Impact of Biological Agents on Postsurgical Complications in Inflammatory Bowel Disease: A Multicentre Study of Geteccu
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Definitions
- −
- Postoperative complications: the presence of superficial wound infection, intraabdominal infection, urinary tract infection, bacteraemia, respiratory infection, fever above 38 °C of unknown origin, anastomosis leak, mechanical obstruction, postoperative ileus, bleeding, thrombosis, fistula or evisceration during the 30 days after the date of surgery.
- −
- −
- Low albumin levels: albumin levels lower than 3 g/dL at any point during the two weeks before the date of surgery [20].
- −
- Low cholesterol levels: serum cholesterol level below 160 mg/dL at any point during the two weeks prior to surgery [10].
- −
- Smoking habit: current smokers included individuals who actively smoked more than seven cigarettes per week, former smokers included individuals who quit smoking more than six months ago and non-smokers included those patients who had never smoked before [21].
- −
- Nutritional risk: a weight loss >10% within six months or body mass index (BMI) <18.5 kg/m2 [22].
2.4. Statistical Analysis
3. Results
3.1. Patient Population
3.2. Postoperative Complications
3.3. Postoperative Complications According to Exposure
3.4. Predictive Factors Associated with the Appearance of Postoperative Complications
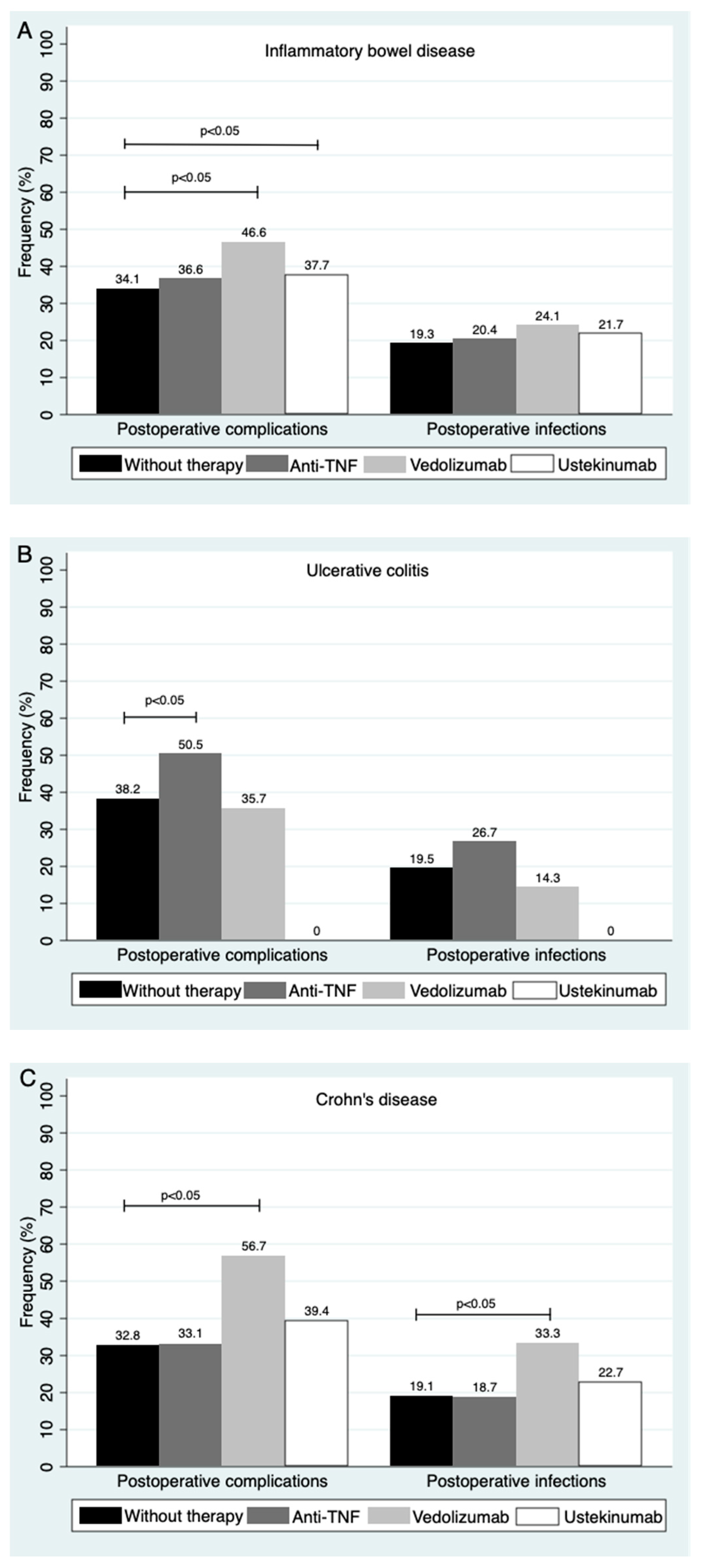
3.5. Type of Biological Therapy during the Preoperative Period and Its Impact on Postoperative Complications
3.6. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Present, D.H.; Rutgeerts, P.; Targan, S.; Hanauer, S.B.; Mayer, L.; van Hogezand, R.A.; Podolsky, D.K.; Sands, B.E.; Braakman, T.; DeWoody, K.L.; et al. Infliximab for the treatment of fistulas in patients with crohn’s disease. N. Engl. J. Med. 1999, 340, 1398–1405. [Google Scholar] [CrossRef]
- Bouguen, G.; Peyrin-Biroulet, L. Surgery for adult Crohn’s disease: What is the actual risk? Gut 2011, 60, 1178–1181. [Google Scholar] [CrossRef]
- van Overstraeten, A.D.; Wolthuis, A.; D’Hoore, A. Surgery for Crohn’s disease in the era of biologicals: A reduced need or delayed verdict? World J. Gastroenterol. 2012, 18, 3828–3832. [Google Scholar] [CrossRef] [PubMed]
- Ramadas, A.V.; Gunesh, S.; Thomas, G.A.O.; Williams, G.T.; Hawthorne, A.B. Natural history of Crohn’s disease in a population-based cohort from Cardiff (1986–2003): A study of changes in medical treatment and surgical resection rates. Gut 2010, 59, 1200–1206. [Google Scholar] [CrossRef] [PubMed]
- Frolkis, A.D.; Dykeman, J.; Negrón, M.E.; Debruyn, J.; Jette, N.; Fiest, K.M.; Frolkis, T.; Barkema, H.W.; Rioux, K.P.; Panaccione, R.; et al. Risk of surgery for inflammatory bowel diseases has decreased over time: A systematic review and meta-analysis of population-based studies. Gastroenterology 2013, 145, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Fumery, M.; Seksik, P.; Auzolle, C.; Munoz-Bongrand, N.; Gornet, J.M.; Boschetti, G.; Cotte, E.; Buisson, A.; Dubois, A.; Pariente, B.; et al. Postoperative complications after ileocecal resection in Crohn’s disease: A prospective study from the REMIND Group. Am. J. Gastroenterol. 2017, 112, 337–345. [Google Scholar] [CrossRef] [PubMed]
- de Silva, S.; Ma, C.; Proulx, M.C.; Crespin, M.; Kaplan, B.S.; Hubbard, J.; Prusinkiewicz, M.; Fong, A.; Panaccione, R.; Ghosh, S.; et al. Postoperative complications and mortality following colectomy for ulcerative colitis. Clin. Gastroenterol. Hepatol. 2011, 9, 972–980. [Google Scholar] [CrossRef]
