Rapid Antigen Test Combined with Chest Computed Tomography to Rule Out COVID-19 in Patients Admitted to the Emergency Department
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COVID-19 | coronavirus disease 2019 |
| ED | emergency department |
| Ag-RDT | antigen-detecting rapid diagnostic tests |
| CT | computed tomography |
References
- Sprivulis, P.C.; Da Silva, J.A.; Jacobs, I.G.; Frazer, A.R.; Jelinek, G.A. The association between hospital overcrowding and mortality among patients admitted via Western Australian emergency departments. Med. J. Aust. 2006, 184, 208–212. [Google Scholar] [CrossRef]
- Thibon, E.; Bobbia, X.; Blanchard, B.; Masia, T.; Palmier, L.; Tendron, L.; de la Coussaye, J.E.; Claret, P.G. Association between Mortality and Waiting Time in Emergency Room among Adults Hospitalized for Medical Etiologies. Ann. Fr. Méd. Urgence 2019, 9, 229–234. [Google Scholar] [CrossRef]
- Chen, J.Y.; Chang, F.Y.; Lin, C.S.; Wang, C.H.; Tsai, S.H.; Lee, C.C.; Chen, S.J. Impact of the COVID-19 Pandemic on the Loading and Quality of an Emergency Department in Taiwan: Enlightenment from a Low-Risk Country in a Public Health Crisis. J. Clin. Med. 2021, 10, 1150. [Google Scholar] [CrossRef] [PubMed]
- Dinnes, J.; Deeks, J.J.; Adriano, A.; Berhane, S.; Davenport, C.; Dittrich, S.; Emperador, D.; Takwoingi, Y.; Cunningham, J.; Beese, S.; et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst. Rev. 2020, 8, CD013705. [Google Scholar]
- Pilarowski, G.; Marquez, C.; Rubio, L.; Peng, J.; Martinez, J.; Black, D.; Chamie, G.; Jones, D.; Jacobo, J.; Tulier-Laiwa, V.; et al. Field performance and public health response using the BinaxNOW TM Rapid SARS-CoV-2 antigen detection assay during community-based testing. Clin. Infect. Dis. 2020, ciaa1890. [Google Scholar] [CrossRef] [PubMed]
- Hanson, K.E.; Caliendo, A.M.; Arias, C.A.; Hayden, M.K.; Englund, J.A.; Lee, M.J.; Loeb, M.; Patel, R.; El Alayli, A.; Altayar, O.; et al. The Infectious Diseases Society of America guidelines on the diagnosis of COVID-19: Molecular diagnostic testing. Clin. Infect. Dis. 2021, ciab048. [Google Scholar] [CrossRef]
- Mak, G.C.K.; Lau, S.S.Y.; Wong, K.K.Y.; Chow, N.L.S.; Lau, C.S.; Lam, E.T.K.; Chan, R.C.W.; Tsang, D.N.C. Evaluation of rapid antigen detection kit from the WHO Emergency Use List for detecting SARS-CoV-2. J Clin Virol. 2021, 134, 104712. [Google Scholar] [CrossRef]
- Rubin, G.D.; Ryerson, C.J.; Haramati, L.B.; Sverzellati, N.; Kanne, J.P.; Raoof, S.; Schluger, N.W.; Volpi, A.; Yim, J.J.; Martin, I.B.; et al. The role of chest imaging in patient management during the COVID-19 pandemic: A multinational consensus statement from the Fleischner Society. Chest 2020, 158, 106–116. [Google Scholar] [CrossRef]
- Schalekamp, S.; Bleeker-Rovers, C.P.; Beenen, L.F.M.; Quarles van Ufford, H.M.E.; Gietema, H.A.; Stöger, J.L.; Harris, V.; Reijers, M.H.E.; Rahamat-Langendoen, J.; Korevaar, D.A.; et al. Chest CT in the emergency department for diagnosis of COVID-19 pneumonia: Dutch experience. Radiology 2021, 298, E98–E106. [Google Scholar] [CrossRef] [PubMed]
- Islam, N.; Salameh, J.P.; Leeflang, M.M.; Hooft, L.; McGrath, T.A.; van der Pol, C.B.; Frank, R.A.; Kazi, S.; Prager, R.; Hare, S.S.; et al. Thoracic imaging tests for the diagnosis of COVID-19. Cochrane Database Syst. Rev. 2020, 11, CD013639. [Google Scholar] [PubMed]
- World Health Organization. RT-PCR Assays for the Detection of SARS-CoV-2. Available online: https://www.who.int/docs/default-source/coronaviruse/real-time-rt-pcr-assays-for-the-detection-of-SARS-CoV-2-institut-pasteur-paris.pdf?sfvrsn=3662fcb6_2 (accessed on 20 July 2021).
