The Assessment of Muscle Mass and Function in Patients with Long-Standing Rheumatoid Arthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Measurement of Body Composition and Muscle Mass Index
2.3. Statistical Analysis
2.4. Ethics Statement
3. Results
3.1. Clinical and Sarcopenic Characteristics of RA Patients
3.2. Comparison of Clinical Factors between RA Patients with Low Muscle Mass and Those Without
3.3. Comparison of Clinical Factors between RA Patients with Sarcopenia by Definition of SARC-F
3.4. Associations of Clinical Factors with Low Muscle Mass or Sarcopenia in RA Patients
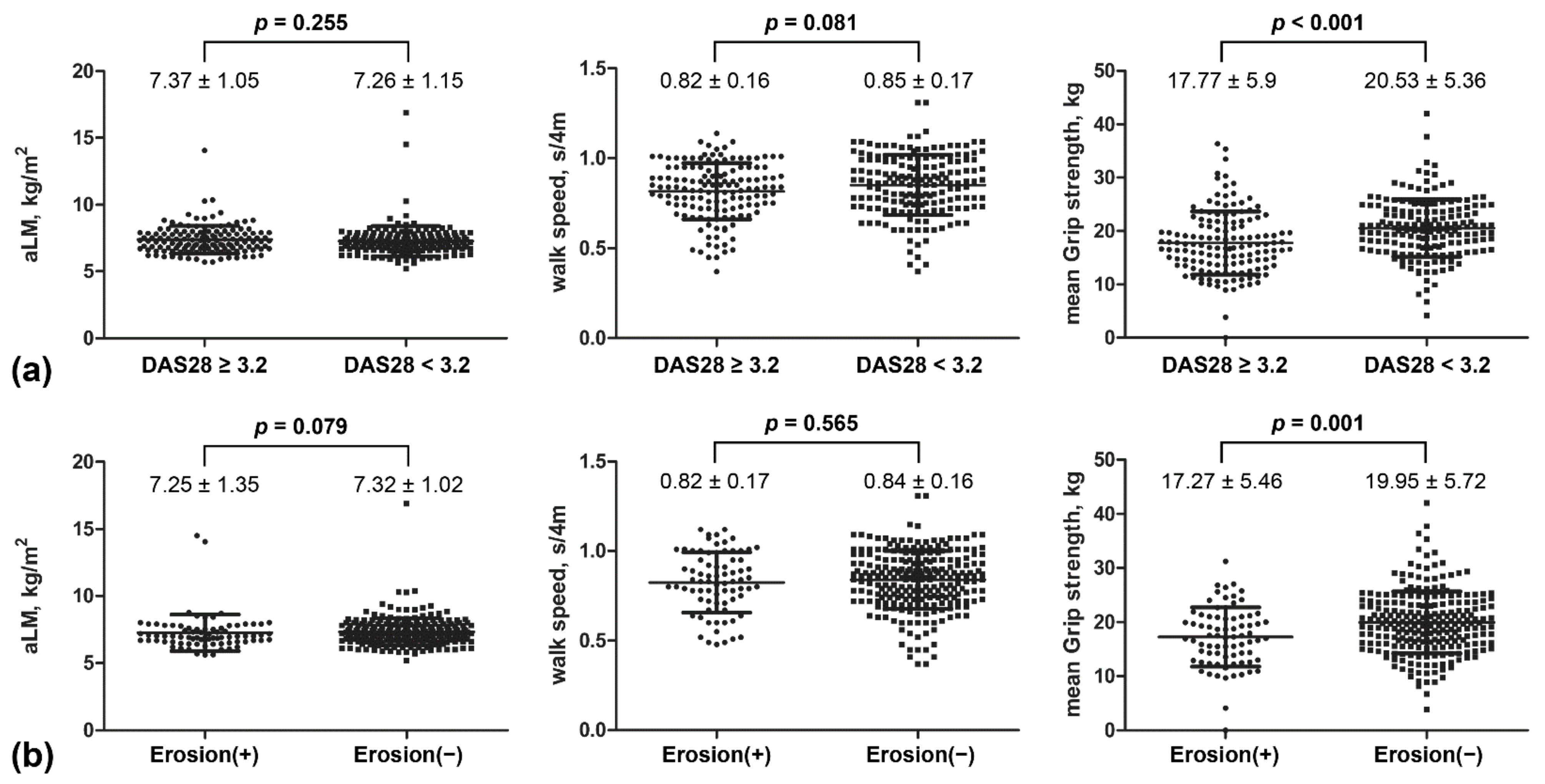
3.5. Comparison of Muscular Parameters by Disease Activity and Joint Damage
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Young, A.; Dixey, J.; Cox, N.; Davies, P.; Devlin, J.; Emery, P.; Gallivan, S.; Gough, A.; James, D.; Prouse, P.; et al. How does functional disability in early rheumatoid arthritis (RA) affect patients and their lives? Results of 5 years of follow-up in 732 patients from the Early RA Study (ERAS). Rheumatology 2000, 39, 603–611. [Google Scholar] [CrossRef]
- Goekoop-Ruiterman, Y.P.; de Vries-Bouwstra, J.K.; Allaart, C.F.; van Zeben, D.; Kerstens, P.J.; Hazes, J.M.; Zwinderman, A.H.; Ronday, H.K.; Han, K.H.; Westedt, M.L.; et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): A randomized, controlled trial. Arthritis Rheum. 2005, 52, 3381–3390. [Google Scholar] [CrossRef] [PubMed]
- Safiri, S.; Kolahi, A.A.; Hoy, D.; Smith, E.; Bettampadi, D.; Mansournia, M.A.; Almasi-Hashiani, A.; Ashrafi-Asgarabad, A.; Moradi-Lakeh, M.; Qorbani, M.; et al. Global, regional and national burden of rheumatoid arthritis 1990–2017: A systematic analysis of the Global Burden of Disease study 2017. Ann. Rheum. Dis. 2019, 78, 1463–1471. [Google Scholar] [CrossRef]
- Takanashi, S.; Kaneko, Y.; Takeuchi, T. Elderly patients with comorbidities in the definition of difficult-to-treat rheumatoid arthritis. Ann. Rheum. Dis. 2021, 1–3. [Google Scholar] [CrossRef]
- Mochizuki, T.; Yano, K.; Ikari, K.; Okazaki, K. Sarcopenia-associated factors in Japanese patients with rheumatoid arthritis: A cross-sectional study. Geriatr. Gerontol. Int. 2019, 19, 907–912. [Google Scholar] [CrossRef]