- Subramanian, V.; Saxena, S.; Kang, J.Y.; Pollok, R.C.G. Preoperative steroid use and risk of postoperative complications in patients with inflammatory bowel disease undergoing abdominal surgery. Am. J. Gastroenterol. 2008, 103, 2373–2381. [Google Scholar] [CrossRef]
- Zhou, W.; Cao, Q.; Qi, W.; Xu, Y.; Liu, W.; Xiang, J.; Xia, B. Prognostic nutritional index predicts short-term postoperative outcomes after bowel resection for Crohn’s disease. Nutr. Clin. Pract. 2017, 32, 92–97. [Google Scholar] [CrossRef]
- Huang, W.; Tang, Y.; Nong, L.; Sun, Y. Risk factors for postoperative intra-abdominal septic complications after surgery in Crohn’s disease: A meta-analysis of observational studies. J. Crohn’s Colitis 2015, 9, 293–301. [Google Scholar] [CrossRef]
- Aberra, F.N.; Lewis, J.D.; Hass, D.; Rombeau, J.L.; Osborne, B.; Lichtenstein, G.R. Corticosteroids and immunomodulators: Postoperative infectious complication risk in inflammatory bowel disease patients. Gastroenterology 2003, 125, 320–327. [Google Scholar] [CrossRef]
- Argollo, M.C.; Kotze, P.G.; Spinelli, A.; Gomes, T.N.F.; Danese, S. The impact of biologics in surgical outcomes in ulcerative colitis. Best Pract. Res. Clin. Gastroenterol. 2018, 32, 79–87. [Google Scholar] [CrossRef]
- Chang, M.I.; Cohen, B.L.; Greenstein, A.J. A review of the impact of biologics on surgical complications in Crohn’s disease. Inflamm. Bowel. Dis. 2015, 21, 1472–1477. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shim, H.H.; Ma, C.; Kotze, P.G.; Panaccione, R. Pre-operative exposure to Ustekinumab: A risk factor for postoperative complications in Crohn’s disease (CD)? Curr. Drug Targets 2019, 20, 1369–1372. [Google Scholar] [CrossRef] [PubMed]
- Moosvi, Z.; Duong, J.T.; Bechtold, M.L.; Nguyen, D.L. Systematic review and meta-analysis: Preoperative vedolizumab and postoperative complications in patients with IBD. South Med. J. 2021, 114, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Lucendo, A.J.; Roncero, Ó.; Serrano-Duenas, M.T.; Hervías, D.; Alcázar, L.M.; Verdejo, C.; Laserna-Mendieta, E.; Lorente, R.; Arias, Á. Effects of anti–TNF-α therapy on hemoglobin levels and anemia in patients with inflammatory bowel disease. Dig. Liver Dis. 2020, 52, 400–407. [Google Scholar] [CrossRef]
- Portela, F.; Lago, P.; Cotter, J.; Gonçalves, R.; Vasconcelos, H.; Ministro, P.; Lopes, S.; Eusébio, M.; Morna, H.; Cravo, M.; et al. Anaemia in patients with inflammatory bowel disease—A nationwide cross-sectional study. Digestion 2016, 93, 214–220. [Google Scholar] [CrossRef]
- Yamamoto, T.; Allan, R.N.; Keighley, M.R.B. Risk factors for intra-abdominal sepsis after surgery in Crohn’s disease. Dis. Colon. Rectum. 2000, 43, 1141–1145. [Google Scholar] [CrossRef]
- Nunes, T.; Etchevers, M.J.; Merino, O.; Gallego, S.; García-Sánchez, V.; Marín-Jiménez, I.; Menchén, L.; Acosta, M.B.; Bastida, G.; García, S.; et al. Does smoking influence Crohn’s disease in the biologic era? the TABACROHN study. Inflamm. Bowel Dis. 2013, 19, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Escher, J.; Hébuterne, X.; Kłęk, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN Guideline ESPEN practical guideline: Clinical nutrition in inflammatory bowel disease. Clin. Nutr. 2019, 39, 632–653. [Google Scholar] [CrossRef] [PubMed]
- Diamond, A.; Sekhon, J.S. Genetic matching for estimating causal effects: A general multivariate matching method for achieving balance in observational studies. Rev. Econ. Stat. 2013, 95, 932–945. [Google Scholar] [CrossRef]
