Neurotransmitter Dysfunction in Irritable Bowel Syndrome: Emerging Approaches for Management
Abstract
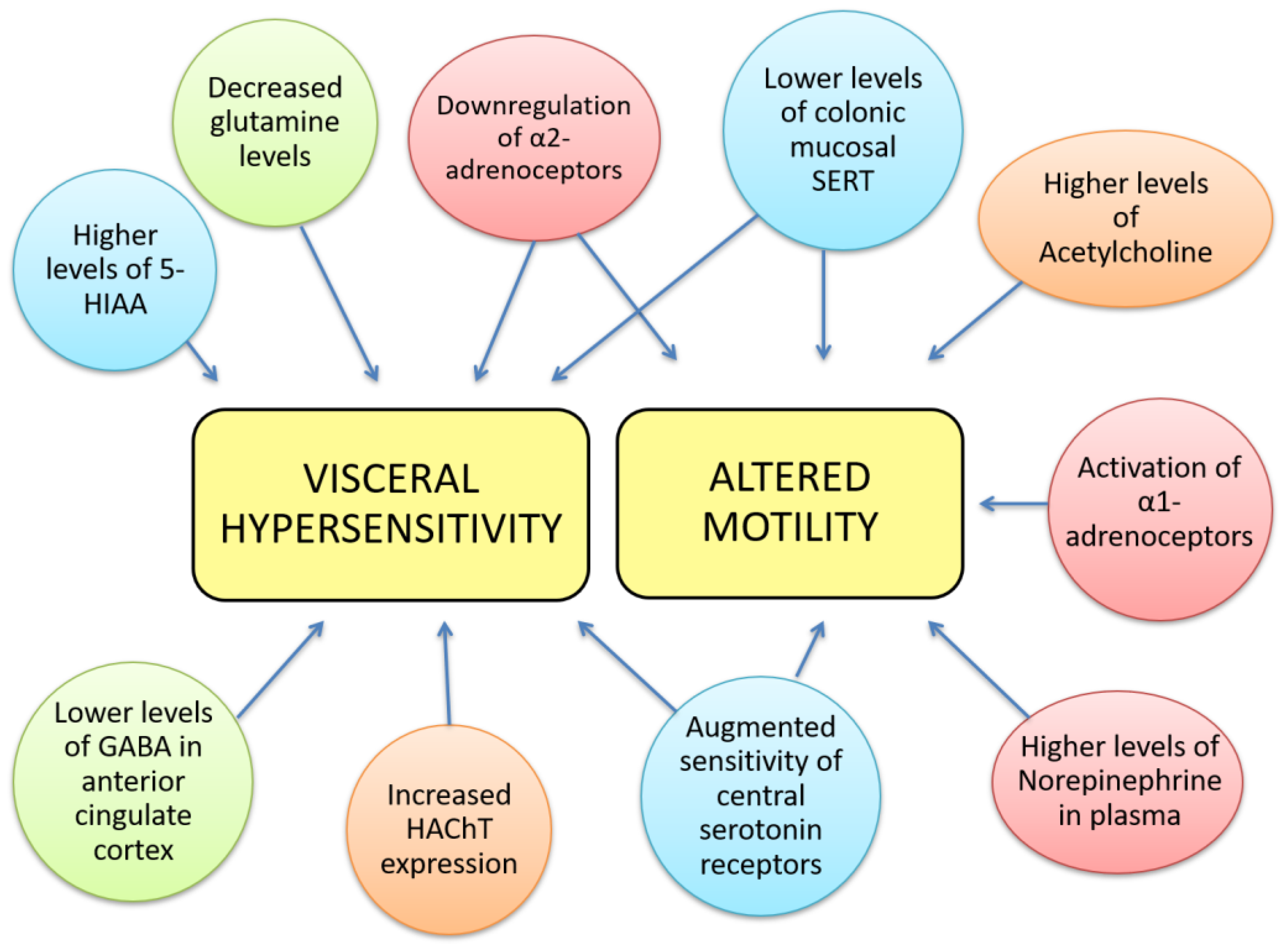
:1. Introduction
2. Norepinephrine
2.1. Norepinephrine in the Central Nervous System
2.2. Norepinephrine’s Role in the Gastrointestinal System
2.3. Norepinephrine as a Target for Treatment
3. Serotonin
3.1. Serotonin’s Role in the Central Nervous System
3.2. Serotonin’s Role in the Gastrointestinal System
3.3. The Serotonergic System as a Target for Treatment
4. Glutamate
4.1. Glutamate in the Central Nervous System
4.2. Glutamate’s Role in the Gastrointestinal Tract
4.3. Glutamate as a Target for Treatment
5. Gamma-Aminobutyric ACID
5.1. GABA in the Central Nervous System
5.2. GABA’s Role in the Gastrointestinal Tract
5.3. GABA as a Target for Treatment
6. Acetylcholine
6.1. Acetylcholine’s in the Central Nervous System
6.2. Acetylcholine’s Role in the Gastrointestinal System
6.3. Acetylcholine as a Target for Treatment
7. Other Neurotransmitters
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Drossman, D.A.; Hasler, W.L. Rome IV-Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology 2016, 150, 1257–1261. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M. Irritable bowel syndrome: Diagnosis and pathogenesis. World J. Gastroenterol. 2012, 18, 5151–5163. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kanazawa, M.; Kano, M.; Tashiro, M.; Fukudo, S. Relationship between sympathoadrenal and pituitary-adrenal response during colorectal distention in the presence of corticotropin- releasing hormone in patients with irritable bowel syndrome and healthy controls. PLoS ONE 2018, 13, e0199698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, A.E.; Labus, J.; Aziz, Q.; Tracey, I.; Kilpatrick, L.; Elsenbruch, S.; Schweinhardt, P.; Van Oudenhove, L.; Borsook, D. Role of brain imaging in disorders of brain-gut interaction: A Rome Working Team Report. Gut 2019, 68, 1701–1715. [Google Scholar] [CrossRef] [PubMed]
- Surdea-Blaga, T.; Băban, A.; Dumitrascu, D.L. Psychosocial determinants of irritable bowel syndrome. World J. Gastroenterol. 2012, 18, 616–626. [Google Scholar] [CrossRef]
- Wang, H.X.; Wang, Y.P. Gut Microbiota-brain Axis. Chin. Med. J. (Engl.) 2016, 129, 2373–2380. [Google Scholar] [CrossRef]
- Labus, J.S.; Osadchiy, V.; Hsiao, E.Y.; Tap, J.; Derrien, M.; Gupta, A.; Tillisch, K.; Le Nevé, B.; Grinsvall, C.; Ljungberg, M.; et al. Evidence for an association of gut microbial Clostridia with brain functional connectivity and gastrointestinal sensorimotor function in patients with irritable bowel syndrome, based on tripartite network analysis. Microbiome 2019, 7, 45. [Google Scholar] [CrossRef]
- Husebye, E.; Hellstrom, P.; Sundler, F.; Chen, J.; Midtvedt, T. Influence of microbial species on small intestinal myoelectric activity and transit in germ-free rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, 368–380. [Google Scholar] [CrossRef] [Green Version]
- Simrén, M.; Barbara, G.; Flint, H.J.; Spiegel, B.M.; Spiller, R.C.; Vanner, S.; Verdu, E.F.; Whorwell, P.J.; Zoetendal, E.G.; Rome Foundation Committee. Intestinal microbiota in functional bowel disorders: A Rome foundation report. Gut 2013, 62, 159–176. [Google Scholar] [CrossRef]
- Rajilić-Stojanović, M.; Jonkers, D.M.; Salonen, A.; Hanevik, K.; Raes, J.; Jalanka, J.; de Vos, W.M.; Manichanh, C.; Golic, N.; Enck, P.; et al. Intestinal Microbiota And Diet in IBS: Causes, Consequences, or Epiphenomena? Am. J. Gastroenterol. 2015, 110, 278–287. [Google Scholar] [CrossRef] [Green Version]
- Ford, A.; Sperber, A.; Corsetti, M.; Camilleri, M. Irritable bowel syndrome. Lancet 2020, 396, 1675–1688. [Google Scholar] [CrossRef]
- Borodovitsyna, O.; Flamini, M.; Chandler, D. Noradrenergic Modulation of Cognition in Health and Disease. Neural Plast. 2017, 2017, 6031478. [Google Scholar] [CrossRef] [Green Version]
- Clark, K.L.; Noudoost, B. The role of prefrontal catecholamines in attention and working memory. Front. Neural Circuits 2014, 8, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alcántara-Hernández, R.; Hernández-Méndez, A. Complejos moleculares de la señalización adrenérgica [Adrenergic signaling molecular complexes]. Gac. Med. Mex. 2018, 154, 223–235. [Google Scholar] [CrossRef]
- Ramos, B.P.; Arnsten, A.F. Adrenergic pharmacology and cognition: Focus on the prefrontal cortex. Pharmacol. Ther. 2007, 113, 523–536. [Google Scholar] [CrossRef] [Green Version]
- Griffen, T.C.; Maffei, A. GABAergic synapses: Their plasticity and role in sensory cortex. Front. Cell. Neurosci. 2014, 8, 91. [Google Scholar] [CrossRef] [Green Version]
- Alhayek, S.; Preuss, C.V. Beta 1 Receptors. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Choudhury, B.K.; Shi, X.Z.; Sarna, S.K. Norepinephrine mediates the transcriptional effects of heterotypic chronic stress on colonic motor function. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, 1238–1247. [Google Scholar] [CrossRef] [Green Version]
- Zou, N.; Lv, H.; Li, J.; Yang, N.; Xue, H.; Zhu, J.; Qian, J. Changes in brain G proteins and colonic sympathetic neural signaling in chronic-acute combined stress rat model of irritable bowel syndrome (IBS). Transl. Res. 2008, 152, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Camilleri, M.; Carlson, P.J.; Cremonini, F.; Ferber, I.; Stephens, D.; McKinzie, S.; Zinsmeister, A.R.; Urrutia, R. Association of distinct alpha(2) adrenoceptor and serotonin transporter polymorphisms with constipation and somatic symptoms in functional gastrointestinal disorders. Gut 2004, 53, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Naitou, K.; Shiina, T.; Kato, K.; Nakamori, H.; Sano, Y.; Shimizu, Y. Colokinetic effect of noradrenaline in the spinal defecation center: Implication for motility disorders. Sci. Rep. 2015, 5, 12623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jubelin, G.; Desvaux, M.; Schüller, S.; Etienne-Mesmin, L.; Muniesa, M.; Blanquet-Diot, S. Modulation of Enterohaemorrhagic Escherichia coli Survival and Virulence in the Human Gastrointestinal Tract. Microorganisms 2018, 6, 115. [Google Scholar] [CrossRef] [Green Version]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693 Pt B, 128–133. [Google Scholar] [CrossRef]