- Yamada, Y.; Tada, M.; Mandai, K.; Hidaka, N.; Inui, K.; Nakamura, H. Glucocorticoid use is an independent risk factor for developing sarcopenia in patients with rheumatoid arthritis: From the CHIKARA study. Clin. Rheumatol. 2020, 39, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Tournadre, A.; Vial, G.; Capel, F.; Soubrier, M.; Boirie, Y. Sarcopenia. Jt. Bone Spine 2019, 86, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Ontan, M.S.; Dokuzlar, O.; Ates Bulut, E.; Soysal, P.; Isik, A.T. The relationship between osteoporosis and sarcopenia, according to EWGSOP-2 criteria, in outpatient elderly. J. Bone Miner. Metab. 2021. [Google Scholar] [CrossRef] [PubMed]
- Tembo, M.C.; Mohebbi, M.; Holloway-Kew, K.L.; Gaston, J.; Brennan-Olsen, S.L.; Williams, L.J.; Kotowicz, M.A.; Pasco, J.A. The Predictability of Frailty Associated with Musculoskeletal Deficits: A Longitudinal Study. Calcif. Tissue Int. 2021, 1–9. [Google Scholar] [CrossRef]
- Granic, A.; Martin-Ruiz, C.; Dodds, R.M.; Robinson, L.; Spyridopoulos, I.; Kirkwood, T.B.; von Zglinicki, T.; Sayer, A.A. Immunosenescence profiles are not associated with muscle strength, physical performance and sarcopenia risk in very old adults: The Newcastle 85+ Study. Mech. Ageing Dev. 2020, 190, 111321. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Jeong, J.B.; Kang, J.; Ahn, D.W.; Kim, J.W.; Kim, B.G.; Lee, K.L.; Oh, S.; Yoon, S.H.; Park, S.J.; et al. Association between sarcopenia level and metabolic syndrome. PLoS ONE 2021, 16, e0248856. [Google Scholar] [CrossRef]
- Koon-Yee Lee, G.; Chun-Ming Au, P.; Hoi-Yee Li, G.; Chan, M.; Li, H.L.; Man-Yung Cheung, B.; Chi-Kei Wong, I.; Ho-Fun Lee, V.; Mok, J.; Hon-Kei Yip, B.; et al. Sarcopenia and mortality in different clinical conditions: A meta-analysis. Osteoporos. Sarcopenia 2021, 7, S19–S27. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef]
- Cao, L.; Chen, S.; Zou, C.; Ding, X.; Gao, L.; Liao, Z.; Liu, G.; Malmstrom, T.K.; Morley, J.E.; Flaherty, J.H.; et al. A pilot study of the SARC-F scale on screening sarcopenia and physical disability in the Chinese older people. J. Nutr. Health Aging 2014, 18, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Malmstrom, T.K.; Miller, D.K.; Simonsick, E.M.; Ferrucci, L.; Morley, J.E. SARC-F: A symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J. Cachexia Sarcopenia Muscle 2016, 7, 28–36. [Google Scholar] [CrossRef]
- Mentzel, M.; Hofmann, F.; Ebinger, T.; Gulke, J.; Kinzl, L.; Wachter, N.J. Measuring grip strength of the hand with a sensor glove by gripping with submaximal and maximal strength. Handchir. Mikrochir. Plast. Chir. Organ. Der Dtsch. Arb. Fur Handchir. Organ. Der Dtsch. Arb. Fur Mikrochir. Der Peripher. Nerven Und Gefasse 2001, 33, 52–57, discussion 57–58. [Google Scholar] [CrossRef]