- Billioud, V.; Ford, A.C.; Tedesco, E.; Colombel, J.F.; Roblin, X.; Peyrin-Biroulet, L. Preoperative use of anti-TNF therapy and postoperative complications in inflammatory bowel diseases: A meta-analysis. J. Crohn’s Colitis 2013, 27, 853–867. [Google Scholar] [CrossRef] [PubMed]
- Narula, N.; Charleton, D.; Marshall, J.K. Meta-analysis: Peri-operative anti-TNFα treatment and post-operative complications in patients with inflammatory bowel disease. Aliment Pharmacol. Ther. 2013, 37, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yang, L.; An, P.; Zhou, B.; Liu, G. Meta-analysis: The influence of preoperative infliximab use on postoperative complications of Crohn’s disease. Inflamm. Bowel. Dis. 2019, 25, 261–269. [Google Scholar] [CrossRef]
- Quaresma, A.B.; Yamamoto, T.; Kotze, P.G. Biologics and surgical outcomes in Crohn’s disease: Is there a direct relationship? Therap. Adv. Gastroenterol. 2020, 13, 1756284820931738. [Google Scholar] [CrossRef]
- El-Hussuna, A.; Qvist, N.; Zangenberg, M.S.; Langkilde, A.; Siersma, V.; Hjort, S.; Gögenur, I. No effect of anti-TNF-α agents on the surgical stress response in patients with inflammatory bowel disease undergoing bowel resections: A prospective multi-center pilot study 11 medical and health sciences 1103 clinical sciences. BMC Surg. 2018, 18, 1–10. [Google Scholar] [CrossRef]
- Cohen, B.L.; Fleshner, P.; Kane, S.V.; Herfarth, H.H.; Palekar, N.; Farraye, F.A.; Leighton, J.A.; Katz, J.; Cohen, R.D.; Gerich, M.E.; et al. 415a—Anti-tumor necrosis factor therapy is not associated with post-operative infection: Results from prospective cohort of ulcerative colitis and Crohn’s disease patients undergoing surgery to identify risk factors for postoperative Infection I (Puccini). Gastroenterology 2019, 156, S-80. [Google Scholar] [CrossRef]
- Lightner, A.L.; McKenna, N.P.; Alsughayer, A.; Harmsen, W.S.; Taparra, K.; Parker, M.E.; Raffals, L.E.; Loftus, E.V., Jr. Biologics and 30-day postoperative complications after abdominal operations for Crohn’s disease: Are there differences in the safety profiles? Dis. Colon. Rectum. 2019, 62, 1352–1362. [Google Scholar] [CrossRef]
- Novello, M.; Stocchi, L.; Holubar, S.; Shawki, S.; Lipman, J.; Gorgun, E.; Hull, T.; Steele, S.R. Surgical outcomes of patients treated with ustekinumab vs. vedolizumab in inflammatory bowel disease: A matched case analysis. Int. J. Colorectal. Dis. 2019, 34, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Komaki, Y.; Patel, N.; Komaki, F.; Aelvoet, A.S.; Tran, A.L.; Pekow, J.; Dalal, S.; Cohen, R.D.; Cannon, L.; et al. Risk of postoperative complications among inflammatory bowel disease patients treated preoperatively with Vedolizumab. Am. J. Gastroenterol. 2017, 112, 1423–1429. [Google Scholar] [CrossRef]
- Kim, J.Y.; Zaghiyan, K.; Lightner, A.; Fleshner, P. Risk of postoperative complications among ulcerative colitis patients treated preoperatively with vedolizumab: A matched case-control study. BMC Surg. 2020, 20, 46. [Google Scholar] [CrossRef]
