Telehealth and Screening Strategies in the Diagnosis and Management of Glaucoma
Abstract
:1. Introduction
2. Equipment
2.1. Visual Acuity Test
2.2. Intraocular Pressure Measurement
2.2.1. Tono-Pen® (Reichert; Depew, New York, NY, USA)
2.2.2. Air Puff Non-Contact Tonometer
2.2.3. iCare (iCare Finland Oy; Helsinki, Finland)
2.2.4. Ocular Response Analyzer (Reichert; Depew, NY, USA)
2.2.5. Sensimed Triggerfish® Contact Lens (Sensimed; Lausanne, Switzerland)
2.2.6. Other Contact Lenses in Development
2.2.7. Eyemate® (Implandata Ophthalmic Products GmbH; Hannover, Germany)
2.2.8. Injectsense (Injectsense, Inc.; Emeryville, CA, USA)
2.2.9. Diaton Transpalpebral Tonometer (DevelopAll Inc.; New York, NY, USA)
2.2.10. Finger Palpation
2.3. Anterior Segment Photography
2.4. Iridocorneal Angle Imaging
2.5. Fundus Photography
2.6. Optical Coherence Tomography of the Retinal Nerve Fiber Layer
2.7. Visual Field
2.7.1. Peristat Online Perimetry
2.7.2. Melbourne Rapid Fields (MRF)
2.7.3. Virtual Reality Headsets
2.8. Artificial Intelligence
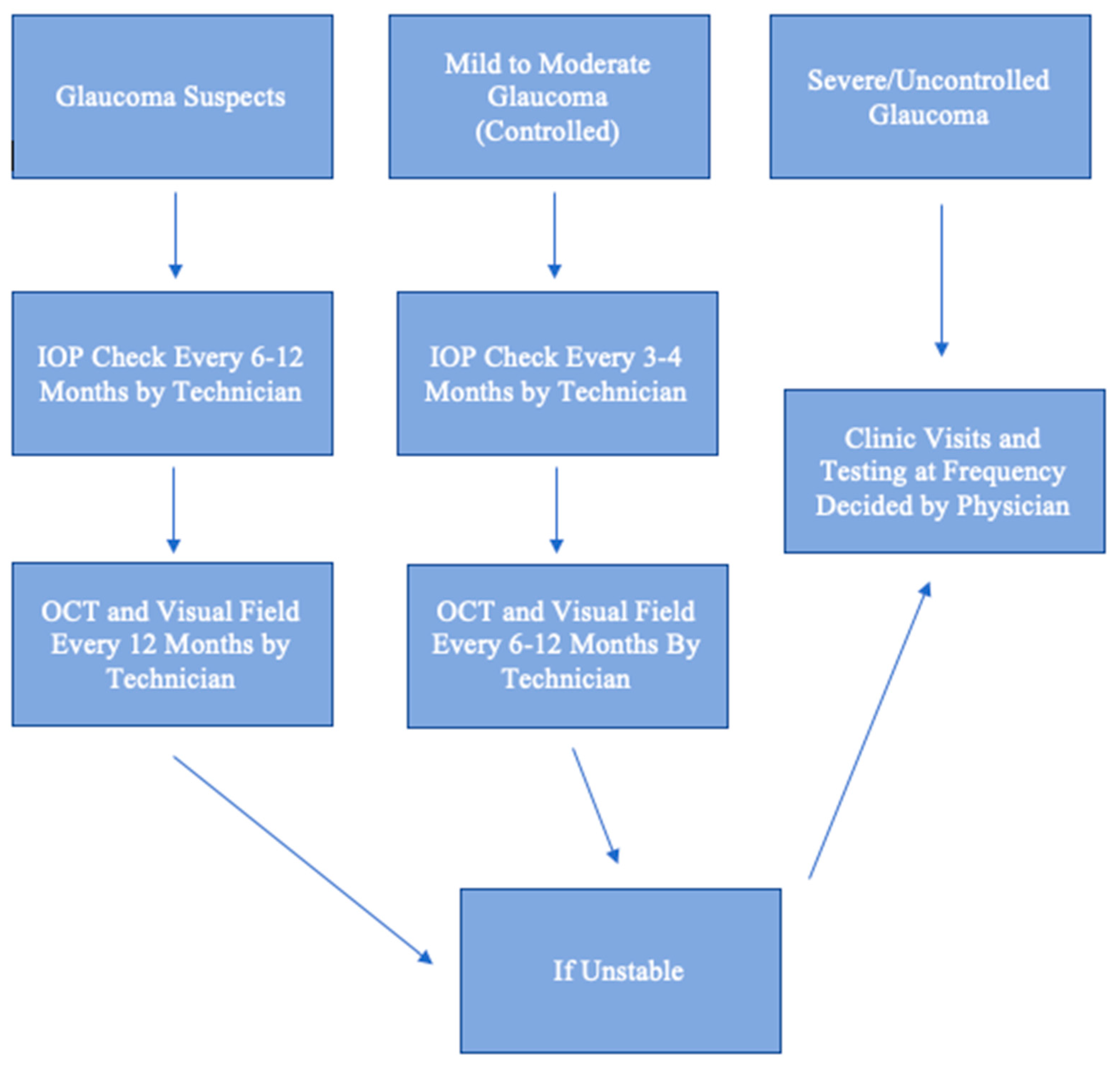
3. Setups for Telehealth Programs
4. How the Coronavirus Pandemic Shaped Telehealth
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Consultant
References
- Tham, Y.-C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.-Y. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040: A Systematic Review and Meta-Analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Dall, T.; Reynolds, R.; Chakrabarti, R.; Jones, K.; Iacobucci, W. The Complexities of Physician Supply and Demand: Projections From 2018 to 2033. Assoc. Am. Med Coll. 2020, 1–92. [Google Scholar]
- Samanta, A.; Mauntana, S.; Barsi, Z.; Yarlagadda, B.; Nelson, P.C. Is Your Vision Blurry? A Systematic Review of Home-Based Visual Acuity for Telemedicine. J. Telemed. Telecare 2020. [Google Scholar] [CrossRef] [PubMed]
- Heijl, A.; Leske, M.C.; Bengtsson, B.; Hyman, L.; Bengtsson, B.; Hussein, M. Early Manifest Glaucoma Trial Group Reduction of Intraocular Pressure and Glaucoma Progression: Results from the Early Manifest Glaucoma Trial. Arch. Ophthalmol 2002, 120, 1268–1279. [Google Scholar] [CrossRef]
- Minckler, D.S.; Baerveldt, G.; Heuer, D.K.; Quillen-Thomas, B.; Walonker, A.F.; Weiner, J. Clinical Evaluation of the Oculab Tono-Pen. Am. J. Ophthalmol. 1987, 104, 168–173. [Google Scholar] [CrossRef]
- Kao, S.F.; Lichter, P.R.; Bergstrom, T.J.; Rowe, S.; Musch, D.C. Clinical Comparison of the Oculab Tono-Pen to the Goldmann Applanation Tonometer. Ophthalmology 1987, 94, 1541–1544. [Google Scholar] [CrossRef]
- Frenkel, R.E.; Hong, Y.J.; Shin, D.H. Comparison of the Tono-Pen to the Goldmann Applanation Tonometer. Arch. Ophthalmol. 1988, 106, 750–753. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.; Diaconita, V.; Schulz, D.C.; Hutnik, C. Tono-Pen versus Goldmann Applanation Tonometry: A Comparison of 898 Eyes. Ophthalmol. Glaucoma 2019, 2, 435–439. [Google Scholar] [CrossRef]
- Bhartiya, S.; Bali, S.J.; Sharma, R.; Chaturvedi, N.; Dada, T. Comparative Evaluation of TonoPen AVIA, Goldmann Applanation Tonometry and Non-Contact Tonometry. Int. Ophthalmol. 2011, 31, 297–302. [Google Scholar] [CrossRef]
- Dohadwala, A.A.; Munger, R.; Damji, K.F. Positive Correlation between Tono-Pen Intraocular Pressure and Central Corneal Thickness. Ophthalmology 1998, 105, 1849–1854. [Google Scholar] [CrossRef]
- Hsu, S.-Y.; Sheu, M.-M.; Hsu, A.-H.; Wu, K.-Y.; Yeh, J.-I.; Tien, J.-N.; Tsai, R.-K. Comparisons of Intraocular Pressure Measurements: Goldmann Applanation Tonometry, Noncontact Tonometry, Tono-Pen Tonometry, and Dynamic Contour Tonometry. Eye 2009, 23, 1582–1588. [Google Scholar] [CrossRef] [PubMed]
- Razeghinejad, M.R.; Salouti, R.; Khalili, M.R. Intraocular Pressure Measurements by Three Different Tonometers in Children with Aphakic Glaucoma and a Thick Cornea. Iran. J. Med. Sci. 2014, 39, 11–19. [Google Scholar] [PubMed]