- Revel, M.P.; Parkar, A.P.; Prosch, H.; Silva, M.; Sverzellati, N.; Gleeson, F.; Brady, A.; European Society of Radiology (ESR) and the European Society of Thoracic Imaging (ESTI). COVID-19 patients and the radiology department—advice from the European Society of Radiology (ESR) and the European Society of Thoracic Imaging (ESTI). Eur. Radiol. 2020, 30, 4903–4909. [Google Scholar] [CrossRef] [Green Version]
- Toulouse, E.; Masseguin, C.; Lafont, B.; McGurk, G.; Harbonn, A.; ARoberts, J.; Granier, S.; Dupeyron, A.; Bazin, J.E. French legal approach to clinical research. Anaesth. Crit. Care Pain Med. 2018, 37, 607–614. [Google Scholar] [CrossRef] [Green Version]
- Pekosz, A.; Parvu, V.; Li, M.; Andrews, J.C.; Manabe, Y.C.; Kodsi, S.; Gary, D.S.; Roger-Dalbert, C.; Leitch, J.; Cooper, C.K. Antigen-based testing but not real-time polymerase chain reaction correlates with severe acute respiratory syndrome coronavirus 2 viral culture. Clin. Infect. Dis. 2021, ciaa1706. [Google Scholar] [CrossRef]
- Turcato, G.; Zaboli, A.; Pfeifer, N.; Ciccariello, L.; Sibilio, S.; Tezza, G.; Ausserhofer, D. Clinical application of a rapid antigen test for the detection of SARS-CoV-2 infection in symptomatic and asymptomatic patients evaluated in the emergency department: A preliminary report. J. Infect. 2021, 82, e14–e16. [Google Scholar] [CrossRef] [PubMed]
- Möckel, M.; Corman, V.M.; Stegemann, M.S.; Hofmann, J.; Stein, A.; Jones, T.C.; Gastmeier, P.; Seybold, J.; Offermann, R.; Bachmann, U.; et al. SARS-CoV-2 antigen rapid immunoassay for diagnosis of COVID-19 in the emergency department. Biomarkers 2021, 26, 213–220. [Google Scholar] [CrossRef]
- Krüger, L.J.; Gaeddert, M.; Tobian, F.; Lainati, F.; Gottschalk, C.; Klein, J.A.F.; Schnitzler, P.; Kräusslich, H.G.; Nikolai, O.; Lindner, A.K.; et al. The Abbott PanBio WHO emergency use listed, rapid, antigen-detecting point-of-care diagnostic test for SARS-CoV-2-Evaluation of the accuracy and ease-of-use. PLoS ONE 2021, 16, e0247918. [Google Scholar] [CrossRef]
- Linares, M.; Pérez-Tanoira, R.; Carrero, A.; Romanyk, J.; Pérez-García, F.; Gómez-Herruz, P.; Arroyo, T.; Cuadros, J. Panbio antigen rapid test is reliable to diagnose SARS-CoV-2 infection in the first 7 days after the onset of symptoms. J. Clin. Virol. 2020, 33, 104659. [Google Scholar] [CrossRef] [PubMed]
- Herpe, G.; Lederlin, M.; Naudin, M.; Ohana, M.; Chaumoitre, K.; Gregory, J.; Vilgrain, V.; Freitag, C.A.; De Margerie-Mellon, C.; Flory, V.; et al. Efficacy of chest CT for COVID-19 pneumonia diagnosis in France. Radiology 2021, 298, E81–E87. [Google Scholar] [CrossRef]
- Lanser, L.; Bellmann-Weiler, R.; Öttl, K.W.; Huber, L.; Griesmacher, A.; Theurl, I.; Weiss, G. Evaluating the clinical utility and sensitivity of SARS-CoV-2 antigen testing in relation to RT-PCR Ct values. Infection 2020, 49, 555–557. [Google Scholar] [CrossRef] [PubMed]
- Merino, P.; Guinea, J.; Muñoz-Gallego, I.; González-Donapetry, P.; Galán, J.C.; Antona, N.; Cilla, G.; Hernáez-Crespo, S.; Díaz-de Tuesta, J.L.; Gual-de Torrella, A.; et al. Multicenter evaluation of the Panbio™ COVID-19 rapid antigen-detection test for the diagnosis of SARS-CoV-2 infection. Clin. Microbiol. Infect. 2021, 27, 758–761. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antigendetection in the Diagnosis of SARS-CoV-2 Infection Using Rapid Immunoassays—Interim Guidance. Available online: https://www.who.int/publications/i/item/antigen-detection-in-the-diagnosis-of-sars-cov-2infection-using-rapid-immunoassays (accessed on 20 July 2021).