- Shah, R.S.; Bachour, S.; Jia, X.; Holubar, S.D.; Hull, T.L.; Achkar, J.P.; Philpott, J.; Qazi, T.; Rieder, F.; Cohen, B.L.; et al. Hypoalbuminemia, not biologic exposure, is associated with postoperative complications in Crohn’s disease patients undergoing ileocolic resection. J. Crohn’s Colitis 2021, 15, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Shim, H.H.; Ma, C.; Kotze, P.G.; Seow, C.H.; Al-Farhan, H.; Al-Darmaki, A.K.; Pang, J.X.Q.; Fedorak, R.N.; Devlin, S.M.; Dieleman, L.A.; et al. Preoperative Ustekinumab Treatment is not associated with increased postoperative complications in Crohn’s disease: A Canadian multi-centre observational Cohort Study. J. Can. Assoc. Gastroenterol. 2018, 1, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Law, C.C.Y.; Koh, D.; Bao, Y.; Jairath, V.; Narula, N. Risk of postoperative infectious complications from medical therapies in inflammatory bowel disease: A systematic review and meta-analysis. Inflamm. Bowel Dis. 2020, 26, 1796–1807. [Google Scholar] [CrossRef] [PubMed]
- Law, C.C.; Bell, C.; Koh, D.; Bao, Y.; Jairath, V.; Narula, N. Risk of postoperative infectious complications from medical therapies in inflammatory bowel disease. Cochrane Database Syst. Rev. 2020, 10, CD013256. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.; Dubinsky, M.; Melmed, G.; Vasiliauskas, E.; Berel, D.; McGovern, D.; Ippoliti, A.; Shih, D.; Targan, S.; Fleshner, P. The impact of preoperative serum anti-TNFα therapy levels on early postoperative outcomes in inflammatory bowel disease surgery. Ann. Surg. 2015, 261, 487–496. [Google Scholar] [CrossRef]
- Lightner, A.L.; Mathis, K.L.; Tse, C.S.; Pemberton, J.H.; Shen, B.; Kochlar, G.; Singh, A.; Dulai, P.S.; Eisenstein, S.; Sandborn, W.J.; et al. Postoperative outcomes in Vedolizumab-treated patients undergoing major abdominal operations for inflammatory bowel disease: Retrospective multicenter Cohort Study. Inflamm. Bowel Dis. 2018, 24, 871–876. [Google Scholar] [CrossRef]
- Novello, M.; Stocchi, L.; Steele, S.R.; Holubar, S.D.; Duraes, L.C.; Kessler, H.; Shawki, S.; Hull, L.T. Case-matched comparison of postoperative outcomes following surgery for inflammatory bowel disease after exposure to Vedolizumab vs. other biologics. J. Crohn’s Colitis 2020, 14, 185–191. [Google Scholar] [CrossRef]
- Ferrante, M.; van Overstraeten, A.D.; Schils, N.; Moens, A.; van Assche, G.; Wolthuis, A.; Vermeire, S.; D’Hoore, A. Perioperative use of vedolizumab is not associated with postoperative infectious complications in patients with ulcerative colitis undergoing colectomy. J. Crohn’s Colitis 2017, 11, 1353–1361. [Google Scholar] [CrossRef]
- Lightner, A.L.; McKenna, N.P.; Tse, C.S.; Hyman, N.; Smith, R.; Ovsepyan, G.; Fleshner, P.; Crowell, K.; Koltun, W.; Ferrante, M.; et al. Postoperative outcomes in Ustekinumab- treated patients undergoing abdominal operations for Crohn’s disease. J. Crohn’s Colitis 2018, 12, 402–407. [Google Scholar] [CrossRef]
- Lightner, A.L.; Grass, F.; Alsughayer, A.; Petersen, M.M.; Raffals, L.E.; Loftus, E.V. Postoperative outcomes in Ustekinumab-treated patients undergoing abdominal operations for Crohn’s disease: Single-center series. Crohn’s Colitis 360 2019, 1, otz018. [Google Scholar] [CrossRef]
- Subramanian, V.; Pollok, R.C.G.; Kang, J.Y.; Kumar, D. Systematic review of postoperative complications in patients with inflammatory bowel disease treated with immunomodulators. Br. J. Surg. 2006, 93, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.F.; Loftus, E.V.; Tremaine, W.J.; Pemberton, J.H.; Wolff, B.G.; Young-Fadok, T.; Harmsen, W.S.; Schleck, C.D.; Sandborn, W.J. Early postoperative complications are not increased in patients with Crohn’s disease treated perioperatively with infliximab or immunosuppressive therapy. Am. J. Gastroenterol. 2004, 99, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Furst, M.B.; Stromberg, B.V.; Blatchford, G.J.; Christensen, M.A.; Thorson, A.G. Colonic anastomoses: Bursting strength after corticosteroid treatment. Dis. Colon Rectum. 1994, 37, 12–15. [Google Scholar] [CrossRef]
