [Google Scholar] [CrossRef]
- Tseng, S.C.G. HC-HA/PTX3 purified from amniotic membrane as novel regenerative matrix: Insight into relationship between inflammation and regeneration. Investig. Opthalmol. Vis. Sci. 2016, 57, ORSFh1–ORSFh8. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Chen, D.; A Sullivan, D.; Xie, H.; Li, Y.; Liu, Y. Expression of lubricin in the human amniotic membrane. Cornea 2020, 39, 118–121. [Google Scholar] [CrossRef]
- Sabater, A.L.; Perez, V.L. Amniotic membrane use for management of corneal limbal stem cell deficiency. Curr. Opin. Ophthalmol. 2017, 28, 363–369. [Google Scholar] [CrossRef]
- Murri, M.S.; Moshirfar, M.; Birdsong, O.C.; Ronquillo, Y.C.; Ding, Y.; Hoopes, P.C. Amniotic membrane extract and eye drops: A review of literature and clinical application. Clin. Ophthalmol. 2018, 12, 1105–1112. [Google Scholar] [CrossRef] [Green Version]
- Utheim, T.P.; Utheim, A.; Salvanos, P.; Jackson, C.; Schrader, S.; Geerling, G.; Sehic, A. concise review: Altered versus unaltered amniotic membrane as a substrate for limbal epithelial cells. Stem Cells Transl. Med. 2018, 7, 415–427. [Google Scholar] [CrossRef]
- Zhu, Y.-T.; Li, F.; Zhang, Y.; Chen, S.-Y.; Tighe, S.; Lin, S.-Y.; Tseng, S.C.G. HC-HA/PTX3 purified from human amniotic membrane reverts human corneal fibroblasts and myofibroblasts to keratocytes by activating BMP signaling. Investig. Opthalmol. Vis. Sci. 2020, 61, 62. [Google Scholar] [CrossRef]
- Kruse, F.E.; Rohrschneider, K.; Völcker, H.E. Multilayer amniotic membrane transplantation for reconstruction of deep corneal ulcers. Ophthalmology 1999, 106, 1504–1511. [Google Scholar] [CrossRef]
- Lambiase, A.; Sacchetti, M. Diagnosis and management of neurotrophic keratitis. Clin. Ophthalmol. 2014, 8, 571–579. [Google Scholar] [CrossRef] [Green Version]
- Saad, S.; Abdelmassih, Y.; Saad, R.; Guindolet, D.; El-Khoury, S.; Doan, S.; Cochereau, I.; Gabison, E.E.; El Khoury, S. Neurotrophic keratitis: Frequency, etiologies, clinical management and outcomes. Ocul. Surf. 2020, 18, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Di Zazzo, A.; Coassin, M.; Varacalli, G.; Galvagno, E.; De Vincentis, A.; Bonini, S. Neurotrophic keratopathy: Pros and cons of current treatments. Ocul. Surf. 2019, 17, 619–623. [Google Scholar] [CrossRef]