- Tonnu, P.-A.; Ho, T.; Newson, T.; El Sheikh, A.; Sharma, K.; White, E.; Bunce, C.; Garway-Heath, D. The Influence of Central Corneal Thickness and Age on Intraocular Pressure Measured by Pneumotonometry, Non-Contact Tonometry, the Tono-Pen XL, and Goldmann Applanation Tonometry. Br. J. Ophthalmol. 2005, 89, 851–854. [Google Scholar] [CrossRef] [Green Version]
- Bang, S.P.; Lee, C.E.; Kim, Y.C. Comparison of Intraocular Pressure as Measured by Three Different Non-Contact Tonometers and Goldmann Applanation Tonometer for Non-Glaucomatous Subjects. BMC Ophthalmol. 2017, 17, 199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Resúa, C.; Giráldez Fernández, M.J.; Yebra-Pimentel, E.; García-Montero, S. Clinical Evaluation of the Canon TX-10 Noncontact Tonometer in Healthy Eyes. Eur. J. Ophthalmol. 2010, 20, 523–530. [Google Scholar] [CrossRef]
- Kutzscher, A.E.; Kumar, R.S.; Ramgopal, B.; Rackenchath, M.V.; Devi, S.; Nagaraj, S.; Moe, C.A.; Fry, D.M.; Stamper, R.L.; Keenan, J.D. Reproducibility of 5 Methods of Ocular Tonometry. Ophthalmol. Glaucoma 2019, 2, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, T.; Balakrishna, N. Effect of Central Corneal Thickness on Intraocular Pressure and Comparison of Topcon CT-80 Non-Contact Tonometry with Goldmann Applanation Tonometry. Clin. Exp. Optom. 2018, 101, 206–212. [Google Scholar] [CrossRef]
- Tonnu, P.-A.; Ho, T.; Sharma, K.; White, E.; Bunce, C.; Garway-Heath, D. A Comparison of Four Methods of Tonometry: Method Agreement and Interobserver Variability. Br. J. Ophthalmol. 2005, 89, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Hubanova, R.; Aptel, F.; Zhou, T.; Arnol, N.; Romanet, J.-P.; Chiquet, C. Comparison of Intraocular Pressure Measurements with the Reichert Pt100, the Keeler Pulsair Intellipuff Portable Noncontact Tonometers, and Goldmann Applanation Tonometry. J. Glaucoma 2015, 24, 356–363. [Google Scholar] [CrossRef]
- Nakakura, S.; Mori, E.; Fujio, Y.; Fujisawa, Y.; Matsuya, K.; Kobayashi, Y.; Tabuchi, H.; Asaoka, R.; Kiuchi, Y. Comparison of the Intraocular Pressure Measured Using the New Rebound Tonometer Icare Ic100 and Icare TA01i or Goldmann Applanation Tonometer. J. Glaucoma 2019, 28, 172–177. [Google Scholar] [CrossRef]
- Gao, F.; Liu, X.; Zhao, Q.; Pan, Y. Comparison of the ICare Rebound Tonometer and the Goldmann Applanation Tonometer. Exp. Ther. Med. 2017, 13, 1912–1916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subramaniam, A.G.; Allen, P.; Toh, T. Comparison of the Icare Ic100 Rebound Tonometer and the Goldmann Applanation Tonometer in 1000 Eyes. Ophthalmic Res. 2021, 64, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Badakere, S.V.; Chary, R.; Choudhari, N.S.; Rao, H.L.; Garudadri, C.; Senthil, S. Agreement of Intraocular Pressure Measurement of Icare Ic200 with Goldmann Applanation Tonometer in Adult Eyes with Normal Cornea. Ophthalmol. Glaucoma 2021, 4, 89–94. [Google Scholar] [CrossRef]
- Takagi, D.; Sawada, A.; Yamamoto, T. Evaluation of a New Rebound Self-Tonometer, Icare HOME: Comparison with Goldmann Applanation Tonometer. J. Glaucoma 2017, 26, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, J.R.; Haseltine, S.; Shimmyo, M.; Radcliffe, N.M. Evaluation of Agreement between Intraocular Pressure Measurements Using Goldmann Applanation Tonometry and Goldmann Correlated Intraocular Pressure by Reichert’s Ocular Response Analyser. Eye 2010, 24, 1555–1560. [Google Scholar] [CrossRef] [Green Version]
- Ogbuehi, K.C.; Almubrad, T.M. Evaluation of the Intraocular Pressure Measured with the Ocular Response Analyzer. Curr. Eye Res. 2010, 35, 587–596. [Google Scholar] [CrossRef]