- Stoner, P.L.; Kamel, A.; Ayoub, F.; Tan, S.; Iqbal, A.; Glover, S.C.; Zimmermann, E.M. Perioperative care of patients with inflammatory bowel disease: Focus on nutritional support. Gastroenterol. Res. Pract. 2018, 2018, 7890161. [Google Scholar] [CrossRef]
- Nguyen, G.C.; Elnahas, A.; Jackson, T.D. The impact of preoperative steroid use on short-term outcomes following surgery for inflammatory bowel disease. J. Crohn’s Colitis 2014, 8, 1661–1667. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.C.; Du, L.; Chong, R.Y.; Jackson, T.D. Hypoalbuminaemia and postoperative outcomes in inflammatory bowel disease: The NSQIP surgical cohort. J. Crohn’s Colitis 2019, 13, 1433–1438. [Google Scholar] [CrossRef]
- Liang, H.; Jiang, B.; Manne, S.; Lissoos, T.; Bennett, D.; Dolin, P. Risk factors for postoperative infection after gastrointestinal surgery among adult patients with inflammatory bowel disease: Findings from a large observational US cohort study. JGH Open 2018, 23, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Michailidou, M.; Nfonsam, V.N. Preoperative anemia and outcomes in patients undergoing surgery for inflammatory bowel disease. Am. J. Surg. 2018, 215, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Bruns, E.R.J.; Borstlap, W.A.; van Duijvendijk, P.; van der Zaag-Loonen, H.J.; Buskens, C.J.; van Munster, B.C.; Bemelman, W.A.; Tanis, P.J. The association of preoperative anemia and the postoperative course and oncological outcome in patients undergoing rectal cancer surgery: A multicenter snapshot study. Dis. Colon. Rectum. 2019, 62, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Luo, J.J.; Pei, K.Y.; Khan, S.A.; Wang, X.X.; Zhao, Z.X.; Yang, M.; Johnson, C.H.; Wang, X.S.; Zhang, Y. Joint effect of pre-operative anemia and perioperative blood transfusion on outcomes of colon-cancer patients undergoing colectomy. Gastroenterol. Rep. 2020, 8, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Kessler, H.; Mudter, J.; Hohenberger, W. Recent results of laparoscopic surgery in inflammatory bowel disease. World J. Gastroenterol. 2011, 17, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.S.; Patel, M.S.; Goldfarb, M.; Ortega, A.; Ault, G.T.; Kaiser, A.M.; Senagore, A.J. Elective versus emergency surgery for ulcerative colitis: A National Surgical Quality Improvement Program analysis. Am. J. Surg. 2013, 205, 333–338. [Google Scholar] [CrossRef]
- Maartense, S.; Dunker, M.S.; Slors, J.F.M.; Cuesta, M.A.; Pierik, E.G.J.M.; Gouma, D.J.; Hommes, D.W.; Sprangers, M.A.; Bemelman, W.A. Laparoscopic-assisted versus open ileocolic resection for Crohn’s disease: A randomized trial. Ann. Surg. 2006, 243, 143–149. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Rivero, M.; Martín-Arranz, M.D.; Garcia Sánchez, V.; Castro, M.; Barrio, J.; de Francisco, R.; Barreiro-de Acosta, M.; Juliá, B.; Cea-Calvo, L.; et al. Perioperative management and early complications after intestinal resection with ileocolonic anastomosis in Crohn’s disease: Analysis from the PRACTICROHN study. Gastroenterol. Rep. 2019, 7, 168–175. [Google Scholar] [CrossRef]
- Ramos Fernández, M.; Rivas Ruiz, F.; Fernández López, A.; Loinaz Segurola, C.; Fernández Cebrián, J.M.; de la Portilla de Juan, F.C. Reactive protein as a predictor of anastomotic leakage in colorectal surgery. Comparison between open and laparoscopic surgery. Cir. Esp. 2017, 95, 529–535. [Google Scholar] [CrossRef]
