Clinical Outcomes of Pediatric Chronic Intestinal Pseudo-Obstruction
Abstract
1. Introduction
2. Materials and Methods
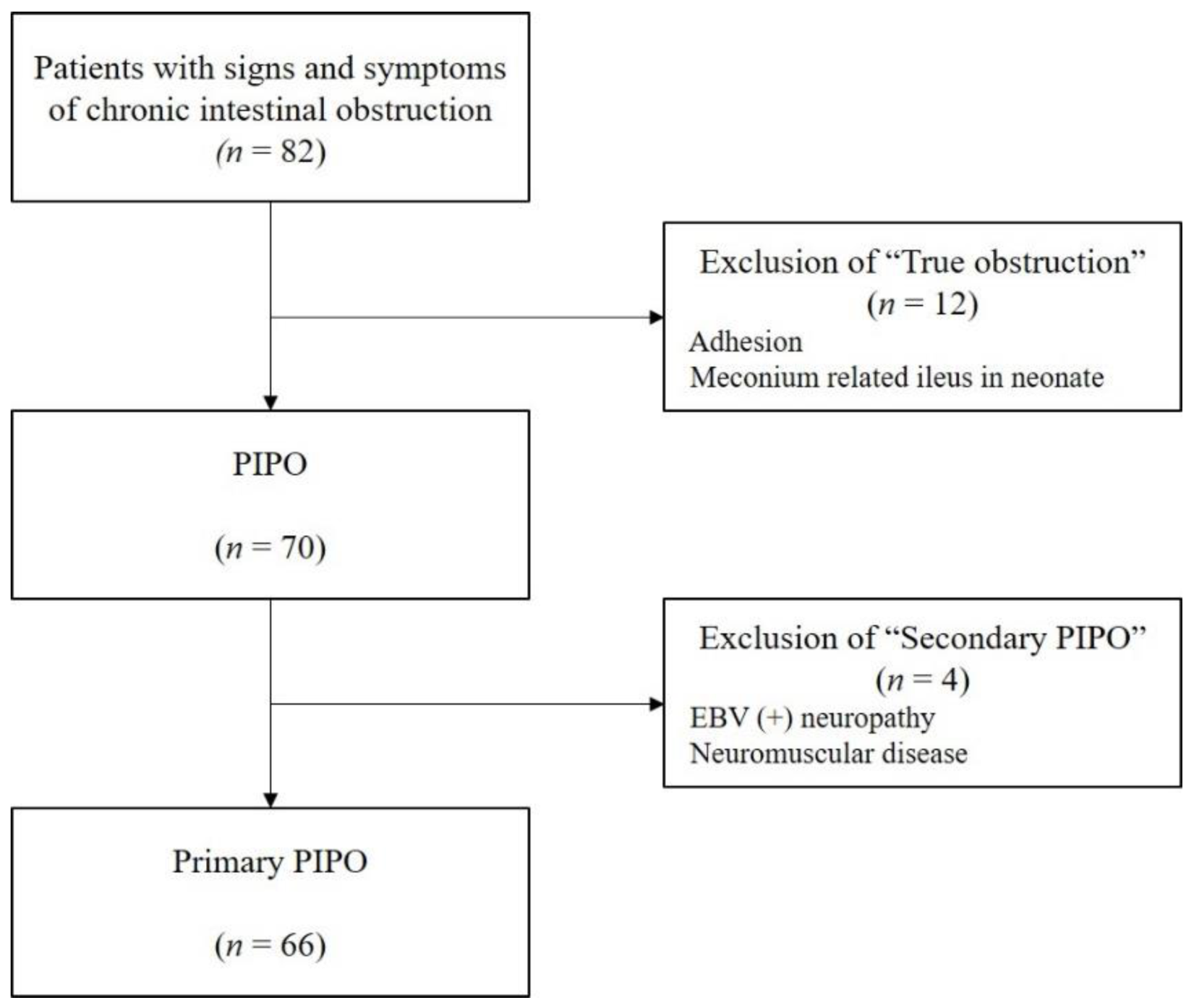
2.1. Patients
2.2. Patients’ Characteristics
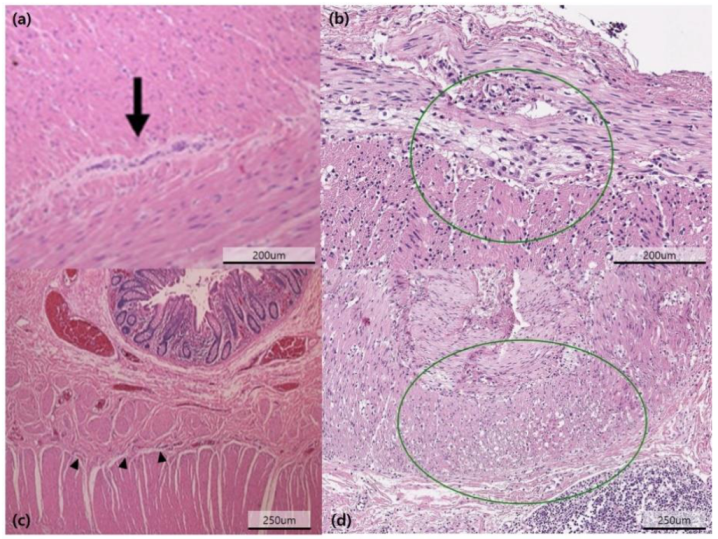
2.3. Diagnostic Examinations
2.4. Patient Groups
2.5. Statistical Analyses
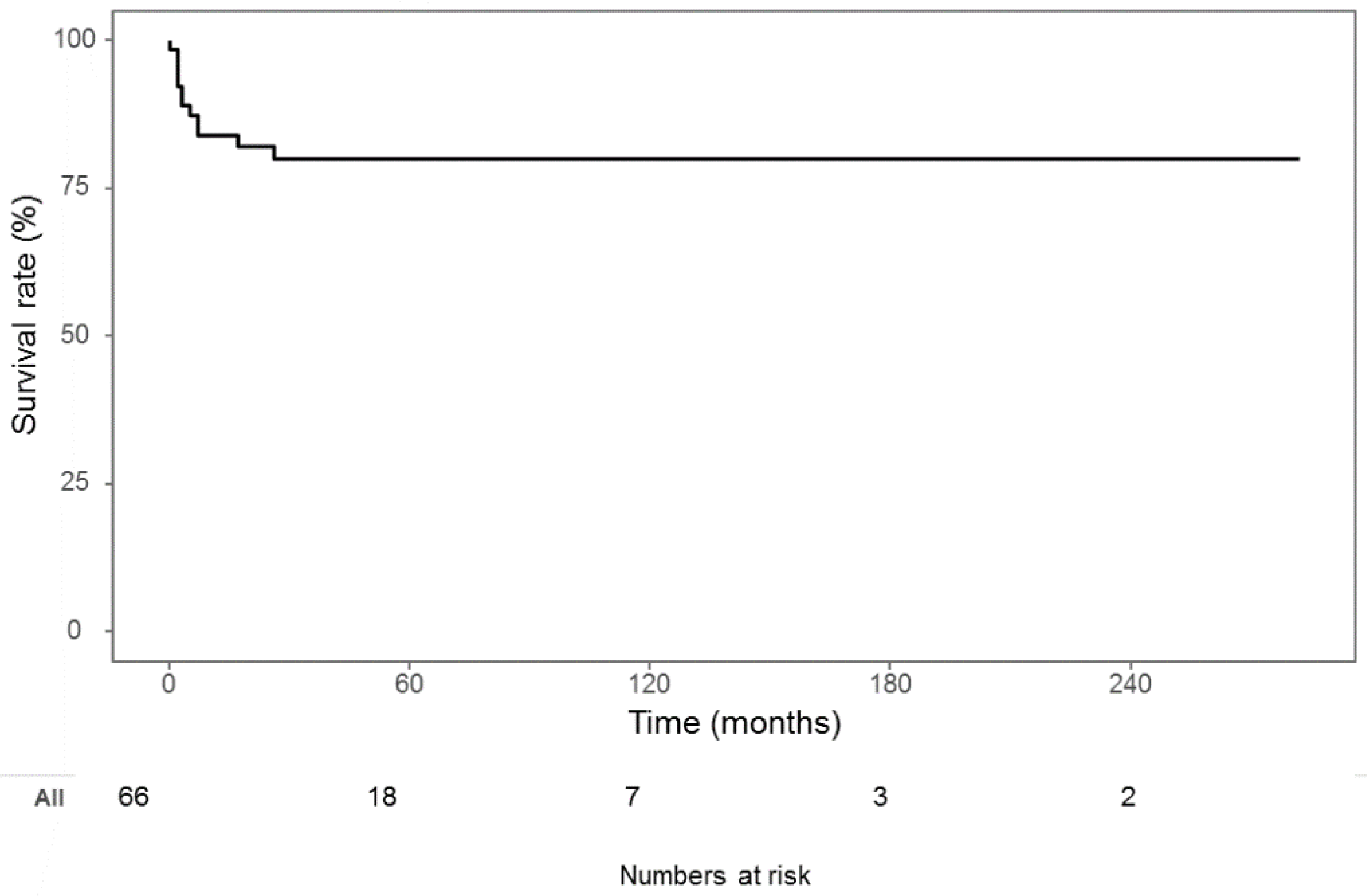
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Byrne, W.J.; Cipel, L.; Euler, A.R.; Halpin, T.C.; Ament, M.E. Chronic idiopathic intestinal pseudo-obstruction syndrome in children-clinical characteristics and prognosis. J. Pediatr. 1977, 90, 585–589. [Google Scholar] [CrossRef]
- Muto, M.; Matsufuji, H.; Tomomasa, T.; Nakajima, A.; Kawahara, H.; Ida, S.; Ushijima, K.; Kubota, A.; Mushiake, S.; Taguchi, T. Pediatric chronic intestinal pseudo-obstruction is a rare, serious, and intractable disease: A report of a nationwide survey in Japan. J. Pediatr. Surg. 2014, 49, 1799–1803. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, G.; Törnblom, H.; Iwarzon, M.; Nyberg, B.; Martin, J.E.; Veress, B. Full-thickness biopsy findings in chronic intestinal pseudo-obstruction and enteric dysmotility. Gut 2009, 58, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Stanghellini, V.; Cogliandro, R.F.; De Giorgio, R.; Barbara, G.; Cremon, C.; Antonucci, A.; Fronzoni, L.; Cogliandro, L.; Naponelli, V.; Serra, M. Natural history of intestinal failure induced by chronic idiopathic intestinal pseudo-obstruction. Transplant. Proc. 2010, 42, 15–18. [Google Scholar] [CrossRef]
- Thapar, N.; Saliakellis, E.; Benninga, M.A.; Borrelli, O.; Curry, J.; Faure, C.; De Giorgio, R.; Gupte, G.; Knowles, C.H.; Staiano, A. Paediatric Intestinal Pseudo-obstruction: Evidence and Consensus-based Recommendations From an ESPGHAN-Led Expert Group. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 991–1019. [Google Scholar] [CrossRef]