- Martinez-de-la-Casa, J.M.; Garcia-Feijoo, J.; Fernandez-Vidal, A.; Mendez-Hernandez, C.; Garcia-Sanchez, J. Ocular Response Analyzer versus Goldmann Applanation Tonometry for Intraocular Pressure Measurements. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4410–4414. [Google Scholar] [CrossRef] [Green Version]
- Renier, C.; Zeyen, T.; Fieuws, S.; Vandenbroeck, S.; Stalmans, I. Comparison of Ocular Response Analyzer, Dynamic Contour Tonometer and Goldmann Applanation Tonometer. Int. Ophthalmol. 2010, 30, 651–659. [Google Scholar] [CrossRef]
- Kotecha, A.; White, E.; Schlottmann, P.G.; Garway-Heath, D.F. Intraocular Pressure Measurement Precision with the Goldmann Applanation, Dynamic Contour, and Ocular Response Analyzer Tonometers. Ophthalmology 2010, 117, 730–737. [Google Scholar] [CrossRef]
- Vandewalle, E.; Vandenbroeck, S.; Stalmans, I.; Zeyen, T. Comparison of ICare, Dynamic Contour Tonometer, and Ocular Response Analyzer with Goldmann Applanation Tonometer in Patients with Glaucoma. Eur. J. Ophthalmol. 2009, 19, 783–789. [Google Scholar] [CrossRef]
- Carbonaro, F.; Andrew, T.; Mackey, D.A.; Spector, T.D.; Hammond, C.J. Comparison of Three Methods of Intraocular Pressure Measurement and Their Relation to Central Corneal Thickness. Eye 2010, 24, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, F.A.; Meira-Freitas, D.; Lisboa, R.; Kuang, T.-M.; Zangwill, L.M.; Weinreb, R.N. Corneal Hysteresis as a Risk Factor for Glaucoma Progression: A Prospective Longitudinal Study. Ophthalmology 2013, 120, 1533–1540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schweitzer, J.A.; Ervin, M.; Berdahl, J.P. Assessment of Corneal Hysteresis Measured by the Ocular Response Analyzer as a Screening Tool in Patients with Glaucoma. Clin. Ophthalmol. 2018, 12, 1809–1813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansouri, K.; Medeiros, F.A.; Tafreshi, A.; Weinreb, R.N. Continuous 24-Hour Monitoring of Intraocular Pressure Patterns with a Contact Lens Sensor: Safety, Tolerability, and Reproducibility in Patients with Glaucoma. Arch. Ophthalmol. 2012, 130, 1534–1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansouri, K.; Weinreb, R.N.; Liu, J.H.K. Efficacy of a Contact Lens Sensor for Monitoring 24-h Intraocular Pressure Related Patterns. PLoS ONE 2015, 10, e0125530. [Google Scholar] [CrossRef]
- De Moraes, C.G.; Mansouri, K.; Liebmann, J.M.; Ritch, R. Association between 24-hour intraocular pressure monitored with contact lens sensor and visual field progression in older adults with glaucoma. JAMA Ophthalmol. 2018, 136, 779–785. [Google Scholar] [CrossRef]
- Kim, J.; Park, J.; Park, Y.-G.; Cha, E.; Ku, M.; An, H.S.; Lee, K.-P.; Huh, M.-I.; Kim, J.; Kim, T.-S.; et al. A Soft and Transparent Contact Lens for the Wireless Quantitative Monitoring of Intraocular Pressure. Nat. Biomed. Eng. 2021, 5, 772–782. [Google Scholar] [CrossRef]
- Koutsonas, A.; Walter, P.; Roessler, G.; Plange, N. Implantation of a Novel Telemetric Intraocular Pressure Sensor in Patients with Glaucoma (ARGOS Study): 1-Year Results. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1063–1069. [Google Scholar] [CrossRef] [Green Version]
- Enders, P.; Hall, J.; Bornhauser, M.; Mansouri, K.; Altay, L.; Schrader, S.; Dietlein, T.S.; Bachmann, B.O.; Neuhann, T.; Cursiefen, C. Telemetric Intraocular Pressure Monitoring after Boston Keratoprosthesis Surgery Using the Eyemate-IO Sensor: Dynamics in the First Year. Am. J. Ophthalmol. 2019, 206, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, J.; Duan, X.; Fan, F. Transpalpebral Measurement of Intraocular Pressure Using the Diaton Tonometer versus Standard Goldmann Applanation Tonometry. Graefes Arch. Clin. Exp. Ophthalmol. 2010, 248, 1765–1770. [Google Scholar] [CrossRef]