- Singhal, D.; Nagpal, R.; Maharana, P.K.; Sinha, R.; Agarwal, T.; Sharma, N.; Titiyal, J.S. Surgical alternatives to keratoplasty in microbial keratitis. Surv. Ophthalmol. 2020, 66. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-J.; Pires, R.T.F.; Tseng, S.C.G. Amniotic membrane transplantation for severe neurotrophic corneal ulcers. Br. J. Ophthalmol. 2000, 84, 826–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dua, H.S.; Said, D.G.; Messmer, E.M.; Rolando, M.; Benitez-Del-Castillo, J.M.; Hossain, P.N.; Shortt, A.J.; Geerling, G.; Nubile, M.; Figueiredo, F.C.; et al. Neurotrophic keratopathy. Prog. Retin. Eye Res. 2018, 66, 107–131. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, R.; Nagpal, R.; Todi, V.; Sharma, N. Descemetocele. Surv. Ophthalmol. 2020, 66, 2–19. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ares, M.T.; Touriño, R.; López-Valladares, M.J.; Gude, F. Multilayer amniotic membrane transplantation in the treatment of corneal perforations. Cornea 2004, 23, 577–583. [Google Scholar] [CrossRef]
- Prabhasawat, P.; Smith, G.T.; Liu, C.S.C. Single and multilayer amniotic membrane transplantation for persistent corneal epithelial defect with and without stromal thinning and perforation. Br. J. Ophthalmol. 2001, 85, 1455–1463. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Wang, M.; Zhong, F. Improvement of amniotic membrane method for the treatment of corneal perforation. BioMed Res. Int. 2016, 2016, 1693815. [Google Scholar] [CrossRef] [Green Version]
- Joseph, A.; Dua, H.S.; King, A. Failure of amniotic membrane transplantation in the treatment of acute ocular burns. Br. J. Ophthalmol. 2001, 85, 1065–1069. [Google Scholar] [CrossRef]
- Navas, A.; Guerrero, F.S.M.; López, A.D.; Chávez-García, C.; Partido, G.; Graue-Hernández, E.O.; Sánchez-García, F.J.; Garfias, Y. Anti-inflammatory and anti-fibrotic effects of human amniotic membrane mesenchymal stem cells and their potential in corneal repair. Stem Cells Transl. Med. 2018, 7, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Kaur, M.; Agarwal, T.; Sangwan, V.S.; Vajpayee, R.B. Treatment of acute ocular chemical burns. Surv. Ophthalmol. 2018, 63, 214–235. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Singh, D.; Maharana, P.K.; Kriplani, A.; Velpandian, T.; Pandey, R.M.; Vajpayee, R.B. Comparison of amniotic membrane transplantation and umbilical cord serum in acute ocular chemical burns: A randomized controlled trial. Am. J. Ophthalmol. 