- Asano, Y.; Hiramoto, T.; Nishino, R.; Aiba, Y.; Kimura, T.; Yoshihara, K.; Koga, Y.; Sudo, N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, 1288–1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrasco, G.A.; Van De Kar, L.D. Neuroendocrine pharmacology of stress. Eur. J. Pharmacol. 2003, 463, 235–272. [Google Scholar] [CrossRef]
- Deechakawan, W.; Heitkemper, M.; Cain, K.; Burr, R.; Jarrett, M. Anxiety, depression, and catecholamine levels after self-management intervention in irritable bowel syndrome. Gastroenterol. Nurs. 2014, 37, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Burr, R.L.; Jarrett, M.E.; Cain, K.C.; Jun, S.; Heitkemper, M.M. Catecholamine and Cortisol Levels during Sleep in Women with Irritable Bowel Syndrome. Neurogastroenterol. Motil. 2010, 21, 1148-e97. [Google Scholar] [CrossRef] [Green Version]
- Pellissier, S.; Dantzer, C.; Mondillon, L.; Trocme, C.; Gauchez, A.S.; Ducros, V.; Mathieu, N.; Toussaint, B.; Fournier, A.; Canini, F.; et al. Relationship between vagal tone, cortisol, TNF-alpha, epinephrine and negative affects in Crohn’s disease and irritable bowel syndrome. PLoS ONE 2014, 9, e105328. [Google Scholar] [CrossRef] [Green Version]
- Elsenbruch, S.; Holtmann, G.; Oezcan, D.; Lysson, A.; Janssen, O.; Goebel, M.U.; Schedlowski, M. Are there alterations of neuroendocrine and cellular immune responses to nutrients in women with irritable bowel syndrome? Am. J. Gastroenterol. 2004, 99, 703–710. [Google Scholar] [CrossRef]
- Berman, S.; Suyenobua, B.; Naliboff, B.D.; Bueller, J.; Stains, J.; Wong, H.; Mandelkern, M.; Fitzgerald, L.; Ohning, G.; Gupta, A.; et al. Evidence for alterations in central noradrenergic signaling in irritable bowel syndrome. Neuroimage 2012, 63, 1854–1863. [Google Scholar] [CrossRef] [Green Version]
- Toral, M.; Robles-Vera, I.; De La Visitación, N.; Romero, M.; Yang, T.; Sánchez, M.; Gómez-Guzmán, M.; Jiménez, R.; Raizada, M.K.; Duarte, J. Critical Role of the Interaction Gut Microbiota—Sympathetic Nervous System in the Regulation of Blood Pressure. Front. Physiol. 2019, 10, 231. [Google Scholar] [CrossRef] [Green Version]
- Miner, L.H.; Jedema, H.P.; Moore, F.W.; Blakely, R.D.; Grace, A.A.; Susan, R. Chronic stress increases the plasmalemmal distribution of the norepinephrine transporter and the coexpression of tyrosine hydroxylase in norepinephrine axons in the prefrontal cortex. J. Neurosci. 2006, 26, 1571–1578. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Zou, N.; Li, J.; Lv, H.; Wei, J.; Fang, X.C.; Qian, J.M. Elevated expression of c-fos in central nervous system correlates with visceral hypersensitivity in irritable bowel syndrome (IBS): A new target for IBS treatment. Int. J. Color Dis. 2011, 26, 1035–1044. [Google Scholar] [CrossRef] [Green Version]
- Hubbard, C.S.; Labus, J.S.; Bueller, J.; Stains, J.; Suyenobu, B.; Dukes, G.E.; Kelleher, D.L.; Tillisch, K.; Naliboff, B.D.; Mayer, E.A. Corticotropin-releasing factor receptor 1 antagonist alters regional activation and effective connectivity in an emotional-arousal circuit during expectation of abdominal pain. J. Neurosci. 2011, 31, 12491–12500. [Google Scholar] [CrossRef] [Green Version]
- Sebastián, J.J.; Sebastián, B. Serotonin and the two brains: Conductor of orchestra of intestinal physiology and mood role in irritable bowel syndrome. Med. Nat. 2018, 12, 11–17. [Google Scholar]
- Spohn, S.N.; Mawe, G.M. Non-conventional features of peripheral serotonin signaling Stephanie. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 412–420. [Google Scholar] [CrossRef]
- Sharp, T.; Barnes, N.M. Central 5-HT receptors and their function; present and future. Neuropharmacology 2020, 177. [Google Scholar] [CrossRef] [PubMed]
- Göthert, M. Serotonin discovery and stepwise disclosure of 5-HT receptor complexity over four decades. Part I. General background and discovery of serotonin as a basis for 5-HT receptor identification. Pharmacol. Rep. 2013, 65, 771–786. [Google Scholar] [CrossRef]
- Green, A.R. Neuropharmacology of 5-hydroxytryptamine. Br. J. Pharmacol. 2006, 147 (Suppl. 1), S145–S152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammad-Zadeh, L.F.; Moses, L.; Gwaltney-Brant, S.M. Serotonin: A review. J. Vet. Pharmacol. Ther. 2008, 31, 187–199. [Google Scholar] [CrossRef]
- Songtachalert, T.; Roomruangwong, C.; Carvalho, A.F.; Bourin, M.; Maes, M. Anxiety Disorders: Sex Differences in Serotonin and Tryptophan Metabolism. Curr. Top. Med. Chem. 2018, 18, 1704–1715. [Google Scholar] [CrossRef]
- Zhuang, X.; Xu, H.; Fang, Z.; Xu, C.; Xue, C.; Hong, X. Platelet serotonin and serotonin transporter as peripheral surrogates in depression and anxiety patients. Eur. J. Pharmacol. 2018, 834, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Zmudzka, E.; Salaciak, K.; Sapa, J.; Pytka, K. Serotonin receptors in depression and anxiety: Insights from animal studies. Life Sci. 2018, 210, 106–124. [Google Scholar] [CrossRef]
- Mawe, G.M.; Hoffman, J.M. Serotonin Signaling in the Gastrointestinal Tract-Functions, dysfunctions, and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 473–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latorre, E.; Layunta, E.; Grasa, L.; Castro, M.; Alcalde, A.I.; Mesonero, J.E. Intestinal Serotonin Transporter Inhibition by Toll-Like Receptor 2 Activation. A Feedback Modulation. PLoS ONE 2016, 11, e0169303. [Google Scholar] [CrossRef] [Green Version]
- Thijssen, A.Y.; Mujagic, Z.; Jonkers, D.M.A.E.; Ludidi, S.; Keszthelyi, D.; Hesselink, M.A.; Clemens, C.H.; Conchillo, J.M.; Kruimel, J.W.; Masclee, A.A. Alterations in serotonin metabolism in the irritable bowel syndrome. Aliment. Pharmacol. Ther. 2016, 43, 272–282. [Google Scholar] [CrossRef]