- Collins, R.R.; Barth, B.; Megison, S.; Pfeifer, C.M.; Rice, L.M.; Harris, S.; Timmons, C.F.; Rakheja, D. ACTG2-Associated Visceral Myopathy With Chronic Intestinal Pseudoobstruction, Intestinal Malrotation, Hypertrophic Pyloric Stenosis, Choledochal Cyst, and a Novel Missense Mutation. Int. J. Surg. Pathol. 2019, 27, 77–83. [Google Scholar] [CrossRef]
- Jenkins, Z.A.; Macharg, A.; Chang, C.Y.; van Kogelenberg, M.; Morgan, T.; Frentz, S.; Wei, W.; Pilch, J.; Hannibal, M.; Foulds, N. Differential regulation of twoFLNAtranscripts explains some of the phenotypic heterogeneity in the loss-of-function filaminopathies. Hum. Mutat. 2018, 39, 103–113. [Google Scholar] [CrossRef]
- Mann, S.D.; Debinski, H.S.; Kamm, M.A. Clinical characteristics of chronic idiopathic intestinal pseudo-obstruction in adults. Gut 1997, 41, 675–681. [Google Scholar] [CrossRef]
- Mousa, H.; Hyman, P.E.; Cocjin, J.; Flores, A.F.; Di Lorenzo, C. Long-term outcome of congenital intestinal pseudoobstruction. Dig. Dis. Sci. 2002, 47, 2298–2305. [Google Scholar] [CrossRef]
- Heneyke, S.; Smith, V.V.; Spitz, L.; Milla, P.J. Chronic intestinal pseudo-obstruction: Treatment and long term follow up of 44 patients. Arch. Dis. Child. 1999, 81, 21–27. [Google Scholar] [CrossRef]
- El-Chammas, K.; Sood, M.R. Chronic Intestinal Pseudo-obstruction. Clin. Colon. Rectal. Surg. 2018, 31, 99–107. [Google Scholar] [CrossRef]
- Rudolph, C.D.; Hyman, P.E.; Altschuler, S.M.; Christensen, J.; Colletti, R.B.; Cucchiara, S.; Di Lorenzo, C.; Flores, A.F.; Hillemeier, A.C.; McCallum, R.W. Diagnosis and Treatment of Chronic Intestinal Pseudo-Obstruction in Children: Report of Consensus Workshop. J. Pediatric Gastroenterol. Nutr. 1997, 24, 102–112. [Google Scholar] [CrossRef]