- An, H.J.; Tizaoui, K.; Terrazzino, S.; Cargnin, S.; Lee, K.H.; Nam, S.W.; Kim, J.S.; Yang, J.W.; Lee, J.Y.; Smith, L.; et al. Sarcopenia in Autoimmune and Rheumatic Diseases: A Comprehensive Review. Int. J. Mol. Sci. 2020, 21, 5678. [Google Scholar] [CrossRef] [PubMed]
- Santo, R.C.E.; Fernandes, K.Z.; Lora, P.S.; Filippin, L.I.; Xavier, R.M. Prevalence of rheumatoid cachexia in rheumatoid arthritis: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2018, 9, 816–825. [Google Scholar] [CrossRef]
- Lin, J.Z.; Liang, J.J.; Ma, J.D.; Li, Q.H.; Mo, Y.Q.; Cheng, W.M.; He, X.L.; Li, N.; Cao, M.H.; Xu, D.; et al. Myopenia is associated with joint damage in rheumatoid arthritis: A cross-sectional study. J. Cachexia Sarcopenia Muscle 2019, 10, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Torii, M.; Hashimoto, M.; Hanai, A.; Fujii, T.; Furu, M.; Ito, H.; Uozumi, R.; Hamaguchi, M.; Terao, C.; Yamamoto, W.; et al. Prevalence and factors associated with sarcopenia in patients with rheumatoid arthritis. Mod. Rheumatol. 2019, 29, 589–595. [Google Scholar] [CrossRef]
- Dogan, S.C.; Hizmetli, S.; Hayta, E.; Kaptanoglu, E.; Erselcan, T.; Guler, E. Sarcopenia in women with rheumatoid arthritis. Eur. J. Rheumatol. 2015, 2, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Ngeuleu, A.; Allali, F.; Medrare, L.; Madhi, A.; Rkain, H.; Hajjaj-Hassouni, N. Sarcopenia in rheumatoid arthritis: Prevalence, influence of disease activity and associated factors. Rheumatol. Int. 2017, 37, 1015–1020. [Google Scholar] [CrossRef]
- Li, T.H.; Chang, Y.S.; Liu, C.W.; Su, C.F.; Tsai, H.C.; Tsao, Y.P.; Liao, H.T.; Chen, M.H.; Chuang, C.C.; Yang, Y.Y.; et al. The prevalence and risk factors of sarcopenia in rheumatoid arthritis patients: A systematic review and meta-regression analysis. Semin. Arthritis Rheum. 2021, 51, 236–245. [Google Scholar] [CrossRef]
- Alabarse, P.V.G.; Lora, P.S.; Silva, J.M.S.; Santo, R.C.E.; Freitas, E.C.; de Oliveira, M.S.; Almeida, A.S.; Immig, M.; Teixeira, V.O.N.; Filippin, L.I.; et al. Collagen-induced arthritis as an animal model of rheumatoid cachexia. J. Cachexia Sarcopenia Muscle 2018, 9, 603–612. [Google Scholar] [CrossRef]