- World Health Organisation. WHO Emergency Use Listing for In Vitro Diagnostics (IVDs) Detecting SARS-CoV-2. 2020. Available online: https://www.who.int/publications/m/item/200922-eul-sars-cov2-product-list (accessed on 20 July 2021).
- World Health Organisation. WHO Emergency Use Assessment Coronavirus Disease (COVID-19) IVDs Public Report 2020. 2020. Available online: https://www.who.int/teams/regulation-prequalification/eul/ (accessed on 20 July 2021).
- Li, B.; Deng, A.; Li, K.; Hu, Y.; Li, Z.; Xiong, Q.; Liu, Z.; Guo, Q.; Zou, L.; Zhang, H.; et al. Viral Infection and Transmission in a Large Well-Traced Outbreak Caused by the Delta SARS-CoV-2 Variant. Virological.org. 2021. Available online: https://www.medrxiv.org/content/10.1101/2021.07.07.21260122v1 (accessed on 20 July 2021).
- Frediani, J.K.; Levy, J.M.; Rao, A.; Bassit, L.; Figueroa, J.; Vos, M.B.; Wood, A.; Jerris, R.; Van Leung-Pineda Gonzalez, M.D.; Rogers, B.B.; et al. Multidisciplinary assessment of the Abbott BinaxNOW SARS-CoV-2 point-of-care antigen test in the context of emerging viral variants and self-administration. Sci. Rep. 2021, 11, 14604. [Google Scholar] [CrossRef] [PubMed]


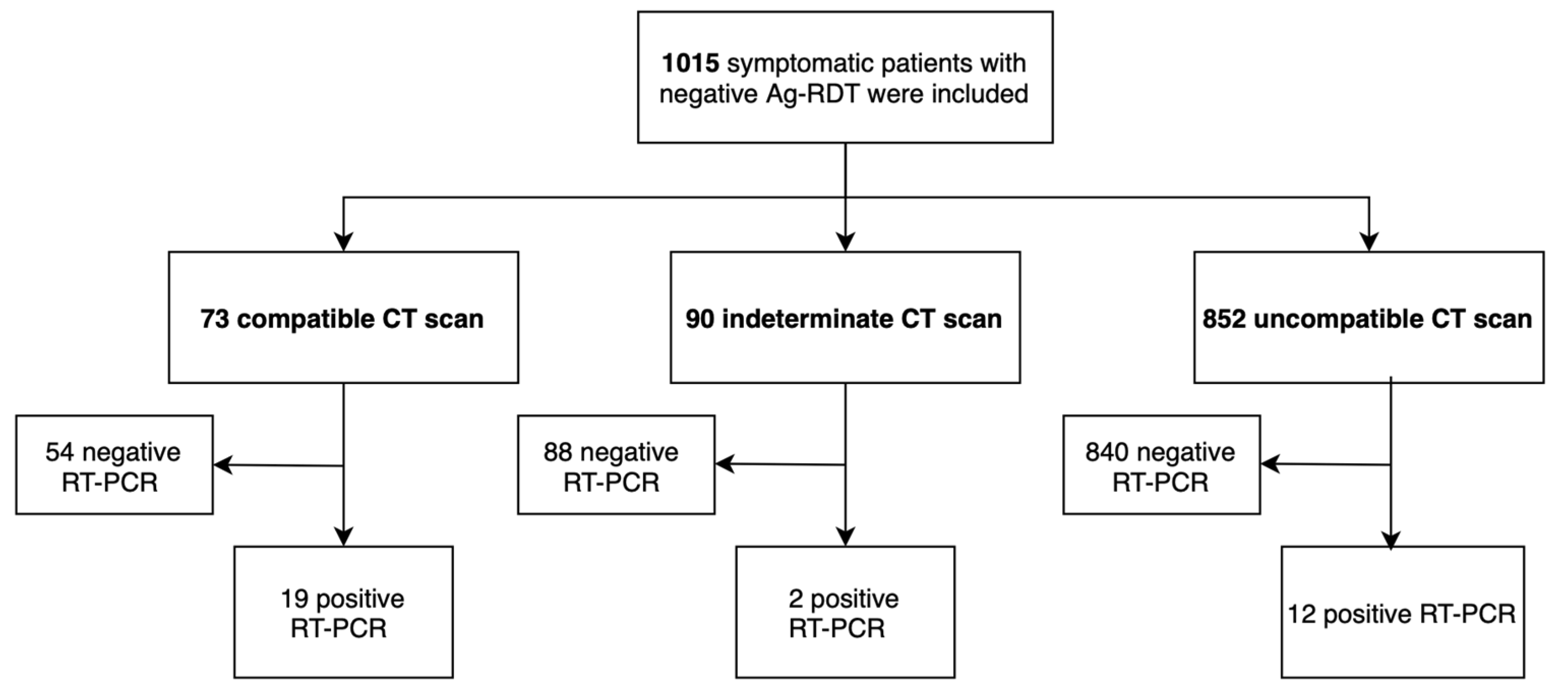
| Characteristics | N (%), M (SD) N = 1015 | |
|---|---|---|
| Age | 70.4 (18.2) | |
| Gender | Men Women | 557 (54.9) 458 (45.1) |
| PCR positive before ED stay | 25 (2.5) | |
| Contact with COVID before ED stay | 6 (0.6) | |
| Chest CT | Compatible | 73 (7.2) |
| Indeterminate | 90 (8.9) | |
| Incompatible | 852 (83.9) | |