- Risma, J.M.; Tehrani, S.; Wang, K.; Fingert, J.H.; Alward, W.L.M.; Kwon, Y.H. The Utility of Diaton Tonometer Measurements in Patients with Ocular Hypertension, Glaucoma, and Glaucoma Tube Shunts: A Preliminary Study for Its Potential Use in Keratoprosthesis Patients. J. Glaucoma 2016, 25, 643–647. [Google Scholar] [CrossRef]
- Bali, S.J.; Bhartiya, S.; Sobti, A.; Dada, T.; Panda, A. Comparative Evaluation of Diaton and Goldmann Applanation Tonometers. Ophthalmologica 2012, 228, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Doherty, M.D.; Carrim, Z.I.; O’Neill, D.P. Diaton Tonometry: An Assessment of Validity and Preference against Goldmann Tonometry. Clin. Exp. Ophthalmol. 2012, 40, e171–e175. [Google Scholar] [CrossRef]
- Chan, E.C.; Roberts, D.K.; Loconte, D.D.; Wernick, M.N. Digital Camera System to Perform Infrared Photography of Iris Transillumination. J. Glaucoma 2002, 11, 426–428. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, C.A.; Murthy, S.I.; Pappuru, R.R.; Jais, A.; Myung, D.J.; Chang, R.T. A Novel Smartphone Ophthalmic Imaging Adapter: User Feasibility Studies in Hyderabad, India. Indian J. Ophthalmol. 2016, 64, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Nongpiur, M.E.; Sakata, L.M.; Friedman, D.S.; He, M.; Chan, Y.-H.; Lavanya, R.; Wong, T.Y.; Aung, T. Novel Association of Smaller Anterior Chamber Width with Angle Closure in Singaporeans. Ophthalmology 2010, 117, 1967–1973. [Google Scholar] [CrossRef]
- Wu, R.-Y.; Nongpiur, M.E.; He, M.-G.; Sakata, L.M.; Friedman, D.S.; Chan, Y.-H.; Lavanya, R.; Wong, T.-Y.; Aung, T. Association of Narrow Angles with Anterior Chamber Area and Volume Measured with Anterior-Segment Optical Coherence Tomography. Arch. Ophthalmol. 2011, 129, 569–574. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Sakata, L.M.; Friedman, D.S.; Chan, Y.-H.; He, M.; Lavanya, R.; Wong, T.-Y.; Aung, T. Quantitative Iris Parameters and Association with Narrow Angles. Ophthalmology 2010, 117, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Nongpiur, M.E.; He, M.; Amerasinghe, N.; Friedman, D.S.; Tay, W.-T.; Baskaran, M.; Smith, S.D.; Wong, T.Y.; Aung, T. Lens Vault, Thickness, and Position in Chinese Subjects with Angle Closure. Ophthalmology 2011, 118, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.S.; He, M.; Zhao, W.; Sakata, L.M.; Li, J.; Nongpiur, M.E.; Lavanya, R.; Friedman, D.S.; Aung, T. Determinants of Lens Vault and Association with Narrow Angles in Patients from Singapore. Am. J. Ophthalmol. 2012, 154, 39–46. [Google Scholar] [CrossRef]
- Foo, L.-L.; Nongpiur, M.E.; Allen, J.C.; Perera, S.A.; Friedman, D.S.; He, M.; Cheng, C.-Y.; Wong, T.Y.; Aung, T. Determinants of Angle Width in Chinese Singaporeans. Ophthalmology 2012, 119, 278–282. [Google Scholar] [CrossRef]
- Nongpiur, M.E.; Haaland, B.A.; Perera, S.A.; Friedman, D.S.; He, M.; Sakata, L.M.; Baskaran, M.; Aung, T. Development of a Score and Probability Estimate for Detecting Angle Closure Based on Anterior Segment Optical Coherence Tomography. Am. J. Ophthalmol. 2014, 157, 32–38. [Google Scholar] [CrossRef]
- Perera, S.A.; Baskaran, M.; Friedman, D.S.; Tun, T.A.; Htoon, H.M.; Kumar, R.S.; Aung, T. Use of EyeCam for Imaging the Anterior Chamber Angle. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2993–2997. [Google Scholar] [CrossRef]
- Shinoj, V.K.; Murukeshan, V.M.; Baskaran, M.; Aung, T. Integrated Flexible Handheld Probe for Imaging and Evaluation of Iridocorneal Angle. J. Biomed. Opt. 2015, 20, 016014. [Google Scholar] [CrossRef]
- Russo, A.; Mapham, W.; Turano, R.; Costagliola, C.; Morescalchi, F.; Scaroni, N.; Semeraro, F. Comparison of Smartphone Ophthalmoscopy with Slit-Lamp Biomicroscopy for Grading Vertical Cup-to-Disc Ratio. J. Glaucoma 2016, 25, e777–e781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wintergerst, M.W.M.; Brinkmann, C.K.; Holz, F.G.; Finger, R.P. Undilated versus Dilated Monoscopic Smartphone-Based Fundus Photography for Optic Nerve Head Evaluation. Sci. Rep. 2018, 8, 10228. [Google Scholar] [CrossRef] [Green Version]