- Tøttrup, A.; Erichsen, R.; Sværke, C.; Laurberg, S.; Srensen, H.T. Thirty-day mortality after elective and emergency total colectomy in Danish patients with inflammatory bowel disease: A population-based nationwide cohort study. BMJ Open 2012, 2, e000823. [Google Scholar] [CrossRef]
- Zangenberg, M.S.; Horesh, N.; Kopylov, U.; El-Hussuna, A. Preoperative optimization of patients with inflammatory bowel disease undergoing gastrointestinal surgery: A systematic review. Int. J. Colorectal Dis. 2017, 32, 1663–1676. [Google Scholar] [CrossRef]
- Ma, C.; Crespin, M.; Proulx, M.C.; DeSilva, S.; Hubbard, J.; Prusinkiewicz, M.; Nguyen, G.C.; Panaccione, R.; Ghosh, S.; Myers, R.P.; et al. Postoperative complications following colectomy for ulcerative colitis: A validation study. BMC Gastroenterol. 2012, 12, 39. [Google Scholar] [CrossRef] [PubMed]
| Exposed Cohort (n = 711) | Non-Exposed Cohort (n = 824) | p-Value | |
|---|---|---|---|
| Gender: male | 51.5 (363) | 53.8 (443) | 0.3 |
| Median age at surgery (years) (mean, SD) | 43.57 (13.48) | 46.26 (15.36) | <0.001 * |
| Median age at IBD onset (years) (mean, SD) | 33.43 (13.74) | 37.40 (16.03) | <0.001 * |
| Mean duration of IBD until surgery (years) (mean, SD) | 10.13 (8.56) | 8.85 (9.05) | <0.05 * |
| Smoking habit (%, n) | <0.05 * | ||
| - Current smokers | 25.2 (170) | 31.6 (242) | |
| - Former smokers | 25.2 (170) | 18.8 (144) | |
| - Non smokers | 49.7 (336) | 49.5 (379) | |
| Type of disease (%, n) | 0.76 | ||
| - Ulcerative colitis | 18.76(132) | 18.1 (149) | |
| - Crohn’s disease | 80.6 (573) | 80.7 (665) | |
| - IBD-unclassified | 0.8 (6) | 1.2 (10) | |
| Location of IBD (%, n) | |||
| - Ulcerative proctitis (UC) | 3.6 (5) | 0.6 (1) | 0.08 |
| - Left-side colitis (UC) | 23.2 (32) | 18.2 (29) | |
| -Extensive colitis (UC) | 73.2 (101) | 81.1 (129) | |
| - Ileum (CD) | 49.2 (282) | 53.4 (355) | 0.13 |
| - Colon (CD) | 5.8 (33) | 7.1 (47) | |
| - Ileocolonic (CD) | 45.0 (258) | 39.6 (263) | |
| - Upper disease (CD) | 10.8 (62) | 7.5 (50) | |
| Behaviour of CD at surgery (%, n) | <0.05 * | ||
| - Inflammatory | 13.3 (76) | 16.5 (110) | |
| - Stricturing | 56.5 (324) | 46.3 (308) | |
| - Penetrating | 30.2 (173) | 37.1 (247) | |
| Perianal disease (yes) (%, n) | 24.4 (140) | 17.1 (14) | <0.05 * |
| Extraintestinal manifestations (yes) (%, n) | 21.9 (156) | 15.7 (129) | <0.05 * |
| Prior surgery for IBD (yes) (%, n) | 31.1 (221) | 35.8 (295) | 0.05 |
| Hospital admission within 3 months prior to surgery (yes) (%, n) | 43.7 (310) | 32.2 (265) | <0.001 * |
| Partial Mayo Score (mean, SD) | 6.89 (2.27) | 4.2 (3.04) | <0.001 * |
| Harvey-Bradshaw Index (mean, SD) | 6.56 (3.59) | 6.38 (3.28) | 0.47 |
| Weight at surgery (kg) (mean, SD) | 64.18 (14.23) | 65.99 (14.49) | 0.08 |
| Weight loss between 6 months and 2 weeks prior to surgery (kg) (mean, SD) | 4.52 (8.73) | 3.09 (7.18) | <0.05 * |
| BMI at surgery (mean, SD) | 22.81 (4.53) | 23.31 (4.48) | 0.13 |
| Haemoglobin (gr/dL) (mean, SD) | 12.19 (1.98) | 12.63 (2.11) | <0.001 * |
| Lymphocyte count (/mL) (mean, SD) | 1895.51 (1096.27) | 1702.5 (1013.08) | <0.001 * |
| C-reactive protein (mg/dL) (mean, SD) | 4.53 (13.61) | 5.05 (8.43) | 0.47 |
| Cholesterol (mg/dL) (mean, SD) | 149.60 (43.40) | 153.66 (43.52) | 0.23 |
| Prealbumin (mg/dL) (mean, SD) | 21.84 (9.20) | 21.41 (10.35) | 0.76 |
| Albumin (mg/dL) (mean, SD) | 3.52 (0.70) | 3.59 (0.78) | 0.14 |
| Malnutrition (yes) (%, n) | 43.7 (151) | 37.53 (158) | 0.08 |
| Blood transfusion (yes) (%, n) | 13.5 (96) | 6.9 (57) | <0.001 * |