- Gershon, M.D.; Tack, J. The Serotonin Signaling System: From Basic Understanding to Drug Development for Functional GI Disorders. Gastroenterology 2007, 132, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Otoshi, C.K.; Walwyn, W.M.; Tillakaratne, N.J.K.; Zhong, H.; Roy, R.R.; Edgerton, V.R. Distribution and localization of 5-HT(1A) receptors in the rat lumbar spinal cord after transection and deafferentation. J. Neurotrauma 2009, 26, 575–584. [Google Scholar] [CrossRef]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Brain-Gut Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry 2018, 9, 44. [Google Scholar] [CrossRef] [Green Version]
- Gunn, D.; Garsed, K.; Lam, C.; Singh, G.; Lingaya, M.; Wahl, V.; Niesler, B.; Henry, A.; Hall, I.P.; Whorwell, P.; et al. Abnormalities of mucosal serotonin metabolism and 5-HT3 receptor subunit 3C polymorphism in irritable bowel syndrome with diarrhoea predict responsiveness to ondansetron. Aliment. Pharmacol. Ther. 2019, 50, 538–546. [Google Scholar] [CrossRef] [Green Version]
- Chojnacki, C.; Błońska, A.; Kaczka, A.; Chojnacki, J.; Stępień, A.; Gąsiorowska, A. Evaluation of serotonin and dopamine secretion and metabolism in patients with irritable bowel syndrome. Pol. Arch. Intern. Med. 2018, 128, 711–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adler, J.R.; Vahora, I.S.; Tsouklidis, N.; Kumar, R.; Soni, R.; Khan, S. How Serotonin Level Fluctuation Affects the Effectiveness of Treatment in Irritable Bowel Syndrome. Cureus 2020, 12, e9871. [Google Scholar] [CrossRef]
- Keszthelyi, D.; Troost, F.; Jonkers, D.M.; van Eijk, H.M.; Dekker, J.; Buurman, W.A.; Masclee, A.A. Visceral hypersensitivity in irritable bowel syndrome: Evidence for involvement of serotonin metabolism--A preliminary study. Neurogastroenterol. Motil. 2015, 27, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Houghton, L.A.; Atkinson, W.; Whitaker, R.P.; Whorwell, P.J.; Rimmer, M.J. Increased platelet depleted plasma 5-hydroxytryptamine concentration following meal ingestion in symptomatic female subjects with diarrhoea predominant irritable bowel syndrome. Gut 2003, 52, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Huang, S.; Zhang, H.; Ye, H.; Chi, H.G.; Zou, Y.; Lv, R.X.; Zheng, X.B. Comparison of 5-hydroxytryptophan signaling pathway characteristics in diarrhea-predominant irritable bowel syndrome and ulcerative colitis. World J. Gastroenterol. 2016, 22, 3451–3459. [Google Scholar] [CrossRef]
- Shi, H.L.; Liu, C.H.; Ding, L.L.; Zheng, Y.; Fei, X.Y.; Lu, L.; Zhou, X.M.; Yuan, J.Y.; Xie, J.Q. Alterations in serotonin, transient receptor potential channels and protease-activated receptors in rats with irritable bowel syndrome attenuated by Shugan decoction. World J. Gastroenterol. 2015, 21, 4852–4863. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Atanasova, E.; Carlson, P.J.; Ahmad, U.; Kim, H.J.; Viramontes, B.E.; McKinzie, S.; Urrutia, R. Serotonin-transporter polymorphism pharmacogenetics in diarrhea-predominant irritable bowel syndrome. Gastroenterology 2002, 123, 425–432. [Google Scholar] [CrossRef]
- Kerckhoffs, A.P.M.; Linde, J.J.M.; Akkermans, L.M.A.; Samsom, M. SERT and TPH-1 mRNA expression are reduced in irritable bowel syndrome patients regardless of visceral sensitivity state in large intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G1053–G1060. [Google Scholar] [CrossRef] [Green Version]
- Jin, D.C.; Cao, H.L.; Xu, M.Q.; Wang, S.N.; Wang, Y.M.; Yan, F.; Wang, B.M. Regulation of the serotonin transporter in the pathogenesis of irritable bowel syndrome. World J. Gastroenterol. 2016, 22, 8137–8148. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M. Is there a SERT-ain association with IBS? Gut 2004, 53, 1396–1399. [Google Scholar] [CrossRef]
- Baj, A.; Moro, E.; Bistoletti, M.; Orlandi, V.; Crema, F.; Giaroni, C. Glutamatergic Signaling along The Microbiota-Gut-Brain Axis. Int. J. Mol. Sci. 2019, 20, 1482. [Google Scholar] [CrossRef] [Green Version]
- Christmas, D.M.; Badawy, A.A.; Hince, D.; Davies, S.J.; Probert, C.; Creed, T.; Smithson, J.; Afzal, M.; Nutt, D.J.; Potokar, J.P. Increased serum free tryptophan in patients with diarrhea-predominant irritable bowel syndrome. Nutr. Res. 2010, 30, 678–688. [Google Scholar] [CrossRef]
- Heitkemper, M.M.; Han, C.J.; Jarrett, M.E.; Gu, H.; Djukovic, D.; Shulman, R.J.; Raftery, D.; Henderson, W.A.; Cain, K.C. Serum Tryptophan Metabolite Levels During Sleep in Patients with and without Irritable Bowel Syndrome (IBS). Biol. Res. Nurs. 2016, 18, 193–198. [Google Scholar] [CrossRef] [Green Version]
- De Ponti, F. Pharmacology of Serotonin: What a Clinician Should Know. Gut 2004, 53, 1520–1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pytliak, M.; Vargová, V.; Mechírová, V.; Felšöci, M. Serotonin receptors—From molecular biology to clinical applications. Physiol. Res. 2011, 60, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Crowell, M.D. The role of serotonin in the pathophysiology of irritable bowel syndrome. Am. J. Manag. Care 2001, 7, 252–260. [Google Scholar] [CrossRef]
- Terry, N.; Margolis, K.G. Serotonergic Mechanisms Regulating the GI Tract—Experimental Evidence and Therapeutic Relevance. Handb. Exp. Pharmacol. 2017, 239, 319–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacy, B.E.; Nicandro, J.P.; Chuang, E.; Earnest, D.L. Alosetron use in clinical practice: Significant improvement in irritable bowel syndrome symptoms evaluated using the US Food and Drug Administration composite endpoint. Ther. Adv. Gastroenterol. 2018, 11, 1756284818771674. [Google Scholar] [CrossRef]
- El-Ayache, N.; Galligan, J.J. 5-HT3 receptor signaling in serotonin transporter-knockout rats a female sex-specific animal model of visceral hypersensitivity. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 316, 132–143. [Google Scholar] [CrossRef] [Green Version]