- Lowry, E.A.; Hou, J.; Hennein, L.; Chang, R.T.; Lin, S.; Keenan, J.; Wang, S.K.; Ianchulev, S.; Pasquale, L.R.; Han, Y. Comparison of Peristat Online Perimetry with the Humphrey Perimetry in a Clinic-Based Setting. Transl. Vis. Sci. Technol. 2016, 5, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prea, S.M.; Kong, Y.X.G.; Mehta, A.; He, M.; Crowston, J.G.; Gupta, V.; Martin, K.R.; Vingrys, A.J. Six-Month Longitudinal Comparison of a Portable Tablet Perimeter with the Humphrey Field Analyzer. Am. J. Ophthalmol. 2018, 190, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.A.; Thapa, S.; George Kong, Y.X.; Robin, A.L. Performance of an IPad Application to Detect Moderate and Advanced Visual Field Loss in Nepal. Am. J. Ophthalmol. 2017, 182, 147–154. [Google Scholar] [CrossRef]
- Kong, Y.X.G.; He, M.; Crowston, J.G.; Vingrys, A.J. A Comparison of Perimetric Results from a Tablet Perimeter and Humphrey Field Analyzer in Glaucoma Patients. Transl. Vis. Sci. Technol. 2016, 5, 2. [Google Scholar] [CrossRef]
- Schulz, A.M.; Graham, E.C.; You, Y.; Klistorner, A.; Graham, S.L. Performance of IPad-Based Threshold Perimetry in Glaucoma and Controls. Clin. Exp. Ophthalmol. 2018, 46, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Tsapakis, S.; Papaconstantinou, D.; Diagourtas, A.; Droutsas, K.; Andreanos, K.; Moschos, M.M.; Brouzas, D. Visual Field Examination Method Using Virtual Reality Glasses Compared with the Humphrey Perimeter. Clin. Ophthalmol. 2017, 11, 1431–1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakanishi, M.; Wang, Y.-T.; Jung, T.-P.; Zao, J.K.; Chien, Y.-Y.; Diniz-Filho, A.; Daga, F.B.; Lin, Y.-P.; Wang, Y.; Medeiros, F.A. Detecting Glaucoma with a Portable Brain-Computer Interface for Objective Assessment of Visual Function Loss. JAMA Ophthalmol. 2017, 135, 550–557. [Google Scholar] [CrossRef]
- Rogers, T.W.; Jaccard, N.; Carbonaro, F.; Lemij, H.G.; Vermeer, K.A.; Reus, N.J.; Trikha, S. Evaluation of an AI System for the Automated Detection of Glaucoma from Stereoscopic Optic Disc Photographs: The European Optic Disc Assessment Study. Eye 2019, 33, 1791–1797. [Google Scholar] [CrossRef] [Green Version]
- Hemelings, R.; Elen, B.; Barbosa-Breda, J.; Lemmens, S.; Meire, M.; Pourjavan, S.; Vandewalle, E.; Van de Veire, S.; Blaschko, M.B.; De Boever, P.; et al. Accurate Prediction of Glaucoma from Colour Fundus Images with a Convolutional Neural Network That Relies on Active and Transfer Learning. Acta Ophthalmol. 2020, 98, e94–e100. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.; Kim, J.H.; Park, K.H. Screening Glaucoma with Red-Free Fundus Photography Using Deep Learning Classifier and Polar Transformation. J. Glaucoma 2019, 28, 258–264. [Google Scholar] [CrossRef]
- Li, F.; Yan, L.; Wang, Y.; Shi, J.; Chen, H.; Zhang, X.; Jiang, M.; Wu, Z.; Zhou, K. Deep Learning-Based Automated Detection of Glaucomatous Optic Neuropathy on Color Fundus Photographs. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 851–867. [Google Scholar] [CrossRef]
- Li, Z.; He, Y.; Keel, S.; Meng, W.; Chang, R.T.; He, M. Efficacy of a Deep Learning System for Detecting Glaucomatous Optic Neuropathy Based on Color Fundus Photographs. Ophthalmology 2018, 125, 1199–1206. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Li, L.; Wormstone, I.M.; Qiao, C.; Zhang, C.; Liu, P.; Li, S.; Wang, H.; Mou, D.; Pang, R.; et al. Development and Validation of a Deep Learning System to Detect Glaucomatous Optic Neuropathy Using Fundus Photographs. JAMA Ophthalmol. 2019, 137, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, C.; Lin, D.; Nie, D.; Zhu, Y.; Chen, C.; Zhao, L.; Wang, J.; Zhang, X.; Dongye, M.; et al. Deep Learning for Automated Glaucomatous Optic Neuropathy Detection from Ultra-Widefield Fundus Images. Br. J. Ophthalmol. 2020. [Google Scholar] [CrossRef]