| Intravenous iron treatment (yes) (%, n) | 22.9 (163) | 13.0 (107) | <0.001 * |
| Type of preoperative nutrition support (%, n) | <0.001 * | ||
| - No supplementary nutrition | 61.6 (438) | 77.3 (637) | |
| - Enteral | 20.4 (145) | 11.5 (95) | |
| - Parenteral | 9.3 (66) | 8.0 (66) | |
| - Enteral and parenteral | 8.7 (62) | 3.2 (26) | |
| Corticosteroids (yes) (%, n) | 38.1 (271) | 28.1 (231) | <0.001 * |
| Immunomodulators (yes) (%, n) | 43.7 (311) | 24.4 (201) | <0.001 * |
| Exposed Cohort | Non-Exposed Cohort | p-Value | |
|---|---|---|---|
| Overall complications (%, n) | 37.6 (267) | 34.0 (280) | 0.15 |
| Superficial wound infection (%, n) | 7.7 (55) | 7.5 (62) | 0.8 |
| Intraabdominal infection (%, n) | 10.4 (74) | 9.3 (77) | 0.5 |
| Other infections (%, n) | 3.4 (24) | 3.9 (32) | 0.5 |
| Anastomosis leak (%, n) | 7.0 (50) | 6.9 (57) | 0.9 |
| Bowel obstruction (%, n) | 2.0 (14) | 1.2 (10) | 0.2 |
| Postoperative ileus (%, n) | 6.5 (46) | 4.6 (38) | 0.1 |
| Bleeding (%, n) | 5.2 (37) | 5.2 (43) | 0.9 |
| Thrombosis (%, n) | 0.4 (3) | 0.7 (6) | 0.4 |
| Fistula (%, n) | 0.8 (6) | 1.0 (8) | 0.8 |
| Evisceration (%, n) | 0.1 (1) | 0.73 (6) | 0.09 |
| Postoperative Complications (547 Surgeries) | Non-Complications (988 Surgeries) | p-Value | ||
|---|---|---|---|---|
| Gender (%, n) | Men | 59.4 (325) | 48.7 (481) | <0.001 * |
| Age at surgery (years) (%, n) | Younger than 40 | 34.9 (191) | 44.3 (438) | <0.001 * |
| Between 40 and 60 | 48.0 (262) | 40.7 (402) | ||
| Older than 60 | 17.2 (94) | 15.0 (148) | ||
| Smoking habit (%, n) | Current smoker | 27.8 (141) | 29.0 (271) | 0.84 |
| Former smoker | 22.45(114) | 21.4 (200) | ||
| Non smoker | 49.7 (252) | 49.6 (463) | ||
| Type of disease (%, n) | Ulcerative colitis | 21.6 (118) | 16.5 (163) | <0.05 * |
| Crohn’s disease | 76.6 (419) | 82.9 (819) | ||
| IBD-unclassified | 1.8 (10) | 0.6 (6) | ||
| Location at surgery (%, n) | Extensive colitis | 83.0 (98) | 74.2 (122) | 0.21 |
| Left-side colitis | 15.2 (18) | 23.3 (38) | ||
| Proctitis | 1.7 (2) | 2.5 (4) | ||
| Ileal (L1) | 44.4 (186) | 55.1 (451) | <0.001 * | |
| Colic (L2) | 47.3 (198) | 39.4 (323) | ||
| Ileocolic (L3) | 8.4 (35) | 5.5 (45) | ||
| Upper (L4) | 8.1 (34) | 9.5 (78) | ||
| Behaviour (only CD) (%, n) | Inflammatory | 18.1 (76) | 13.4 (110) | 0.07 |
| Stricturing | 48.0 (201) | 52.6 (431) | ||
| Penetrating | 33.9 (142) | 33.9 (278) | ||
| Perianal disease (%, n) | Yes | 19.9 (109) | 16.7 (165) | 0.12 |
| No | 80.1 (438) | 83.3 (823) | ||
| Prior IBD surgery (%, n) | Yes | 35.3 (193) | 32.7 (323) | 0.3 |
| No | 64.7 (355) | 67.3 (665) | ||
| Prior non-IBD surgery (%, n) | Yes | 18.1 (99) | 17.5 (173) | 0.77 |
| No | 81.9 (448) | 82.5 (815) | ||
| Extraintestinal manifestations (%, n) | Yes | 19.9 (109) | 17.8 (176) | 0.3 |
| No | 80.0 (438) | 82.2 (812) | ||
| Severe anaemia (%, n) | Yes | 17.7 (81) | 10.0 (81) | <0.001 * |
| No | 82.3 (376) | 90.0 (732) | ||
| Low albumin levels (%, n) | Yes | 28.7 (93) | 14.9 (84) | <0.001 * |
| No | 71.3 (231) | 85.1 (479) | ||
| Low cholesterol levels (%, n) | Yes | 64.9 (163) | 55.8 (235) | <0.05 * |
| No | 35.1 (88) | 44.2. (186) | ||
| Intravenous iron treatment (%, n) | Yes | 21.4 (117) | 15.5 (153) | <0.05 * |
| No | 78.6 (430) | 84.5 (835) | ||
| Blood transfusion (%, n) | Yes | 15.2 (83) | 7.1 (70) | <0.001 * |
| No | 84.8 (464) | 92.9 (918) | ||
| Type of nutritional support (%, n) | Enteral | 41.4 (72) | 58.7 (168) | <0.001 * |
| Parenteral | 33.3 (58) | 25.9 (74) | ||
| Enteral and parenteral | 25.3 (44) | 15.4 (44) | ||