- Downes, T.J.; Cheruvu, M.S.; Karunaratne, T.B.; De Giorgio, R.; Farmer, A.D. Pathophysiology, Diagnosis, and Management of Chronic Intestinal Pseudo-Obstruction. J. Clin. Gastroenterol. 2018, 52, 477–489. [Google Scholar] [CrossRef]
- Faure, C.; Goulet, O.; Ategbo, S.; Breton, A.; Tounian, P.; Ginies, J.L.; Roquelaure, B.; Despres, C.; Scaillon, M.; Maurage, C. Chronic intestinal pseudoobstruction syndrome: Clinical analysis, outcome, and prognosis in 105 children. French-Speaking Group of Pediatric Gastroenterology. Dig. Dis. Sci. 1999, 44, 953–959. [Google Scholar] [CrossRef]
- Di Nardo, G.; Di Lorenzo, C.; Lauro, A.; Stanghellini, V.; Thapar, N.; Karunaratne, T.B.; Volta, U.; De Giorgio, R. Chronic intestinal pseudo-obstruction in children and adults: Diagnosis and therapeutic options. Neurogastroenterol. Motil. 2017, 29, e12945. [Google Scholar] [CrossRef]
- Merritt, R.J.; Cohran, V.; Raphael, B.P.; Sentongo, T.; Volpert, D.; Warner, B.W.; Goday, P.S. Intestinal Rehabilitation Programs in the Management of Pediatric Intestinal Failure and Short Bowel Syndrome. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 588–596. [Google Scholar] [CrossRef]
- Mutanen, A.; Wales, P.W. Etiology and prognosis of pediatric short bowel syndrome. Semin. Pediatr. Surg. 2018, 27, 209–217. [Google Scholar] [CrossRef]
- Goulet, O.; Jobert-Giraud, A.; Michel, J.L.; Jaubert, F.; Lortat-Jacob, S.; Colomb, V.; Cuenod-Jabri, B.; Jan, D.; Brousse, N.; Gaillard, D. Chronic intestinal pseudo-obstruction syndrome in pediatric patients. Eur. J. Pediatr. Surg. 1999, 9, 83–89. [Google Scholar] [CrossRef]
- Stanghellini, V.; Cogliandro, R.F.; De Giorgio, R.; Barbara, G.; Salvioli, B.; Corinaldesi, R. Chronic intestinal pseudo-obstruction: Manifestations, natural history and management. Neurogastroenterol. Motil. 2007, 19, 440–452. [Google Scholar] [CrossRef]