- Asaoka, R.; Tanito, M.; Shibata, N.; Mitsuhashi, K.; Nakahara, K.; Fujino, Y.; Matsuura, M.; Murata, H.; Tokumo, K.; Kiuchi, Y. Validation of a Deep Learning Model to Screen for Glaucoma Using Images from Different Fundus Cameras and Data Augmentation. Ophthalmol. Glaucoma 2019, 2, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Al-Aswad, L.A.; Kapoor, R.; Chu, C.K.; Walters, S.; Gong, D.; Garg, A.; Gopal, K.; Patel, V.; Sameer, T.; Rogers, T.W.; et al. Evaluation of a Deep Learning System For Identifying Glaucomatous Optic Neuropathy Based on Color Fundus Photographs. J. Glaucoma 2019, 28, 1029–1034. [Google Scholar] [CrossRef]
- Medeiros, F.A.; Jammal, A.A.; Thompson, A.C. From Machine to Machine: An OCT-Trained Deep Learning Algorithm for Objective Quantification of Glaucomatous Damage in Fundus Photographs. Ophthalmology 2019, 126, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Jammal, A.A.; Mariottoni, E.B.; Medeiros, F.A. Predicting Glaucoma Development with Longitudinal Deep Learning Predictions from Fundus Photographs. Am. J. Ophthalmol. 2021, 225, 86–94. [Google Scholar] [CrossRef]
- Medeiros, F.A.; Jammal, A.A.; Mariottoni, E.B. Detection of Progressive Glaucomatous Optic Nerve Damage on Fundus Photographs with Deep Learning. Ophthalmology 2021, 128, 383–392. [Google Scholar] [CrossRef]
- Christopher, M.; Belghith, A.; Weinreb, R.N.; Bowd, C.; Goldbaum, M.H.; Saunders, L.J.; Medeiros, F.A.; Zangwill, L.M. Retinal Nerve Fiber Layer Features Identified by Unsupervised Machine Learning on Optical Coherence Tomography Scans Predict Glaucoma Progression. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2748–2756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.E.; Kim, J.M.; Song, J.E.; Kee, C.; Han, J.C.; Hyun, S.H. Development and Validation of a Deep Learning System for Diagnosing Glaucoma Using Optical Coherence Tomography. J. Clin. Med. 2020, 9, 2167. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.K.; Park, K.H.; Jeoung, J.W. Diagnosing Glaucoma with Spectral-Domain Optical Coherence Tomography Using Deep Learning Classifier. J. Glaucoma 2020, 29, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.C.; Jammal, A.A.; Berchuck, S.I.; Mariottoni, E.B.; Medeiros, F.A. Assessment of a Segmentation-Free Deep Learning Algorithm for Diagnosing Glaucoma From Optical Coherence Tomography Scans. JAMA Ophthalmol. 2020, 138, 333–339. [Google Scholar] [CrossRef]
- Wang, P.; Shen, J.; Chang, R.; Moloney, M.; Torres, M.; Burkemper, B.; Jiang, X.; Rodger, D.; Varma, R.; Richter, G.M. Machine Learning Models for Diagnosing Glaucoma from Retinal Nerve Fiber Layer Thickness Maps. Ophthalmol. Glaucoma 2019, 2, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Xie, X.; Huang, L.; Chen, B.; Yang, J.; Lu, J.; Qiao, T.; Fan, Z.; Zhang, M. Detecting Glaucoma Based on Spectral Domain Optical Coherence Tomography Imaging of Peripapillary Retinal Nerve Fiber Layer: A Comparison Study between Hand-Crafted Features and Deep Learning Model. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 577–585. [Google Scholar] [CrossRef]
- Christopher, M.; Bowd, C.; Belghith, A.; Goldbaum, M.H.; Weinreb, R.N.; Fazio, M.A.; Girkin, C.A.; Liebmann, J.M.; Zangwill, L.M. Deep Learning Approaches Predict Glaucomatous Visual Field Damage from OCT Optic Nerve Head En Face Images and Retinal Nerve Fiber Layer Thickness Maps. Ophthalmology 2020, 127, 346–356. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Asaoka, R.; Kiwaki, T.; Sugiura, H.; Asano, S.; Murata, H.; Fujino, Y.; Matsuura, M.; Miki, A.; Mori, K.; et al. Deep Learning Model to Predict Visual Field in Central 10° from Optical Coherence Tomography Measurement in Glaucoma. Br. J. Ophthalmol. 2021, 105, 507–513. [Google Scholar] [CrossRef]