- Min, Y.W.; Rhee, P.L. The clinical potential of ramosetron in the treatment of irritable bowel syndrome with. Ther. Adv. Gastroenterol. 2015, 8, 136–142. [Google Scholar] [CrossRef] [Green Version]
- Eom, S.; Jung, W.; Lee, J.; Yeom, H.D.; Lee, S.; Kim, C.; Park, H.D.; Lee, J.H. Differential Regulation of Human Serotonin Receptor Type 3A by Chanoclavine and Ergonovine. Molecules 2021, 26, 1211. [Google Scholar] [CrossRef]
- Tack, J.; Coulie, B.; Wilmer, A.; Andrioli, A.; Janssens, J. Influence of sumatriptan on gastric fundus tone and on the perception of gastric distension in man. Gut 2000, 46, 468–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulak, A.; Paradowski, L. Effect of 5-HT1 agonist (sumatriptan) on anorectal function in irritable bowel syndrome patients. World J. Gastroenterol. 2006, 12, 1591–1596. [Google Scholar] [CrossRef]
- James, G.M.; Baldinger-Melich, P.; Phillippe, C.; Kranz, G.S.; Vanicek, T.; Hahn, A.; Gryglewski, G.; Hienert, M.; Spies, M.; Traub-Weidinger, T.; et al. Effects of Selective Serotonin Reuptake Inhibitors on Interregional Relation of Serotonin Transporter Availability in Major Depression. Front. Hum. Neurosci. 2017, 6, 48. [Google Scholar] [CrossRef] [Green Version]
- Grover, M.; Camilleri, M. Effects on gastrointestinal functions and symptoms of serotonergic psychoactive agents used in functional gastrointestinal diseases. J. Gastroenterol. 2013, 48, 177–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tack, J.; Broekaert, D.; Fischer, B.; Van Oudenhove, L.; Gevers, A.M.; Janssens, J. A controlled crossover study of the selective serotonin reuptake inhibitor citalopram in irritable bowel syndrome. Gut 2006, 55, 1095–1103. [Google Scholar] [CrossRef] [Green Version]
- Ladabaum, U.; Sharabidze, A.; Levin, T.R.; Zhao, W.K.; Chung, E.; Bacchetti, P.; Jin, C.; Grimes, B.; Pepin, C.J. Citalopram is not Effective Therapy for Non-Depressed Patients with Irritable Bowel Syndrome. Clin. Gastroenterol. Hepatol. 2010, 8, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, W.; Liao, Y.; Peng, Y.C.; Chang, C.H.; Lin, C.H.; Yeh, H.Z.; Chang, C.S. Relationship between use of selective serotonin reuptake inhibitors and irritable bowel syndrome: A population- based cohort study. World J. Gastroenterol. 2017, 23, 3513–3521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuiken, S.D.; Tytgat, G.N.J.; Boeckxstaens, G.E.E. The selective serotonin reuptake inhibitor fluoxetine does not change rectal sensitivity and symptoms in patients with irritable bowel syndrome: A double blind, randomized, placebo-controlled study. Clin. Gastroenterol. Hepatol. 2003, 1, 219–228. [Google Scholar] [CrossRef]
- Lacy, B.E.; Weiser, K.; De Lee, R. The Treatment of Irritable Bowel Syndrome. Ther. Adv. Gastroenterol. 2009, 2, 221–238. [Google Scholar] [CrossRef] [Green Version]
- Mujagic, Z.; Keszthelyi, D.; Thijssen, A.Y.; Jonkers, D.M.A.E.; Masclee, A.A.M. Editorial: Serotonin and irritable bowel syndrome—Reconciling pharmacological effects with basic biology; authors’ reply. Aliment. Pharmacol. Ther. 2016, 43, 643–653. [Google Scholar] [CrossRef]
- Zhou, Y.; Danbolt, N.C. Glutamate as a neurotransmitter in the healthy brain. J. Neural. Transm. 2014, 121, 799–817. [Google Scholar] [CrossRef] [Green Version]
- Fontana, A.C.K. Current approaches to enhance glutamate transporter function and expression. J. Neurochem. 2015, 134, 982–1007. [Google Scholar] [CrossRef]
- Nakamura, E.; Uneyama, H.; Torii, K. Gastrointestinal nutrient chemosensing and the gut-brain axis: Significance of glutamate signaling for normal digestion. J. Gastroenterol. Hepatol. 2013, 28 (Suppl. 4), 2–8. [Google Scholar] [CrossRef] [Green Version]
- Petroff, O.A.C. GABA and glutamate in the human brain. Neuroscientist 2002, 8, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Young, V.R.; Ajami, A.M. Glutamate: An amino acid of particular distinction. J. Nutr. 2000, 130, 892S–900S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peres, M.F.P.; Zukerman, E.; Soares, C.A.S.; Alonso, E.O.; Santos, B.F.C.; Faulhaber, M.H.W. Cerebrospinal fluid glutamate levels in chronic migraine. Cephalalgia 2004, 24, 735–739. [Google Scholar] [CrossRef]
- Ramos-Vicente, D.; Ji, J.; Gratacòs-Batlle, E.; Reig-Viader, R.; Luís, J.; Burguera, D.; Navas-Perez, E.; García-Fernández, J.; Fuentes-Prior, P.; Escriva, H.; et al. Metazoan evolution of glutamate receptors reveals unreported phylogenetic groups and divergent lineage-specific events. Elife 2018, 7, e35774. [Google Scholar] [CrossRef] [PubMed]
- Goodwani, S.; Saternos, H.; Alasmari, F.; Sari, Y. Metabotropic and ionotropic glutamate receptors as potential targets for the treatment of alcohol use disorder. Neurosci. Biobehav. Rev. 2017, 77, 14–31. [Google Scholar] [CrossRef]
- Hornby, P.J. Receptors and transmission in the brain-gut axis II. Excitatory amino acid receptors in the brain-gut axis. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G1055–G1060. [Google Scholar] [CrossRef] [Green Version]
- Mathews, D.; Henter, I.; Zarate, C.A. Targeting the Glutamatergic System to Treat Major Depressive Disorder. Drugs 2012, 72, 1313–1333. [Google Scholar] [CrossRef]
- Tsurugizawa, T.; Uematsu, A.; Nakamura, E.; Hasumura, M.; Hirota, M.; Kondoh, T.; Uneyama, H.; Torii, K. Mechanisms of neural response to gastrointestinal nutritive stimuli. Gastroenterology 2009, 137, 262–273. [Google Scholar] [CrossRef]
- Chandra, R.; Liddle, R.A. Modulation of pancreatic exocrine and endocrine secretion. Curr. Opin. Gastroenterol. 2013, 29, 517–522. [Google Scholar] [CrossRef] [Green Version]
- Julio-Pieper, M.; Hyland, N.P.; Bravo, J.A.; Dinan, T.G.; Cryan, J.F. A novel role for the metabotropic glutamate receptor-7: Modulation of faecal water content and colonic electrolyte transport in the mouse. Br. J. Pharmacol. 2010, 160, 367–375. [Google Scholar] [CrossRef] [Green Version]