| PCR positive in ED | 33 (3.2) | |
| Hospitalization in medical ward | 600 (59.1) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kepka, S.; Ohana, M.; Séverac, F.; Muller, J.; Bayle, E.; Ruch, Y.; Laugel, E.; Oberlin, M.; Solis, M.; Hansmann, Y.; et al. Rapid Antigen Test Combined with Chest Computed Tomography to Rule Out COVID-19 in Patients Admitted to the Emergency Department. J. Clin. Med. 2021, 10, 3455. https://doi.org/10.3390/jcm10163455
Kepka S, Ohana M, Séverac F, Muller J, Bayle E, Ruch Y, Laugel E, Oberlin M, Solis M, Hansmann Y, et al. Rapid Antigen Test Combined with Chest Computed Tomography to Rule Out COVID-19 in Patients Admitted to the Emergency Department. Journal of Clinical Medicine. 2021; 10(16):3455. https://doi.org/10.3390/jcm10163455
Chicago/Turabian StyleKepka, Sabrina, Mickaël Ohana, François Séverac, Joris Muller, Eric Bayle, Yvon Ruch, Elodie Laugel, Mathieu Oberlin, Morgane Solis, Yves Hansmann, and et al. 2021. "Rapid Antigen Test Combined with Chest Computed Tomography to Rule Out COVID-19 in Patients Admitted to the Emergency Department" Journal of Clinical Medicine 10, no. 16: 3455. https://doi.org/10.3390/jcm10163455
APA StyleKepka, S., Ohana, M., Séverac, F., Muller, J., Bayle, E., Ruch, Y., Laugel, E., Oberlin, M., Solis, M., Hansmann, Y., Bilbault, P., & Fafi Kremer, S. (2021). Rapid Antigen Test Combined with Chest Computed Tomography to Rule Out COVID-19 in Patients Admitted to the Emergency Department. Journal of Clinical Medicine, 10(16), 3455. https://doi.org/10.3390/jcm10163455