2016, 168, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yan, X.-M.; Wu, H.-R.; Rong, B. An experimental study on the fate of the amniotic membrane after amniotic membrane transplantation for acute alkaline burn of rat cornea. Zhonghua Yan Ke Za Zhi 2012, 48, 27–32. [Google Scholar]
- Eslani, M.; Baradaran-Rafii, A.; Cheung, A.Y.; Kurji, K.H.; Hasani, H.; Djalilian, A.R.; Holland, E.J. Amniotic membrane transplantation in acute severe ocular chemical injury: A randomized clinical trial. Am. J. Ophthalmol. 2019, 199, 209–215. [Google Scholar] [CrossRef]
- Sahay, P.; Goel, S.; Maharana, P.K.; Sharma, N. Amniotic membrane transplantation in acute severe ocular chemical injury: A randomized clinical trial. Am. J. Ophthalmol. 2019, 205, 202–203. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, C.E.; Frings, C.; Rohloff, N.; Harmsen-Aasman, C.; Schmitz, R.; Kiesel, L.; Eter, N.; Busse, H.; Alex, A.F. Long-term efficacy of glycerine-processed amniotic membrane transplantation in patients with corneal ulcer. Acta Ophthalmol. 2015, 93, e481–e487. [Google Scholar] [CrossRef] [Green Version]
- Letko, E.; Stechschulte, S.U.; Kenyon, K.R.; Sadeq, N.; Romero, T.R.; Samson, C.M.; Nguyen, Q.D.; Harper, S.L.; Primack, J.D.; Azar, D.T.; et al. Amniotic membrane inlay and overlay grafting for corneal epithelial defects and stromal ulcers. Arch. Ophthalmol. 2001, 119, 659. [Google Scholar] [CrossRef] [Green Version]
- Dekaris, I.; Gabrić, N.; Mravicić, I.; Karaman, Z.; Katusić, J.; Lazić, R.; Spoljarić, N. Multilayer vs. monolayer amniotic membrane transplantation for deep corneal ulcer treatment. Coll. Antropol. 2001, 25, 23–28. [Google Scholar]
- Liu, J.; Li, L.; Li, X. Effectiveness of cryopreserved amniotic membrane transplantation in corneal ulceration: A meta-analysis. Cornea 2019, 38, 454–462. [Google Scholar] [CrossRef]
- Rodríguez-Ares, M.T.; López-Valladares, M.J.; Touriño, R.; Vieites, B.; Gude, F.; Silva, M.T.; Couceiro, J. Effects of lyophilization on human amniotic membrane. Acta Ophthalmol. 2009, 87, 396–403. [Google Scholar] [CrossRef]
- Samsom, M.; Iwabuchi, Y.; Sheardown, H.; Schmidt, T.A. Proteoglycan 4 and hyaluronan as boundary lubricants for model contact lens hydrogels. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 106, 1329–1338. [Google Scholar] [CrossRef]