- Uneyama, H. Nutritional and physiological significance of luminal glutamate-sensing in the gastrointestinal functions. Yakugaku Zasshi 2011, 131, 1699–1709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Icenhour, A.; Tapper, S.; Bednarska, O.; Witt, S.T.; Tisell, A.; Lundberg, P.; Elsenbruch, S.; Walter, S. Elucidating the putative link between prefrontal neurotransmission, functional connectivity, and affective symptoms in irritable bowel syndrome. Sci. Rep. 2019, 9, 13590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, R.E.; Sundgren, P.C.; Craig, A.D.; Kirshenbaum, E.; Sen, A.; Napadow, V.; Clauw, D.J. Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis Reum. 2009, 60, 3146–3152. [Google Scholar] [CrossRef] [Green Version]
- Bednarska, O.; Icenhour, A.; Tapper, S.; Witt, S.T.; Tisell, A.; Lundberg, P.; Elsenbruch, S.; Engström, M.; Walter, S. Reduced excitatory neurotransmitter levels in anterior insulae are associated with abdominal pain in irritable bowel syndrome. Pain 2019, 160, 2004–2012. [Google Scholar] [CrossRef] [Green Version]
- Shao, L.; Liu, Y.; Ciao, J.; Wang, Q.; Liu, F.; Ding, J. Activating metabotropic glutamate receptor-7 attenuates visceral hypersensitivity in neonatal maternally separated rats. Int. J. Mol. Med. 2019, 43, 761–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stachowicz, K.; Brañski, P.; Kłak, K.; van der Putten, H.; Cryan, J.F.; Flor, P.J.; Andrzej, P. Selective activation of metabotropic G-protein-coupled glutamate 7 receptor elicits anxiolytic-like effects in mice by modulating GABAergic neurotransmission. Behav. Pharmacol. 2008, 19, 597–603. [Google Scholar] [CrossRef]
- Filpa, V.; Moro, E.; Protasoni, M.; Crema, F.; Frigo, G.; Giaroni, C. Role of glutamatergic neurotransmission in the enteric nervous system and brain-gut axis in health and disease. Neuropharmacology 2016, 111, 14–33. [Google Scholar] [CrossRef]
- Holton, K.F.; Taren, D.L.; Thomson, C.A.; Bennett, R.M.; Jones, K.D. The effect of dietary glutamate on fibromyalgia and irritable bowel symptoms. Clin. Exp. Rheumatol. 2012, 30 (Suppl. 74), 10–17. [Google Scholar]
- Zhou, Q.; Verne, M.L.; Fields, J.Z.; Lefante, J.J.; Basra, S.; Salameh, H.; Verne, G.N. Randomised placebo-controlled trial of dietary glutamine supplements for postinfectious irritable bowel syndrome. Gut 2019, 68, 996–1002. [Google Scholar] [CrossRef]
- Ferrigno, A.; Berardo, C.; Di Pasqua, L.G.; Siciliano, V.; Richelmi, P.; Vairetti, M. Localization and role of metabotropic glutamate receptors subtype 5 in the gastrointestinal tract. World J. Gastroenterol. 2017, 23, 4500–4507. [Google Scholar] [CrossRef]
- Moloney, R.D.; O’Mahony, S.M.; Dinan, T.G.; Cryan, J. Stress-induced visceral pain: Toward animal models of irritable-bowel syndrome and associated comorbidities. Front. Psychiatry 2015, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- Meymandi, M.S.; Keyhanfar, F.; Sepehri, G.R.; Heravi, G.; Yazdanpanah, O. The Contribution of NMDA Receptors in Antinociceptive Effect of Pregabalin: Comparison of Two Models of Pain Assessment. Anesth. Pain Med. 2017, 7, e14602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolton, M.M.; Pittman, A.J.; Lo, D.C. Brain-derived neurotrophic factor differentially regulates excitatory and inhibitory synaptic transmission in hippocampal cultures. J. Neurosci. 2000, 20, 3221–3232. [Google Scholar] [CrossRef] [Green Version]
- Roth, F.C.; Draguhn, A. GABA metabolism and transport: Effects on synaptic efficacy. Neural Plast. 2012, 2012, 805830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuypers, K.; Maes, C.; Swinnen, S.P. Aging and GABA. Aging 2018, 10, 1186–1187. [Google Scholar] [CrossRef]
- Siucinska, E. Γ-Aminobutyric acid in adult brain: An update. Behav. Brain Res. 2019, 376, 112224. [Google Scholar] [CrossRef]
- Olsen, R.W.; Sieghart, W. GABA A receptors: Subtypes provide diversity of function and pharmacology. Neuropharmacology 2009, 56, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Tomita, S. Molecular constituents and localization of the ionotropic GABA receptor complex in vivo. Curr. Opin. Neurobiol. 2019, 57, 81–86. [Google Scholar] [CrossRef]
- Li, Y.; Xiang, Y.; Lu, W.; Liu, C.; Li, J. A novel role of intestine epithelial GABAergic signaling in regulating intestinal fluid secretion. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G453–G460. [Google Scholar] [CrossRef] [Green Version]
- Fischer, A.U.; Nicolas, I.C.M.; Deller, T.; Del Turco, D.; Fisch, J.O.; Griesemer, D.; Kattler, K.; Maraslioglu, A.; Roemer, V.; Xu-Friedman, M.A.; et al. GABA is a modulator, rather than a classical transmitter, in the medial nucleus of the trapezoid body—Lateral superior olive sound localization circuit. J. Physiol. 2019, 8, 2269–2295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naffaa, M.M.; Hung, S.; Chebib, M.; Johnston, G.A.R.; Hanrahan, J.R. GABA-ρ receptors: Distinctive functions and molecular pharmacology. Br. J. Pharmacol. 2017, 174, 1881–1894. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Danbolt, N.C. GABA and Glutamate Transporters in Brain. Front. Endocrinol. 2013, 4, 165–179. [Google Scholar] [CrossRef] [Green Version]
- Loeza-Alcocer, E.; McPherson, T.P.; Gold, M.S. Peripheral GABA receptors regulate colonic afferent excitability and visceral nociception. J. Physiol. 2019, 597, 3425–3439. [Google Scholar] [CrossRef]
- Hyland, N.P.; Cryan, J.F. A Gut Feeling about GABA: Focus on GABA B receptors. Front. Pharmacol. 2010, 1, 124. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, S.; Ahuja, V.; Paul, J. Dysregulation of GABAergic Signalling Contributes in the Pathogenesis of Diarrhea-predominant Irritable Bowel Syndrome. Neurogastroenterol. Motil. 2018, 24, 422–430. [Google Scholar] [CrossRef]