- Filippin, L.I.; Teixeira, V.N.; Viacava, P.R.; Lora, P.S.; Xavier, L.L.; Xavier, R.M. Temporal development of muscle atrophy in murine model of arthritis is related to disease severity. J. Cachexia Sarcopenia Muscle 2013, 4, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Lupoli, R.; Pizzicato, P.; Scalera, A.; Ambrosino, P.; Amato, M.; Peluso, R.; Di Minno, M.N. Impact of body weight on the achievement of minimal disease activity in patients with rheumatic diseases: A systematic review and meta-analysis. Arthritis Res. Ther. 2016, 18, 297. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, M.E.; Bengtsson, C.; Kallberg, H.; Wesley, A.; Klareskog, L.; Alfredsson, L.; Saevarsdottir, S. Overweight decreases the chance of achieving good response and low disease activity in early rheumatoid arthritis. Ann. Rheum. Dis. 2014, 73, 2029–2033. [Google Scholar] [CrossRef]
- Baker, J.F.; Ostergaard, M.; George, M.; Shults, J.; Emery, P.; Baker, D.G.; Conaghan, P.G. Greater body mass independently predicts less radiographic progression on X-ray and MRI over 1–2 years. Ann. Rheum. Dis. 2014, 73, 1923–1928. [Google Scholar] [CrossRef] [PubMed]
- Rydell, E.; Forslind, K.; Nilsson, J.A.; Jacobsson, L.T.H.; Turesson, C. Smoking, body mass index, disease activity, and the risk of rapid radiographic progression in patients with early rheumatoid arthritis. Arthritis Res. Ther. 2018, 20, 82. [Google Scholar] [CrossRef]
- Webster, J.M.; Kempen, L.; Hardy, R.S.; Langen, R.C.J. Inflammation and Skeletal Muscle Wasting During Cachexia. Front. Physiol. 2020, 11, 597675. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.E.; Newcomb, P.V.; Holly, J.M. Multifaceted roles of TNF-alpha in myoblast destruction: A multitude of signal transduction pathways. J. Cell. Physiol. 2004, 198, 237–247. [Google Scholar] [CrossRef]
- Li, Y.P.; Schwartz, R.J.; Waddell, I.D.; Holloway, B.R.; Reid, M.B. Skeletal muscle myocytes undergo protein loss and reactive oxygen-mediated NF-kappaB activation in response to tumor necrosis factor alpha. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1998, 12, 871–880. [Google Scholar] [CrossRef]
- Szalay, K.; Razga, Z.; Duda, E. TNF inhibits myogenesis and downregulates the expression of myogenic regulatory factors myoD and myogenin. Eur. J. Cell Biol. 1997, 74, 391–398. [Google Scholar]
- Chen, S.E.; Jin, B.; Li, Y.P. TNF-alpha regulates myogenesis and muscle regeneration by activating p38 MAPK. Am. J. Physiol. Cell Physiol. 2007, 292, C1660–C1671. [Google Scholar] [CrossRef]