- Ghosh, S.; Salvador-Culla, B.; Kotagiri, A.; Pushpoth, S.; Tey, A.; Johnson, Z.K.; Figueiredo, F. Acute chemical eye injury and limbal stem cell deficiency—A prospective study in the United Kingdom. Cornea 2019, 38, 8–12. [Google Scholar] [CrossRef]
- Nubile, M.; Dua, H.S.; Lanzini, T.E.-M.; Carpineto, P.; Ciancaglini, M.; Toto, L.; Mastropasqua, L. Amniotic membrane transplantation for the management of corneal epithelial defects: An in vivo confocal microscopic study. Br. J. Ophthalmol. 2007, 92, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Sotozono, C.; Ang, L.P.; Koizumi, N.; Higashihara, H.; Ueta, M.; Inatomi, T.; Yokoi, N.; Kaido, M.; Dogru, M.; Shimazaki, J.; et al. New grading system for the evaluation of chronic ocular manifestations in patients with Stevens-Johnson syndrome. Ophthalmology 2007, 114, 1294–1302. [Google Scholar] [CrossRef]
- Rabbettts RB: Visual acuity and contrast sensitivity. In Clinical Visual Optics; Rabbetts, R.B. (Ed.) Buttwerworth Heineman: Oxford, UK, 1998; pp. 19–61. [Google Scholar]
- Risse, J.F. Acuité visuelle. In Exploration de la Fonction Visuelle; Risse, J.F., Ed.; Masson: Paris, France, 1999; pp. 99–128. [Google Scholar]
- Yokogawa, H.; Kobayashi, A.; Yamazaki, N.; Masaki, T.; Sugiyama, K. Surgical therapies for corneal perforations: 10 years of cases in a tertiary referral hospital. Clin. Ophthalmol. 2014, 8, 2165–2170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdulhalim, B.-E.H.; Wagih, M.M.; Gad, A.A.M.; Boghdadi, G.; Nagy, R.R.S. Amniotic membrane graft to conjunctival flap in treatment of non-viral resistant infectious keratitis: A randomised clinical study. Br. J. Ophthalmol. 2015, 99, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Rim, T.H.; Kim, D.W.; Chung, E.J.; Kim, S.S. Nationwide incidence of blindness in South Korea: A 12-year study from 2002 to 2013. Clin. Exp. Ophthalmol. 2017, 45, 773–778. [Google Scholar] [CrossRef]
- Lee, C.M.; Afshari, N.A. The global state of cataract blindness. Curr. Opin. Ophthalmol. 2017, 28, 98–103. [Google Scholar] [CrossRef]