- Harper, D.E.; Ichesco, E.; Schrepf, A.; Halvorson, M.; Puiu, T.; Clauw, D.J.; Harris, R.E.; Harte, S.E.; MAPP Research Network. Relationships between brain metabolite levels, functional connectivity, and negative mood in urologic chronic pelvic pain syndrome patients compared to controls: A MAPP research network study. NeuroImage Clin. 2018, 17, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.M.; Liu, S.B.; Chen, T.; Koga, K.; Zhang, T.; Li, Y.Q.; Zhuo, M. Effects of NB001 and gabapentin on irritable bowel syndrome-induced behavioral anxiety and spontaneous pain. Mol. Brain. 2014, 7, 47. [Google Scholar] [CrossRef] [Green Version]
- Saito, Y.A.; Almazar, A.E.; Tilkes, K.E.; Choung, R.S.; Van Norstrand, M.D.; Schleck, C.D.; Zinsmeister, A.R.; Talley, N.J. Randomised Clinical Trial: Pregabalin Versus Placebo for Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2019, 49, 389–397. [Google Scholar] [CrossRef]
- Needham, K.; Bron, R.; Hunne, B.; Nguyen, T.V.; Turner, K.; Nash, M.; Furnes, S.J.B. Identification of subunits of voltage-gated calcium channels and actions of pregabalin on intrinsic primary afferent neurons in the guinea-pig ileum. Neurogastroenterol. Motil. 2010, 22, e301–e308. [Google Scholar] [CrossRef]
- Nissen, T.; Brock, C.; Lykkesfeldt, J.; Lindström, E.; Hultin, L. Pharmacological modulation of colorectal distension evoked potentials in conscious rats. Neuropharmacology 2018, 15, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Sun, Q.; Sun, X.; Chen, D.; Wei, C.; Yu, X.; Liu, C.; Li, Y.; Li, J. Activation of GABAA Receptors in Colon Epithelium Exacerbates Acute Colitis. Front. Immunol. 2018, 9, 987. [Google Scholar] [CrossRef] [Green Version]
- Park, K.B.; Ji, G.E.; Park, M.S.; Oh, S.H. Expression of Rice Glutamate Decarboxylase in Bifidobacterium Longum Enhances γ-Aminobutyric Acid Production. Biotechnol. Lett. 2005, 27, 1681–1684. [Google Scholar] [CrossRef] [PubMed]
- Minervini, F.; Bilancia, M.; Siragusa, S.; Gobbetti, M.; Caponio, F. Fermented goats’ milk produced with selected multiple starters as a potentially functional food. Food Microbiol. 2009, 26, 559–564. [Google Scholar] [CrossRef]
- Racke, K.; Matthiesen, S. The airway cholinergic system: Physiology and pharmacology. Pulm. Pharmacol. Ther. 2004, 17, 181–198. [Google Scholar] [CrossRef]
- Oda, A.; Tanaka, H. Activities of nicotinic acetylcholine receptors modulate neurotransmission and synaptic architecture. Neural Regen. Res. 2014, 9, 2128–2131. [Google Scholar] [CrossRef]
- Kruse, A.C.; Hu, J.; Pan, A.C.; Arlow, D.H.; Rosenbaum, D.M.; Rosemond, E.; Green, H.F.; Liu, T.; Chae, P.S.; Dror, R.O.; et al. Structure and dynamics of the M3 muscarinic acetylcholine receptor. Nature 2012, 482, 552–556. [Google Scholar] [CrossRef] [Green Version]
- Brown, D.A. Acetylcholine and cholinergic receptors. Brain Neurosci. Adv. 2019, 3, 2398212818820506. [Google Scholar] [CrossRef]
- Verma, S.; Kumar, A.; Tripathi, T.; Kumar, A. Muscarinic and nicotinic acetylcholine receptor agonists: Current scenario in Alzheimer’s disease therapy. J. Pharm. Pharmacol. 2018, 70, 985–993. [Google Scholar] [CrossRef] [Green Version]
- Picciotto, M.R.; Higley, M.J.; Mineur, Y.S. Acetylcholine as a neuromodulator: Cholinergic signaling shapes nervous system function and behavior. Neuron 2012, 76, 116–129. [Google Scholar] [CrossRef] [Green Version]
- Yu, A.J.; Dayan, P. Uncertainty, neuromodulation, and attention. Neuron 2005, 46, 681–692. [Google Scholar] [CrossRef] [Green Version]
- Cox, M.A.; Bassi, C.; Saunders, M.E.; Nechanitzky, R.; Morgado-Palacin, I.; Zheng, C.; Mak, T.W. Beyond neurotransmission: Acetylcholine in immunity and inflammation. J. Intern. Med. 2020, 287, 120–133. [Google Scholar] [CrossRef]
- Grossberg, S. Acetylcholine Neuromodulation in Normal and Abnormal Learning and Memory: Vigilance Control in Waking, Sleep, Autism, Amnesia and Alzheimer’s Disease. Front. Neural Circuits 2017, 11, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Deb, B.; Prichard, D.O.; Bharucha, A.E. Constipation and Fecal Incontinence in the Elderly. Curr Gastroenterol. Rep. 2020, 22, 54. [Google Scholar] [CrossRef]
- Russell, J.P.; Mohammadi, E.; Ligon, C.; Latorre, R.; Johnson, A.C.; Hoang, B.; Krull, D.; Ho, M.W.; Eidam, H.S.; DeMartino, M.P.; et al. Enteric RET inhibition attenuates gastrointestinal secretion and motility via cholinergic signaling in rat colonic mucosal preparations. Neurogastroenterol. Motil. 2019, 31, e13479. [Google Scholar] [CrossRef] [Green Version]
- Hirota, C.L.; McKay, D.M. Cholinergic regulation of epithelial ion transport in the mammalian intestine. Br. J. Pharmacol. 2006, 149, 463–479. [Google Scholar] [CrossRef]
- Bonaz, B.; Bazin, T.; Pellissier, S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef] [Green Version]
- Leng, Y.X.; Wei, Y.Y.; Chen, H.; Zhou, S.P.; Yang, Y.L.; Duan, L.P. Alteration of cholinergic and peptidergic neurotransmitters in rat ileum induced by acute stress following transient intestinal infection is mast cell dependent. Chin. Med. J. 2010, 123, 227–233. [Google Scholar]
- Hod, K.; Sperber, A.D.; Maharshak, N.; Ron, Y.; Shapira, I.; David, Z.; Rogowski, O.; Berliner, S.; Shenhar-Tsarfaty, S.; Dekel, R. Serum cholinesterase activity is elevated in female diarrhea-predominant irritable bowel syndrome patients compared to matched controls. Neurogastroenterol. Motil. 2018, 30, e13464. [Google Scholar] [CrossRef]
- Fujikawa, Y.; Tominaga, K.; Tanaka, F.; Tanigawa, T.; Watanabe, T.; Fujiwara, Y.; Arakawa, T. Enteric glial cells are associated with stress-induced colonic hyper-contraction in maternally separated rats. Neurogastroenterol. Motil. 2015, 27, 1010–1023. [Google Scholar] [CrossRef] [PubMed]