| Glucocorticoids (%, n) | Yes | 36.3 (198) | 30.8 (304) | <0.05 * |
| No | 63.7 (347) | 69.2 (683) | ||
| Immunomodulator therapy (%, n) | Yes | 32.9 (180) | 33.6 (332) | 0.78 |
| No | 67.1 (367) | 66.4 (656) | ||
| Biological therapy (%, n) | Yes | 48.8 (267) | 44.9 (444) | 0.15 |
| No | 51.2 (280) | 55.1 (544) | ||
| Temporality of surgery (%, n) | Urgent | 23.8 (130) | 15.3 (151) | <0.001 * |
| Elective | 76.2 (417) | 84.7 (837) | ||
| Surgical approach (%, n) | Laparotomy | 73.5 (402) | 67.3 (665) | <0.05 * |
| Laparoscopy | 26.5 (145) | 32.7 (323) | ||
| Hospital level | 2nd, 3rd or 4st category | 42.7 (234) | 36.6 (362) | <0.05 * |
| 5th Category | 57.2 (313) | 63.4 (626) | ||
| Unadjusted Odds Ratio | 95% Confidence Interval | |
|---|---|---|
| Ileocecal resection | 0.58 | 0.47–0.73 |
| Bowel resection | 0.90 | 0.63–1.27 |
| Strictureplasty | 1.68 | 0.70–4.03 |
| Partial colonic resection | 1.45 | 1.03–2.04 |
| Subtotal colectomy | 1.62 | 1.56–2.30 |
| Total colectomy | 1.72 | 1.06–2.79 |
| Proctectomy | 1.93 | 1.29–2.90 |
| Pouch surgery | 1.69 | 1.05–2.70 |
| Postoperative Complications | Adjusted Odds Ratio | 95% Confidence Interval |
| Exposure to biological therapy | 1.24 | 0.97–1.58 |
| Gender: male | 1.54 | 1.21–1.95 |
| Severe anaemia | 1.83 | 1.30–2.57 |
| Urgent surgery | 1.61 | 1.21–2.16 |
| Surgical approach: laparotomy | 1.45 | 1.11–1.90 |
| Hospital level: 5th category | 0.69 | 0.54–0.88 |
| Postoperative Infections | Adjusted Odds Ratio | Confidence Interval 95% |
| Exposure to biological therapy | 1.50 | 1.03–2.17 |
| C-reactive protein | 1.04 | 1.01–1.06 |
| Hypoalbuminemia | 1.92 | 1.27–2.90 |
| Surgical approach: laparotomy | 2.15 | 1.39–3.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García, M.J.; Rivero, M.; Miranda-Bautista, J.; Bastón-Rey, I.; Mesonero, F.; Leo-Carnerero, E.; Casas-Deza, D.; Cagigas Fernández, C.; Martin-Cardona, A.; El Hajra, I.; et al. Impact of Biological Agents on Postsurgical Complications in Inflammatory Bowel Disease: A Multicentre Study of Geteccu. J. Clin. Med. 2021, 10, 4402. https://doi.org/10.3390/jcm10194402
García MJ, Rivero M, Miranda-Bautista J, Bastón-Rey I, Mesonero F, Leo-Carnerero E, Casas-Deza D, Cagigas Fernández C, Martin-Cardona A, El Hajra I, et al. Impact of Biological Agents on Postsurgical Complications in Inflammatory Bowel Disease: A Multicentre Study of Geteccu. Journal of Clinical Medicine. 2021; 10(19):4402. https://doi.org/10.3390/jcm10194402
Chicago/Turabian StyleGarcía, María José, Montserrat Rivero, José Miranda-Bautista, Iria Bastón-Rey, Francisco Mesonero, Eduardo Leo-Carnerero, Diego Casas-Deza, Carmen Cagigas Fernández, Albert Martin-Cardona, Ismael El Hajra, and et al. 2021. "Impact of Biological Agents on Postsurgical Complications in Inflammatory Bowel Disease: A Multicentre Study of Geteccu" Journal of Clinical Medicine 10, no. 19: 4402. https://doi.org/10.3390/jcm10194402
APA StyleGarcía, M. J., Rivero, M., Miranda-Bautista, J., Bastón-Rey, I., Mesonero, F., Leo-Carnerero, E., Casas-Deza, D., Cagigas Fernández, C., Martin-Cardona, A., El Hajra, I., Hernández-Aretxabaleta, N., Pérez-Martínez, I., Fuentes-Valenzuela, E., Jiménez, N., Rubín de Célix, C., Gutiérrez, A., Suárez Ferrer, C., Huguet, J. M., Fernández-Clotet, A., ... on behalf of the Young Group of GETECCU. (2021). Impact of Biological Agents on Postsurgical Complications in Inflammatory Bowel Disease: A Multicentre Study of Geteccu. Journal of Clinical Medicine, 10(19), 4402. https://doi.org/10.3390/jcm10194402