- Chirapapaisan, C.; Prabhasawat, P.; Srivannaboon, S.; Roongpoovapatr, V.; Chitsuthipakorn, P. Ocular injury due to potassium permanganate granules. Case Rep. Ophthalmol. 2018, 9, 132–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westekemper, H.; Figueiredo, F.; Siah, W.F.; Wagner, N.; Steuhl, K.-P.; Meller, D. Clinical outcomes of amniotic membrane transplantation in the management of acute ocular chemical injury. Br. J. Ophthalmol. 2017, 101, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Paolin, A.; Cogliati, E.; Trojan, D.; Griffoni, C.; Grassetto, A.; Elbadawy, H.; Ponzin, D. Amniotic membranes in ophthalmology: Long term data on transplantation outcomes. Cell Tissue Bank. 2016, 17, 51–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouchard, C.S.; John, T. Amniotic membrane transplantation in the management of severe ocular surface disease: Indications and outcomes. Ocul. Surf. 2004, 2, 201–211. [Google Scholar] [CrossRef]
- Röck, T.; Bartz-Schmidt, K.U.; Landenberger, J.; Bramkamp, M.; Röck, D. Amniotic membrane transplantation in reconstructive and regenerative ophthalmology. Ann. Transplant. 2018, 23, 160–165. [Google Scholar] [CrossRef]
- Sabater-Cruz, N.; Figueras-Roca, M.; Ventosa, A.G.; Padró-Pitarch, L.; Tort, J.; Casaroli-Marano, R.P. Current clinical application of sclera and amniotic membrane for ocular tissue bio-replacement. Cell Tissue Bank. 2020, 21, 597–603. [Google Scholar] [CrossRef]
- Brocks, D.; Mead, O.G.; Tighe, S.; Tseng, S.C.G. Self-retained cryopreserved amniotic membrane for the management of corneal ulcers. Clin. Ophthalmol. 2020, 14, 1437–1443. [Google Scholar] [CrossRef]
- Yin, H.Y.; Cheng, A.M.S.; Tighe, S.; Kurochkin, P.; Nord, J.; Dhanireddy, S.; Swan, R.; Alpert, S. Self-retained cryopreserved amniotic membrane for treating severe corneal ulcers: A comparative, retrospective control study. Sci. Rep. 2020, 10, 17008. [Google Scholar] [CrossRef] [PubMed]
- Resch, M.D.; Schlötzer-Schrehardt, U.; Hofmann-Rummelt, C.; Sauer, R.; Kruse, F.E.; Beckmann, M.W.; Seitz, B. Integration patterns of cryopreserved amniotic membranes into the human cornea. Ophthalmology 2006, 113, 1927–1935. [Google Scholar] [CrossRef] [PubMed]
- Resch, M.D.; Schlötzer-Schrehardt, U.; Hofmann-Rummelt, C.; Sauer, R.; Cursiefen, C.; Kruse, F.E.; Beckmann, M.W.; Seitz, B. Adhesion structures of amniotic membranes integrated into human corneas. Investig. Opthalmol. Vis. Sci. 2006, 47, 1853–1861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-H.; Tseng, S.C. Amniotic membrane transplantation for persistent epithelial defects with ulceration. Am. J. Ophthalmol. 1997, 123, 303–312. [Google Scholar] [CrossRef]