- Miampamba, M.; Million, M.; Yuana, P.Q.; Larauchea, M.; Tache, Y. Water avoidance stress activates colonic myenteric neurons in female rats. Neuroreport 2007, 18, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.A.; Edogawa, S.; Sundt, W.J.; Dyer, R.B.; Dalenberg, D.A.; Mazzone, A.; Singh, R.J.; Moses, N.; Smyrk, T.C.; Weber, C.; et al. Constipation-Predominant Irritable Bowel Syndrome Females Have Normal Colonic Barrier and Secretory Function. Am. J. Gastroenterol. 2017, 112, 913–923. [Google Scholar] [CrossRef] [Green Version]
- Medland, J.E.; Pohl, C.S.; Edwards, L.L.; Frandsen, S.; Bagley, K.; Li, Y.; Moeser, A.J. Early life adversity in piglets induces long-term upregulation of the enteric cholinergic nervous system and heightened, sex-specific secretomotor neuron responses. Neurogastroenterol. Motil. 2016, 28, 1317–1329. [Google Scholar] [CrossRef] [Green Version]
- Balestra, B.; Vicini, R.; Cremon, C.; Zecchi, L.; Dothel, G.; Vasina, V.; De Giorgio, R.; Paccapelo, A.; Pastoris, O.; Stanghellini, V.; et al. Colonic mucosal mediators from patients with irritable bowel syndrome excite enteric cholinergic motor neurons. Neurogastroenterol. Motil. 2012, 24, 1118-e570. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.J.; Yu, B.P. Role of High-affinity Choline Transporter 1 in Colonic Hypermotility in a Rat Model of Irritable Bowel Syndrome. J. Neurogastroenterol. Motil. 2018, 24, 643–655. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Lin, M.; Pan, Y.; Yu, B. Blockage of High-Affinity Choline Transporter Increases Visceral Hypersensitivity in Rats with Chronic Stress. Gastroenterol. Res. Pract. 2018, 2018, 9252984. [Google Scholar] [CrossRef] [Green Version]
- Lin, M.J.; Yu, B.P. Upregulation of the high-affinity choline transporter in colon relieves stress-induced hiperalgesia. J. Pain Res. 2018, 11, 1971–1982. [Google Scholar] [CrossRef] [Green Version]
- Bharucha, A.E.; Ravi, K.; Zinsmeister, A.R. Comparison of selective M3 and nonselective muscarinic receptor antagonists on gastrointestinal transit and bowel habits in humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G215–G219. [Google Scholar] [CrossRef]
- Dumitrascu, D.L.; Chira, A.; Bataga, S.; Diculescu, M.; Drug, V.; Gheorghe, C.; Goldis, A.; Nedelcu, L.; Porr, P.J.; Sporea, I.; et al. The Use of Mebeverine in Irritable Bowel Syndrome. A Position Paper of the Romanian Society of Neurogastroenterology based on Evidence. J. Gastrointestin. Liver Dis. 2014, 23, 431–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, L.; Lai, Y.; Lu, W.; Li, B.; Fan, H.; Yan, Z.; Gong, C.; Wan, X.; Wu, J.; Huang, D.; et al. Pinaverium Reduces Symptoms of Irritable Bowel Syndrome in a Multicenter, Randomized, Controlled Trial. Clin. Gastroenterol. Hepatol. 2015, 13, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- De Schryver, A.M.P.; Samsom, M. New developments in the treatment of Irritable Bowel Syndrome. Scand. J. Gastroenterol. 2000, 35 (Suppl. 232), 38–42. [Google Scholar]
- Bharucha, A.E.; Seide, B.; Guan, G.; Andrews, C.; Zinsmeister, A.R. Effect of tolterodine, on gastrointestinal transit and bowel habits in healthy subjects. Neurogastroenterol. Motil. 2008, 20, 643–648. [Google Scholar] [CrossRef]
- Bulchandani, S.; Toozs-Hobson, P.; Parsons, M.; McCooty, S.; Perkins, K.; Latthe, P. Effect of anticholinergics on the overactive bladder and bowel domain of the electronic personal assessment questionnaire (ePAQ). Int. Urogynecol. J. 2015, 26, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Houghton, L.A.; Rogers, J.R.; Whorwell, P.J.; Campbell, F.C.; Williams, N.S.; Goka, J. Zamifenacin (UK-76, 654), a potent gut M 3 selective muscarinic antagonist, reduces colonic motor activity in patients with irritable bowel syndrome. Aliment. Pharmacol. Ther. 1997, 11, 561–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, B.S.; Camilleri, M.; Busciglio, I.; Carlson, P.; Szarka, L.A.; Burton, D.; Zinsmeister, A.R. Pharmacogenetic Trial of a Cannabinoid Agonist Shows Reduced Bowel Syndrome. Gastroenterology 2011, 141, 1638–1647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousavi, T.; Nikfar, S.; Abdollahi, M. An update on efficacy and safety considerations for the latest drugs used to treat irritable bowel syndrome. Expert Opin. Drug Metab. Toxicol. 2020, 16, 583–604. [Google Scholar] [CrossRef]
- Lieberman, P. The basics of histamine biology. Ann. Allergy Asthma Immunol. 2011, 106, S2–S5. [Google Scholar] [CrossRef]
- Keshteli, A.H.; Madsen, K.L.; Mandal, R.; Boeckxstaens, G.E.; Bercik, P.; De Palma, G.; Reed, D.E.; Wishart, D.; Vanner, S.; Dieleman, L.A. Comparison of the metabolomic profiles of irritable bowel syndrome patients with ulcerative colitis patients and healthy controls: New insights into pathophysiology and potential biomarkers. Aliment. Pharmacol. Ther. 2019, 49, 723–732. [Google Scholar] [CrossRef]
- Hattori, T.; Watanabe, S.; Kano, M.; Kanazawa, M.; Fukudo, S. Differential responding of autonomic function to histamine H 1 antagonism in irritable bowel syndrome. Neurogastroenterol. Motil. 2010, 22, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Deiteren, A.; De Man, J.G.; Ruyssers, N.E.; Moreels, T.G.; Pelckmans, P.A.; De Winter, B.Y. Histamine H4 and H1 receptors contribute to postin fl ammatory visceral hypersensitivity. Gut 2014, 63, 1873–1882. [Google Scholar] [CrossRef]
- Wouters, M.M.; Balemans, D.; Van Wanrooy, S.; Dooley, J.; Cibert-Goton, V.; Alpizar, Y.A.; Valdez-Morales, E.E.; Nasser, Y.; Van Veldhoven, P.P.; Vanbrabant, W.; et al. Histamine Receptor H1-Mediated Sensitization of TRPV1 Mediates Visceral Hypersensitivity and Symptoms in Patients with Irritable Bowel Syndrome. Gastroenterology 2016, 150, 875–887.e9. [Google Scholar] [CrossRef] [Green Version]
- Klooker, T.K.; Braak, B.; Koopman, K.E.; Welting, O.; Wouters, M.M.; van der Heide, S.; Schemann, M.; Bischoff, S.C.; van den Wijngaard, R.M.; Boeckxstaens, G.E. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut 2010, 59, 1213–1221. [Google Scholar] [CrossRef]