- Granado, M.; Martin, A.I.; Priego, T.; Lopez-Calderon, A.; Villanua, M.A. Tumour necrosis factor blockade did not prevent the increase of muscular muscle RING finger-1 and muscle atrophy F-box in arthritic rats. J. Endocrinol. 2006, 191, 319–326. [Google Scholar] [CrossRef][Green Version]
- Subramaniam, K.; Fallon, K.; Ruut, T.; Lane, D.; McKay, R.; Shadbolt, B.; Ang, S.; Cook, M.; Platten, J.; Pavli, P.; et al. Infliximab reverses inflammatory muscle wasting (sarcopenia) in Crohn’s disease. Aliment. Pharmacol. Ther. 2015, 41, 419–428. [Google Scholar] [CrossRef]
- Malmstrom, T.K.; Morley, J.E. SARC-F: A simple questionnaire to rapidly diagnose sarcopenia. J. Am. Med. Dir. Assoc. 2013, 14, 531–532. [Google Scholar] [CrossRef] [PubMed]
- Ida, S.; Kaneko, R.; Imataka, K.; Okubo, K.; Shirakura, Y.; Azuma, K.; Fujiwara, R.; Takahashi, H.; Murata, K. Verification of the predictive validity for mortality of the SARC-F questionnaire based on a meta-analysis. Aging Clin. Exp. Res. 2021, 33, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Smolen, J.S.; Landewe, R.B.M.; Bijlsma, J.W.J.; Burmester, G.R.; Dougados, M.; Kerschbaumer, A.; McInnes, I.B.; Sepriano, A.; van Vollenhoven, R.F.; de Wit, M.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 Update. Ann. Rheum. Dis. 2020, 79, 685–699. [Google Scholar] [CrossRef] [PubMed]
| Mean ± SD or n (%) | |
|---|---|
| Age, years | 60.5 ± 9.8 |
| Male, n (%) | 20 (6.6) |
| Postmenopause, n (%) | 259 (86.3) |
| Body weight, kg | 57.8 ± 10.0 |
| Height, cm | 156.7 ± 6.4 |
| BMI, kg/m2 | 23.5 ± 3.7 |
| Smoker, n (%) | 21 (6.0) |
| Duration of RA, months | 104.5 ± 67.5 |
| Hypertension, n (%) | 85 (26.6) |
| Dyslipidemia, n (%) | 104 (32.5) |
| Diabetes, n (%) | 26 (8.1) |
| Tender joint count | 3.9 ± 4.2 |
| Swollen joint count | 1.1 ± 2.4 |
| DAS28 | 3.0 ± 1.1 |
| Erosion, n (%) | 77 (24.1) |
| Visual analogue score | 6.4 ± 2.1 |
| ESR, mm/hr | 15.7 ± 15.1 |
| CRP, mg/dL | 0.8 ± 1.6 |
| Positive rheumatoid factor, n (%) | 244 (76.3) |
| Medication history | |
| Current dose of glucocorticoids, mg | 1.4 ± 1.3 |
| Cumulative dose of glucocorticoids, g | 3.6 ± 4.1 |
| MTX, n (%) | 192 (59.8) |
| Hydroxychloroquine, n (%) | 163 (50.8) |
| Leflunomide, n (%) | 39 (12.2) |
| TNF inhibitor, n (%) | 23 (7.2) |
| JAK inhibitor, n (%) | 45 (14.0) |
| NSAID use, n (%) | 240 (74.8) |
| Osteoporosis, n (%) | 141 (44.1) |
| Total muscle mass, kg/m2 | 26.87 ± 11.1 |
| Fat free mass index, kg/m2 | 18.1 ± 5.8 |
| aLM, kg/m2 | 7.3 ± 1.1 |