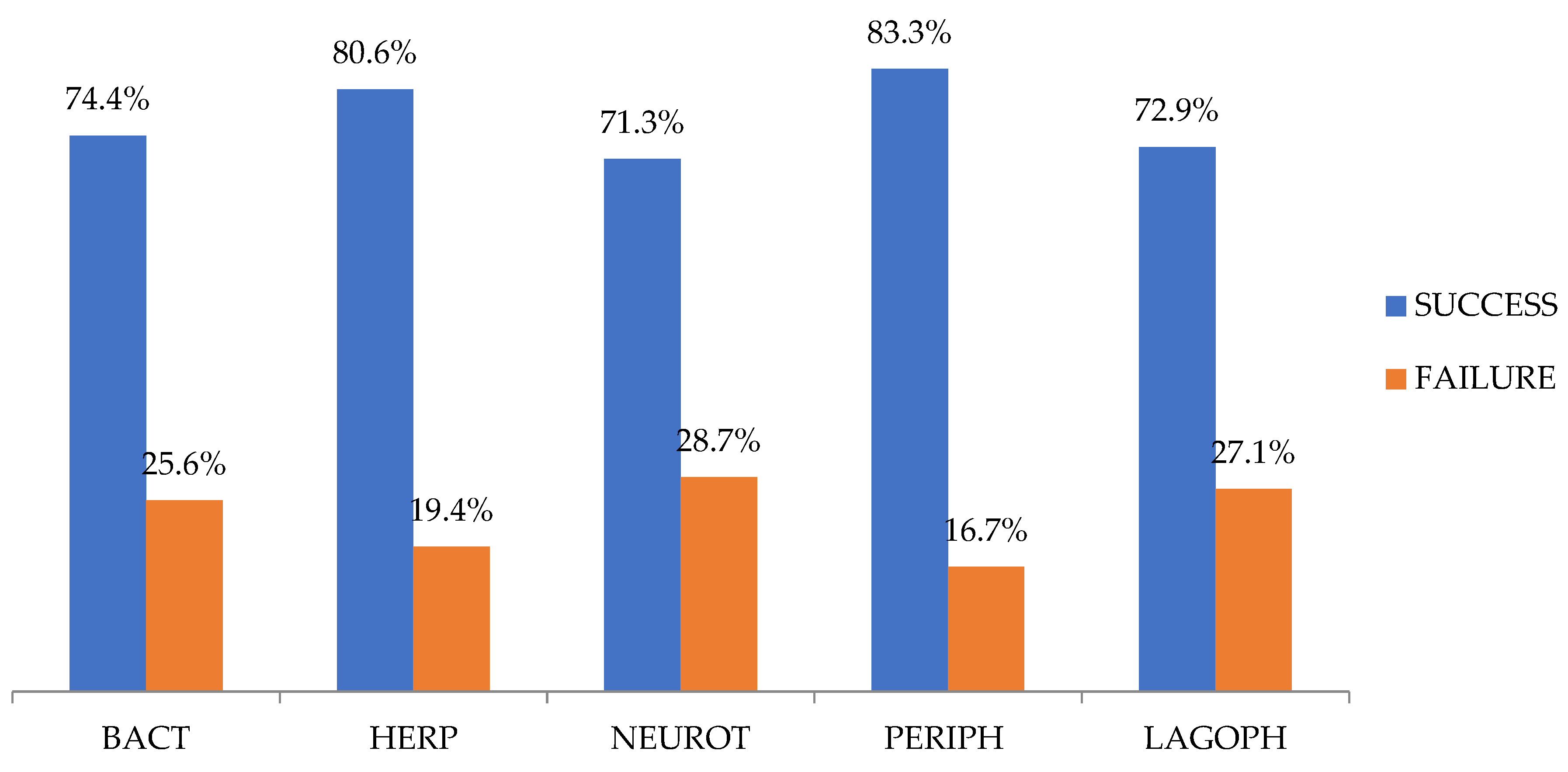
| BACT | HERP | NEUROT | PERIPH | LAGOPH | |
|---|---|---|---|---|---|
| N = 39 | N = 31 | N = 87 | N = 18 | N = 48 | |
| −17.50% | −13.90% | −39.00% | −8.10% | −21.50% | |
| Sex | |||||
| Male | 21 (53.8%) | 16 (51.6%) | 36 (41.4%) | 9 (50%) | 22 (45.8%) |
| Female | 18 (46.2%) | 15 (48.4%) | 51 (58.6%) | 9 (50%) | 26 (54.2%) |
| Age | 66.4 ± 16.9 | 63.6 ± 15.5 | 66.4 ± 17.8 | 65.4 ± 16.9 | 61.9 ± 22.4 |
| (mean ± SD) | |||||
| 1a surgical option | 32 (82.1%) | 24 (77.4%) | 73 (83.9%) | 14 (77.8%) | 42 (87.5%) |
| Corneal opacification | |||||
| Grade 0 | 9 (23.1%) | 1 (3.2%) | 17 (19.5%) | 12 (66.7%) | 12 (25%) |
| Grade 1–2 | 6 (15.4%) | 1 (3.2%) | 24 (27.6%) | 2 (11.1%) | 9 (18.8%) |
| Grade 3 | 24 (61.5%) | 29 (93.5%) | 46 (52.9%) | 4 (22,2%) | 27 (56.2%) |
| BACT | HERP | NEUROT | PERIPH | LAGOPH | |
|---|---|---|---|---|---|
| VA at baseline | |||||
| - Amaurosis | 6 (15.4%) | 1 (3.2%) | 3 (3.4%) | 1 (5.6%) | 2 (4.2%) |
| - LP | 7 (17.9%) | 12 (38.8%) | 13 (14.9%) | 2 (11.1%) | 16 (33.3%) |
| - HM | 10 (25.6%) | 5 (16.1%) | 22 (25.3%) | 1 (5.6%) | 6 (12.5%) |
| - CF | 4 (10.3%) | 5 (16.1%) | 10 (11.5%) | 0 (0%) | 0 (0%) |
| - ≥0.05 a 1 Snellen | 12 (30.8%) | 8 (25.8%) | 39 (44.8%) | 14 (77.8%) | 24 (50%) |
| VA at last follow-up | |||||
| - Amaurosis | 6 (15.4%) | 1 (3.2%) | 4 (4.6%) | 1 (5.5%) | 2 (4.2%) |
| - LP | 5 (12.8%) | 8 (25.8%) | 11 (12.6%) | 0 (0%) | 14 (29.2%) |
| - HM | 7 (17.9%) | 5 (16.1%) | 19 (21.9%) | 0 (0%) | 5 (10.4%) |
| - CF | 1 (2.6%) | 7 (22.7%) | 13 (14.9%) | 0 (0%) | 1 (2.1%) |
| - ≥0.05 a 1 | 20 (51.3%) | 10 (32.3%) | 40 (46%) | 17 (94.5%) | 26 (54.2%) |
| Changes in VA | |||||
| Worsening | 1 (2.6%) | 5 (16.1%) | 8 (9.2%) | 1 (5.6%) | 3 (6.3%) |
| Equal | 22 (56.4%) | 15 (48.4%) | 54 (62.1%) | 2 (11.1%) | 30 (62.5%) |
| Improvement | 16 (41%) | 11 (35.5%) | 25 (28.7%) | 15 (83.3%) | 15 (31.3%) |
| BACT | HERP | NEUROT | PERIPH | LAGOPH | |
|---|---|---|---|---|---|
| MON | 88.9% | 90.9% | 75.0% | 77.8% | 75.7% |
| MUL | 61.9% | 55.6% | 63.0% | 88.9% | 63.6% |
| TOTAL | 74.4% | 80.6% | 71.3% | 85.3% | 72.9% |
| p | p = 0.058 | p = 0.043 | p = 0.186 | p = 0.500 | p = 0.334 |
| BACT | HERP | NEUROT | PERIPH | LAGOPH | ||