- Tack, J.F.; Miner, P.B.; Fischer, L.; Harris, M.S. Randomised clinical trial: The safety and efficacy of AST-120 in non-constipating irritable bowel syndrome—A double-blind, placebo-controlled study. Aliment. Pharmacol. Ther. 2011, 34, 868–877. [Google Scholar] [CrossRef]
- Zizzo, M.G.; Bellanca, A.; Amato, A.; Serio, R. Opposite effects of dopamine on the mechanical activity of circular and longitudinal muscle of human colon. Neurogastroenterol. Motil. 2020, 32, e13811. [Google Scholar] [CrossRef]
- Prakash, S.; Prakash, A. Dopa responsive irritable bowel syndrome: Restless bowel syndrome or a gastrointestinal variant of restless legs syndrome? BMJ Case Rep. 2021, 14, e240686. [Google Scholar] [CrossRef]
- Nozu, T.; Miyagishi, S.; Kumei, S.; Nozu, R.; Takakusaki, K.; Okumura, T. Metformin inhibits visceral allodynia and increased gut permeability induced by stress in rats. J. Gastroenterol. Hepatol. 2019, 34, 186–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nozu, T.; Miyagishi, S.; Nozu, R.; Takakusaki, K.; Okumura, T. Butyrate inhibits visceral allodynia and colonic hyperpermeability in rat models of irritable bowel syndrome. Sci. Rep. 2019, 9, 19603. [Google Scholar] [CrossRef] [PubMed]
- Latorre, E.; Mesonero, J.E.; Harries, L.W. Alternative splicing in serotonergic system: Implications in neuropsychiatric disorders. J. Psychopharmacol. 2019, 33, 1352–1363. [Google Scholar] [CrossRef]
- Carco, C.; Young, W.; Gearry, R.B.; Talley, N.J.; McNabb, W.C.; Roy, N.C. Increasing Evidence That Irritable Bowel Syndrome and Functional Gastrointestinal Disorders Have a Microbial Pathogenesis. Front. Cell. Infect. Microbiol. 2020, 10, 468. [Google Scholar] [CrossRef] [PubMed]
- Halkjær, S.I.; Christensen, A.H.; Lo, B.Z.S.; Browne, P.D.; Günther, S.; Hansen, L.H.; Petersen, A.M. Faecal microbiota transplantation alters gut microbiota in patients with irritable bowel syndrome: Results from a randomised, double-blind placebo-controlled study. Gut 2018, 67, 2107–2115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Neurotransmitter | Drug | Receptor | Effect | Pharmacological Use | References |
|---|---|---|---|---|---|
| SEROTONIN | CISAPRIDE | 5-HT4 agonist and 5-HT3 antagonist | Prokinetic | Use for the treatment of Gastroesophageal reflux, functional dyspepsia and gastroparesis | Pytliak et al. 2011 [65] |
| TEGASEROD | 5-HT4 agonist | Prokinetic | Use for the treatment of IBS-C | Crowell et al. 2001 [66] | |
| VELUSETRAG | 5-HT4 agonist | Prokinetic | Clinical trials have to be done for its approvement | Terry et al. 2017 [67] | |
| PRUCALOPRIDE | 5-HT4 agonist | Prokinetic | Used for the treatment of IBS-C | Terry et al. 2017 [67] | |
| ALOSETRON | 5-HT3 antagonist | Decreases GI motility | Approved in the USA for the treatment of IBS-D | Lacy et al. 2018 [68] | |
| ONDASETRON | 5-HT3 antagonist | Antiemetic, it reduces abdominal pain | Used as antiemetic | Min et al. 2015 [70] | |
| RAMOSETRON | 5-HT3 antagonist | Antiemetic | Used as antiemetic in Asia | Min et al. 2015 [70] | |
| SUMATRIPTAN | 5-HT1B/D agonist | Delays gastric emptying | Many side effects to be approved | Mulak et al. 2006 [73] | |
| GABA | PREGABALIN | GABA analogous | Analgesic and anxiolytic | Use for the treatment of neuropathic pain | Saito et al. 2019 [122] |
| GABAPENTIN | GABA analogous | Analgesic and anxiolytic | Use for the treatment of neuropathic pain | Zhang et al. 2014 [121] | |
| CGP7930 | GABA-B receptor agonist | Reduces visceral pain | Clinical trials have to be done for its approvement | Hyland et al. 2010 [118] | |
| BACLOFEN | GABA-B receptor agonist | Reduces visceromotor response | Use for the treatment of spasticity and muscle spasms | Nissen et al. 2018 [124] | |
| GLUTAMATE | RILUZOLE | Glutamate reuptake activator | Improves visceral hypersensitivity | Use for the treatment of Amyotrophic Lateral Sclerosis | Moloney et al. 2015 [105] |
| MPEP | mGluR5 antagonist | Reduces allodynia | Clinical trials have to be done for its approvement | Ferrigno et al. 2017 [104] | |
| AMN082 | mGluR7 agonist | Reduces visceral hypersensitivitiy induced by colorectal distension | Clinical trials have to be done for its approvement | Shao et al. 2019 [99] | |
| ACETYLCHOLINE | ZAMIFENACIN | Partially selected muscarinic M3 antagonist | Decreases colonic contractility | Clinical trials have to be done for its approvement | Houghton et al. 1997 [157] |
| TOLTERODINE | Non-selective muscarinic antagonist | Induces constipation | Use for the treatment of overactive bladder syndrome | Bharucha et al. 2008 [155] | |
| MEBEVERINE | Muscarinic antagonist | Improves bowel transit and abdominal pain | Approved in Australia for IBS treatment | Dumitrascu et al. 2014 [152] | |
| DARIFENACIN | M3 antagonist | Improves IBS bowel habits | Use for the treatment of overactive bladder syndrome | De Schryver et al. 2000 [154] | |
| PINAVERIUM | Anticholinergic effect | Antispasmodic | Approved for the treatment of functional gastrointestinal diseases, as IBS | Zheng et al. 2015 [153] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gros, M.; Gros, B.; Mesonero, J.E.; Latorre, E. Neurotransmitter Dysfunction in Irritable Bowel Syndrome: Emerging Approaches for Management. J. Clin. Med. 2021, 10, 3429. https://doi.org/10.3390/jcm10153429
Gros M, Gros B, Mesonero JE, Latorre E. Neurotransmitter Dysfunction in Irritable Bowel Syndrome: Emerging Approaches for Management. Journal of Clinical Medicine. 2021; 10(15):3429. https://doi.org/10.3390/jcm10153429
Chicago/Turabian StyleGros, Mónica, Belén Gros, José Emilio Mesonero, and Eva Latorre. 2021. "Neurotransmitter Dysfunction in Irritable Bowel Syndrome: Emerging Approaches for Management" Journal of Clinical Medicine 10, no. 15: 3429. https://doi.org/10.3390/jcm10153429
APA StyleGros, M., Gros, B., Mesonero, J. E., & Latorre, E. (2021). Neurotransmitter Dysfunction in Irritable Bowel Syndrome: Emerging Approaches for Management. Journal of Clinical Medicine, 10(15), 3429. https://doi.org/10.3390/jcm10153429