- Fu, H.; Baskaran, M.; Xu, Y.; Lin, S.; Wong, D.W.K.; Liu, J.; Tun, T.A.; Mahesh, M.; Perera, S.A.; Aung, T. A Deep Learning System for Automated Angle-Closure Detection in Anterior Segment Optical Coherence Tomography Images. Am. J. Ophthalmol. 2019, 203, 37–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, B.Y.; Chiang, M.; Chaudhary, S.; Kulkarni, S.; Pardeshi, A.A.; Varma, R. Deep Learning Classifiers for Automated Detection of Gonioscopic Angle Closure Based on Anterior Segment OCT Images. Am. J. Ophthalmol. 2019, 208, 273–280. [Google Scholar] [CrossRef]
- Asaoka, R.; Murata, H.; Iwase, A.; Araie, M. Detecting Preperimetric Glaucoma with Standard Automated Perimetry Using a Deep Learning Classifier. Ophthalmology 2016, 123, 1974–1980. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.C.; Lee, C.S.; Keane, P.A.; Xiao, S.; Rokem, A.S.; Chen, P.P.; Wu, Y.; Lee, A.Y. Forecasting Future Humphrey Visual Fields Using Deep Learning. PLoS ONE 2019, 14, e0214875. [Google Scholar] [CrossRef] [Green Version]
- Yousefi, S.; Kiwaki, T.; Zheng, Y.; Sugiura, H.; Asaoka, R.; Murata, H.; Lemij, H.; Yamanishi, K. Detection of Longitudinal Visual Field Progression in Glaucoma Using Machine Learning. Am. J. Ophthalmol. 2018, 193, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Hark, L.A.; Myers, J.S.; Pasquale, L.R.; Razeghinejad, M.R.; Maity, A.; Zhan, T.; Hegarty, S.E.; Leiby, B.E.; Waisbourd, M.; Burns, C.; et al. Philadelphia Telemedicine Glaucoma Detection and Follow-up Study: Intraocular Pressure Measurements Found in a Population at High Risk for Glaucoma. J. Glaucoma 2019, 28, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Hark, L.A.; Kresch, Y.S.; De Moraes, C.G.; Horowitz, J.D.; Park, L.; Auran, J.D.; Gorroochurn, P.; Stempel, S.; Maruri, S.C.; Stidham, E.M.; et al. Manhattan Vision Screening and Follow-up Study in Vulnerable Populations (NYC-SIGHT): Design and Methodology. J. Glaucoma 2021, 30, 388–394. [Google Scholar] [CrossRef]
- Newman-Casey, P.A.; Musch, D.C.; Niziol, L.M.; Elam, A.R.; Zhang, J.; Moroi, S.E.; Johnson, L.; Kershaw, M.; Saadine, J.; Winter, S.; et al. Michigan Screening and Intervention for Glaucoma and Eye Health Through Telemedicine (MI-SIGHT): Baseline Methodology for Implementing and Assessing a Community-Based Program. J. Glaucoma 2021, 30, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, L.A.; Register, S.; Asif, I.; McGwin, G.; Saaddine, J.; Nghiem, V.T.H.; Owsley, C.; Girkin, C.A. Alabama Screening and Intervention for Glaucoma and Eye Health Through Telemedicine (AL-SIGHT): Study Design and Methodology. J. Glaucoma 2021, 30, 371–379. [Google Scholar] [CrossRef]
- Verma, S.; Arora, S.; Kassam, F.; Edwards, M.C.; Damji, K.F. Northern Alberta Remote Teleglaucoma Program: Clinical Outcomes and Patient Disposition. Can. J. Ophthalmol. 2014, 49, 135–140. [Google Scholar] [CrossRef]
- Modjtahedi, B.S.; Chu, K.; Luong, T.Q.; Hsu, C.; Mattox, C.; Lee, P.P.; Nakla, M.L.; Fong, D.S. Two-Year Outcomes of a Pilot Glaucoma Suspect Telemedicine Monitoring Program. Clin. Ophthalmol. 2018, 12, 2095–2102. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekaran, S.; Kass, W.; Thangamathesvaran, L.; Mendez, N.; Khouri, P.; Szirth, B.C.; Khouri, A.S. Tele-Glaucoma versus Clinical Evaluation: The New Jersey Health Foundation Prospective Clinical Study. J. Telemed. Telecare 2020, 26, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.; Puertas, R.; Kotecha, A.; Foster, P.J.; Barton, K. Virtual Clinics in Glaucoma Care: Face-to-Face versus Remote Decision-Making. Br. J. Ophthalmol. 2017, 101, 892–895. [Google Scholar] [CrossRef]
- Thomas, S.; Hodge, W.; Malvankar-Mehta, M. The Cost-Effectiveness Analysis of Teleglaucoma Screening Device. PLoS ONE 2015, 10, e0137913. [Google Scholar] [CrossRef]