| Mean strength of grip, kg | 19.3 ± 5.8 |
| Walk speed, m/s | 0.8 ± 0.2 |
| Presarcopenia by the EWGS, n (%) | 54 (16.9) |
| Sarcopenia by the EWGS, n (%) | 21 (6.6) |
| Sarcopenia by the AWGS, n (%) | 7 (2.2) |
| Sarcopenia by the SARC-F, n (%) | 38 (11.9) |
| Without Low Muscle Mass n = 266 | With Low Muscle Mass * n = 54 | p-Value | |
|---|---|---|---|
| Age, year | 60.1 ± 9.7 | 62.4 ± 10.3 | 0.061 |
| Male, n (%) | 9 (3.4) | 11 (20.4) | <0.001 |
| Height, cm | 156.7 ± 6.4 | 157.0 ± 6.4 | 0.561 |
| Weight, kg | 59.6 ± 9.3 | 48.7 ± 8.6 | <0.001 |
| BMI, kg/m2 | 24.3 ± 3.3 | 19.7 ± 2.8 | <0.001 |
| Duration of RA, month | 103.9 ± 67.8 | 107.8 ± 66.9 | 0.676 |
| Erosion, n (%) | 57 (21.4) | 20 (37.0) | 0.015 |
| ESR mm/hr | 15.5 ± 15.1 | 16.9 ± 15.1 | 0.559 |
| CRP mg/dL | 0.7 ± 1.2 | 0.7 ± 1.2 | 0.837 |
| Visual analogue score | 27.2 ± 17.8 | 26.3 ± 14.6 | 0.904 |
| Tender joint count | 4.0 ± 4.4 | 3.8 ± 3.3 | 0.671 |
| Swollen joint count | 1.1 ± 2.5 | 1.2 ± 1.7 | 0.058 |
| DAS28 | 3.0 ± 1.2 | 3.1 ± 1.0 | 0.669 |
| Current dose of GC, mg | 1.3 ± 1.3 | 1.8 ± 1.3 | 0.011 |
| Cumulative dose of GC, g | 3.3 ± 3.6 | 5.3 ± 6.0 | 0.002 |
| MTX, n (%) | 158 (59.4) | 34 (63.0) | 0.626 |
| TNF inhibitor, n (%) | 15 (5.6) | 8 (14.8) | 0.017 |
| Synthetic DMARDs, n (%) | 82 (30.8) | 23 (42.6%) | 0.094 |
| Osteoporosis, n (%) | 107 (40.2) | 34 (63.0) | 0.002 |
| Mean strength of grip | 19.6 ± 5.6 | 18.1 ± 6.2 | 0.038 |
| Walk speed, m/s | 0.84 ± 0.16 | 0.82 ± 0.17 | 0.418 |
| No Sarcopenia by SARC-F n = 282 | Sarcopenia by SARC-F n = 38 | p-Value | |
|---|---|---|---|
| Age, year | 59.6 ± 9.7 | 67.3 ± 8.7 | <0.001 |
| Male, n (%) | 20 (7.1) | 0 | NA |
| Height, cm | 157.4 ± 6.1 | 152.1 ± 6.8 | <0.001 |
| Weight, kg | 57.8 ± 10.1 | 57.8 ± 9.2 | 0.575 |
| BMI, kg/m2 | 23.3 ± 3.7 | 24.9 ± 3.5 | 0.007 |
| Duration of RA, month | 104.7 ± 6.4 | 103.2 ± 69.8 | 0.867 |
| Erosion, n (%) | 66 (23.4) | 11 (28.9) | 0.454 |
| ESR mm/hr | 15.4 ± 15.2 | 18.1 ± 14.1 | 0.071 |
| CRP mg/dL | 0.8 ± 1.7 | 0.6 ± 1.4 | 0.348 |
| Visual analogue score | 26.0 ± 16.4 | 35.3 ± 21.3 | 0.015 |
| Tender joint count | 3.5 ± 3.5 | 7.0 ± 7.0 | 0.001 |
| Swollen joint count | 0.9 ± 1.8 | 2.9 ± 4.6 | <0.001 |
| DAS28 | 2.9 ± 1.1 | 3.8 ± 1.4 | <0.001 |
| Current dose of GC, mg | 1.2 ± 1.0 | 1.4 ± 1.3 | 0.011 |
| Cumulative dose of GC, g | 3.7 ± 4.2 | 2.8 ± 3.0 | 0.002 |
| MTX, n (%) | 167 (59.2) | 25 (65.8) | 0.438 |
| TNF inhibitor, n (%) | 20 (7.1) | 3 (7.9) | 0.858 |