- Hukkinen, M.; Merras-Salmio, L.; Pakarinen, M.P. Health-related quality of life and neurodevelopmental outcomes among children with intestinal failure. Semin. Pediatr. Surg. 2018, 27, 273–279. [Google Scholar] [CrossRef]
- Fell, J.M.; Smith, V.V.; Milla, P.J. Infantile chronic idiopathic intestinal pseudo-obstruction: The role of small intestinal manometry as a diagnostic tool and prognostic indicator. Gut 1996, 39, 306. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, J.H.; Jung, S.E.; Lee, S.C.; Park, K.W.; Kim, W.K. Surgical treatment and prognosis of chronic intestinal pseudo-obstruction in children. J. Pediatric Surg. 2005, 40, 1753–1759. [Google Scholar] [CrossRef]
- Gosemann, J.H.; Puri, P. Megacystis microcolon intestinal hypoperistalsis syndrome: Systematic review of outcome. Pediatr. Surg. Int. 2011, 27, 1041–1046. [Google Scholar] [CrossRef]
- Lu, W.; Xiao, Y.; Huang, J.; Lu, L.; Tao, Y.; Yan, W.; Cao, Y.; Cai, W. Causes and prognosis of chronic intestinal pseudo-obstruction in 48 subjects: A 10-year retrospective case series. Medicine 2018, 97, e12150. [Google Scholar] [CrossRef]
| n (%) | |
|---|---|
| Sex: male | 34 (51.5%) |
| Birth weight (kg) | 3.4 ± 0.5 |
| Gestational age (day) | 254.5 ± 28.5 |
| Onset age (month) | 14.4 ± 33.1 |
| Early (≤1 month) | 42 (63.6%) |
| Late (>1 month) | 24 (36.4%) |
| Gastrointestinal symptom | |
| Abdominal distension | 49 (75.4%) |
| Vomiting | 29 (44.6%) |
| Constipation | 19 (29.2%) |
| Feeding difficulty | 7 (10.8%) |
| Diarrhea | 4 (6.2%) |
| Abdominal pain | 4 (6.2%) |
| Urologic symptom | 14 (21.2%) |
| Megacystis | 8 (12.1%) |
| Hydronephrosis | 3 (4.5%) |
| Vesicoureteral reflux | 2 (3.0%) |
| Neurogenic bladder | 1 (1.5%) |
| n (%) | |
|---|---|
| Pathology | |
| Neuropathy | 47 (71.2%) |
| Hypoganglionosis | 21 (31.8%) |
| IND-B § | 9 (13.6%) |
| Others | 17 (25.8%) |
| Myopathy | 11 (16.7%) |
| Neuropathy, myopathy | 3 (4.6%) |
| Undetermined | 5 (7.6%) |