- Saleem, S.M.; Pasquale, L.R.; Sidoti, P.A.; Tsai, J.C. Virtual Ophthalmology: Telemedicine in a COVID-19 Era. Am. J. Ophthalmol. 2020, 216, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Vinod, K.; Sidoti, P.A. Glaucoma Care during the Coronavirus Disease 2019 Pandemic. Curr. Opin. Ophthalmol. 2021, 32, 75–82. [Google Scholar] [CrossRef]
| Utility | Disadvantages | |
|---|---|---|
| Visual Acuity | Changes can be due to a new central scotoma, refractive error, cataract, and other ocular pathologies. | Glaucoma typically presents with peripheral visual field loss which visual acuity does not assess. Only very advanced glaucoma affects visual acuity. |
| Intraocular Pressure (IOP) | A very important parameter to assess the efficacy of treatment and a major risk factor for disease progression. | Goldmann applanation, the gold standard for measuring IOP, is only performed in clinic. Portable tonometers can significantly differ from Goldmann measurements for IOPs outside the normal range. |
| Anterior Segment Photography | In lieu of the slit lamp examination, the camera can capture abnormalities of the external and anterior parts of the eye. | The camera may miss subtle pathologies such as a pigment deposition on the corneal endothelium or iris neovascularization. In addition, it cannot capture the anterior chamber cell and flare. |
| Iridocorneal Angle Imaging | Identifies eyes with anatomic narrow angles at risk for acute angle closure glaucoma. | Angle camera devices and UBM require instillation of topical anesthetic. |
| Fundus Photography | Captures images of the optic nerve head and macula. Progressive cupping of the optic nerve is a sign of uncontrolled glaucoma. | Although many cameras do not require pharmacologic dilation, the brightness and resolution of the images may be affected by pupil size. |
| Ocular Coherence Tomography (OCT) | Measures retinal nerve fiber layer thickness using near-infrared light. A reference database is available for comparison. An abnormally thin nerve fiber layer or progressive thinning is a sign of uncontrolled glaucoma. | The device is not portable and is only available in clinic. |
| Visual Field | Testing is important to detect early peripheral visual field loss and to monitor for expansion of the scotoma. The amount of visual field loss determines the severity of disease and plays a role in setting the target IOP. | Traditional standard automated perimetry is not portable and is only available in clinic. Visual field monitoring at home can only be performed using a web-based program on a computer, a tablet, or with virtual reality glasses. |
| Artificial Intelligence (Deep Learning) | By self-learning via an artificial neural network, the technology has demonstrated remarkable accuracy in diagnosing glaucoma and monitoring for disease progression using fundus images, OCT, and visual field. | It is still under development and not available to the public yet. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, S.H.; Tsai, J.C. Telehealth and Screening Strategies in the Diagnosis and Management of Glaucoma. J. Clin. Med. 2021, 10, 3452. https://doi.org/10.3390/jcm10163452
Wong SH, Tsai JC. Telehealth and Screening Strategies in the Diagnosis and Management of Glaucoma. Journal of Clinical Medicine. 2021; 10(16):3452. https://doi.org/10.3390/jcm10163452
Chicago/Turabian StyleWong, Sze H., and James C. Tsai. 2021. "Telehealth and Screening Strategies in the Diagnosis and Management of Glaucoma" Journal of Clinical Medicine 10, no. 16: 3452. https://doi.org/10.3390/jcm10163452
APA StyleWong, S. H., & Tsai, J. C. (2021). Telehealth and Screening Strategies in the Diagnosis and Management of Glaucoma. Journal of Clinical Medicine, 10(16), 3452. https://doi.org/10.3390/jcm10163452