| Synthetic DMARDs, n (%) | 96 (34) | 10 (26.3) | 0.343 |
| Osteoporosis, n (%) | 120 (42.6) | 21 (55.3) | 0.139 |
| Total mass index, kg/m2 | 27.5 ± 11.2 | 22.2 ± 9.9 | 0.005 |
| Fat free mass index, kg/m2 | 17.9 ± 5.8 | 19.7 ± 5.3 | 0.032 |
| aLM, kg/m2 | 7.3 ± 1.1 | 7.6 ± 1.3 | 0.036 |
| Walk speed, m/s | 0.84 ± 0.16 | 0.78 ± 0.16 | 0.035 |
| Mean grip strength, kg | 19.8 ± 5.6 | 15.4 ± 5.4 | <0.001 |
| Low Muscle Mass | Sarcopenia by EWGS | Sarcopenia by AWGS | Sarcopenia by SARC-F | |||||
|---|---|---|---|---|---|---|---|---|
| OR | p-Value | OR | p-Value | OR | p-Value | OR | p-Value | |
| Sex, male | 140.65 (20.32–973.54) | <0.001 | 3.58 (0.71–18.1) | 0.12 | 41.03 (3.41–193.79) | 0.003 | NA | |
| Age | 1.05 (1.0–1.1) | 0.074 | 1.04 (0.99–1.1) | 0.14 | 1.19 (1.01–1.39) | 0.038 | 1.11 (1.06–1.17) | <0.001 |
| BMI | 0.41 (0.31–0.53) | <0.001 | 0.66 (0.55–0.8) | <0.001 | 0.47 (0.3–0.75) | 0.002 | 1.13 (1.02–1.24) | 0.015 |
| RA duration | - | 1.01 (1.0–1.01) | 0.09 | NA | NA | |||
| Erosion | 1.79 (0.72–4.54) | 0.247 | 1.41 (0.51–3.96) | 0.51 | NA | NA | ||
| DAS28 | 0.84 (0.58–1.24) | 0.844 | 1.02 (0.64–1.62) | 0.93 | NA | 1.95 (1.38–2.73) | <0.001 | |
| Cumulative dose of GC | 1.0 (1.0–1.0) | 0.437 | 1.0 (1.0–1.0) | 0.36 | NA | 1.0 (1.0–1.0) | 0.48 | |
| TNF inhibitor use | 4.84 (1.1–21.37) | 0.037 | NA | NA | NA | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, H.-W.; Kim, C.-J.; Kim, J.-W.; Kim, H.-A.; Suh, C.-H.; Jung, J.-Y. The Assessment of Muscle Mass and Function in Patients with Long-Standing Rheumatoid Arthritis. J. Clin. Med. 2021, 10, 3458. https://doi.org/10.3390/jcm10163458
Yun H-W, Kim C-J, Kim J-W, Kim H-A, Suh C-H, Jung J-Y. The Assessment of Muscle Mass and Function in Patients with Long-Standing Rheumatoid Arthritis. Journal of Clinical Medicine. 2021; 10(16):3458. https://doi.org/10.3390/jcm10163458
Chicago/Turabian StyleYun, Hye-Won, Chun-Ja Kim, Ji-Won Kim, Hyoun-Ah Kim, Chang-Hee Suh, and Ju-Yang Jung. 2021. "The Assessment of Muscle Mass and Function in Patients with Long-Standing Rheumatoid Arthritis" Journal of Clinical Medicine 10, no. 16: 3458. https://doi.org/10.3390/jcm10163458
APA StyleYun, H.-W., Kim, C.-J., Kim, J.-W., Kim, H.-A., Suh, C.-H., & Jung, J.-Y. (2021). The Assessment of Muscle Mass and Function in Patients with Long-Standing Rheumatoid Arthritis. Journal of Clinical Medicine, 10(16), 3458. https://doi.org/10.3390/jcm10163458