|---|---|---|---|---|---|---|
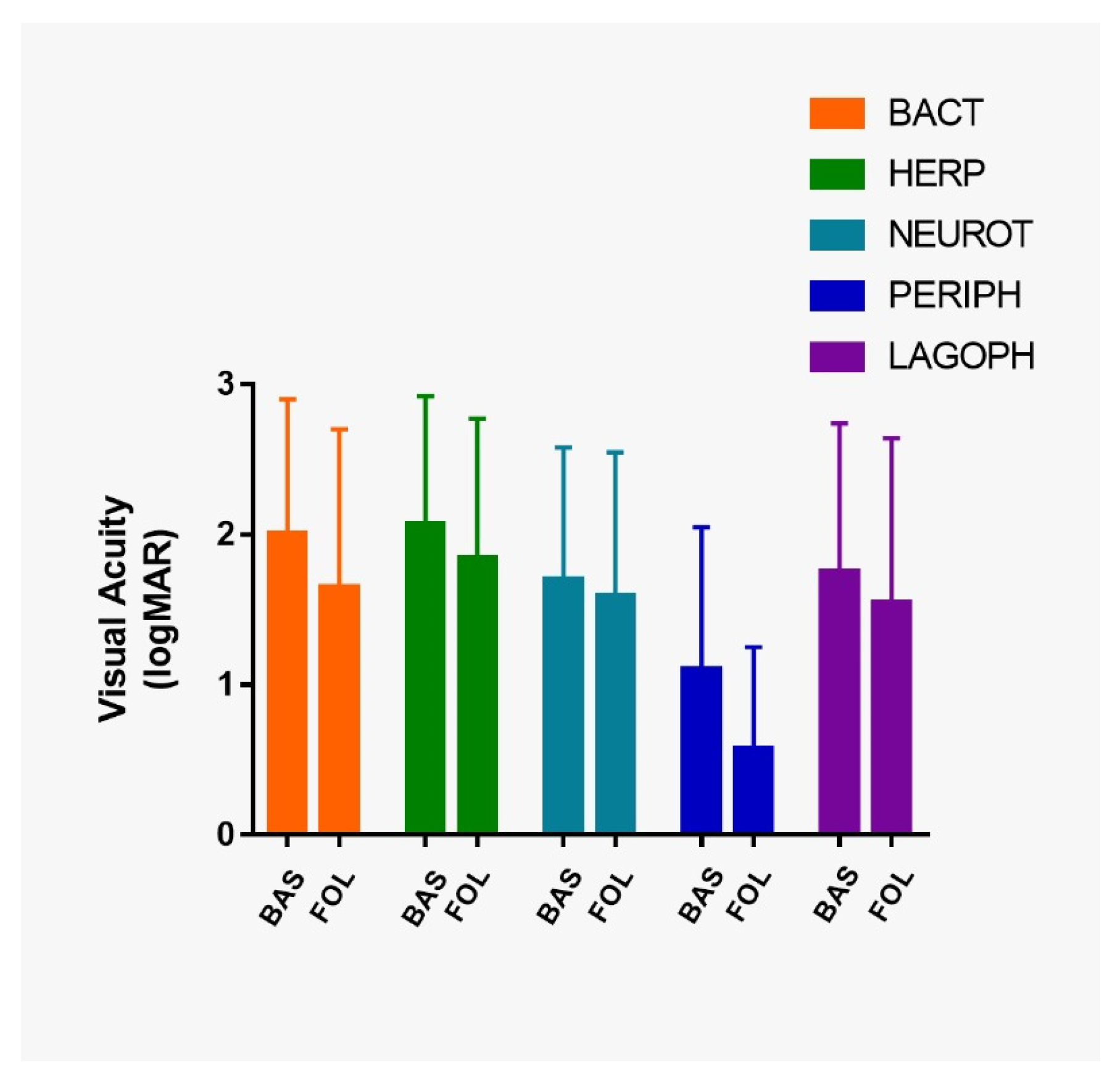
| MON | logMAR VA at baseline (mean ± SD) | 2.19 ± 0.93 | 2.20 ± 0.79 | 1.89 ± 0.87 | 1.56 ± 1.05 | 2.21 ± 0.86 |
| logMAR VA last follow-up (mean ± SD) | 1.88 ± 1.14 | 1.84 ± 0.97 | 1.82 ± 0.94 | 0.61 ± 0.33 | 2.11 ± 0.97 | |
| MUL | logMAR VA at baseline (mean ± SD) | 1.69 ± 0.79 | 1.81 ± 0.94 | 1.48 ± 0.85 | 0.81 ± 0.80 | 1.52 ± 0.98 |
| logMAR VA last follow-up (mean ± SD) | 1.28 ± 0.79 | 1.84 ± 0.89 | 1.35 ± 0.93 | 0.55 ± 0.85 | 1.25 ± 1.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacorzana, J.; Campos, A.; Brocal-Sánchez, M.; Marín-Nieto, J.; Durán-Carrasco, O.; Fernández-Núñez, E.C.; López-Jiménez, A.; González-Gutiérrez, J.L.; Petsoglou, C.; Serrano, J.L.G. Visual Acuity and Number of Amniotic Membrane Layers as Indicators of Efficacy in Amniotic Membrane Transplantation for Corneal Ulcers: A Multicenter Study. J. Clin. Med. 2021, 10, 3234. https://doi.org/10.3390/jcm10153234
Lacorzana J, Campos A, Brocal-Sánchez M, Marín-Nieto J, Durán-Carrasco O, Fernández-Núñez EC, López-Jiménez A, González-Gutiérrez JL, Petsoglou C, Serrano JLG. Visual Acuity and Number of Amniotic Membrane Layers as Indicators of Efficacy in Amniotic Membrane Transplantation for Corneal Ulcers: A Multicenter Study. Journal of Clinical Medicine. 2021; 10(15):3234. https://doi.org/10.3390/jcm10153234
Chicago/Turabian StyleLacorzana, Javier, Antonio Campos, Marina Brocal-Sánchez, Juan Marín-Nieto, Oswaldo Durán-Carrasco, Esly C. Fernández-Núñez, Andrés López-Jiménez, Jose L. González-Gutiérrez, Constantinos Petsoglou, and Jose L. García Serrano. 2021. "Visual Acuity and Number of Amniotic Membrane Layers as Indicators of Efficacy in Amniotic Membrane Transplantation for Corneal Ulcers: A Multicenter Study" Journal of Clinical Medicine 10, no. 15: 3234. https://doi.org/10.3390/jcm10153234
APA StyleLacorzana, J., Campos, A., Brocal-Sánchez, M., Marín-Nieto, J., Durán-Carrasco, O., Fernández-Núñez, E. C., López-Jiménez, A., González-Gutiérrez, J. L., Petsoglou, C., & Serrano, J. L. G. (2021). Visual Acuity and Number of Amniotic Membrane Layers as Indicators of Efficacy in Amniotic Membrane Transplantation for Corneal Ulcers: A Multicenter Study. Journal of Clinical Medicine, 10(15), 3234. https://doi.org/10.3390/jcm10153234