| Extent of involvement | |
| Generalized | 55 (83.3%) |
| Localized | 11 (16.7%) |
| Stomach | 3 (4.6%) |
| Small bowel | 3 (4.6%) |
| Colon | 5 (7.6%) |
| Genetic mutation | |
| ACTG-2 mutation | 4 of 18 |
| SOX10 mutation | 1 of 18 |
| CLMP, FLNA, MYH-11, RAD21, SGOL1 | 0 of 18 |
| Number of operations | 3.6 ± 2.2 |
| Name of operation | |
| Bowel resection | |
| Gastrectomy | 4 |
| Colectomy | 15 |
| No bowel resection | |
| Full-thickness intestinal biopsy | 2 |
| Full-thickness intestinal biopsy with enterostomy | 45 |
| Outcome | |
| PN * duration (month) | 11.8 ± 21.0 |
| PN ≥ 6 months | 21 (31.8%) |
| Home PN | 16 (24.2%) |
| Mortality | 12 (18.2%) |
| Gene | Mutation |
|---|---|
| ACTG-2 mutation | c.533G > A, p.Arg178His, Heterozygote c.188G > A, p.Arg63Gln, Heterozygote c.188G > A, p.Arg63Gln, Heterozygote c.769C > T, p.Arg257Cys, Heterozygote |
| SOX10 mutation | c.1164T > A, p.Tyhr388, Heterozygote |
| Onset Age | Urologic Symptom | Pathology | Involvement Extent | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Early (n = 42) | Late (n = 24) | p | Yes (n = 14) | No (n = 52) | p | Neuropathy (n = 47) | Myopathy (n = 11) | Undetermined (n = 5) | N + M * (n = 3) | p | Generalized (n = 55) | Localized (n = 11) | p | |
| PN duration | 10 (23.8%) | 9 (37.5%) | 0.369 | 8.0 (2.0–48.0) | 1.0 (0.0–6.0) | 0.011 | 1.5 (0.0–19.0) | 4.0 (1.0–34.0) | 0.5 (0.0–2.0) | 1.0 (0.5–40.5) | 0.509 | 2.0 (0.0–15.5) | 0 (0–0) | 0.001 |
| PN > 6 months | 11 (26.2%) | 5 (20.8%) | 0.849 | 7 (50.0%) | 12 (29.3%) | 0.020 | 0 (0.0%) | 4 (40.0%) | 14 (36.8%) | 1 (33.3%) | 0.580 | 19 (34.5%) | 0 (0.0%) | 0.026 |
| Home PN | 7 (16.7%) | 5 (20.8%) | 0.928 | 9 (64.3%) | 7 (13.5%) | <0.001 | 12 (25.5%) | 2 (18.2%) | 1 (20.0%) | 1 (33.3%) | 1.000 | 16 (29.1%) | 0 (0.0%) | 0.053 |
| Mortality | 3.4 ± 2.3 | 3.9 ± 2.1 | 0.394 | 1 (7.1%) | 11 (21.2%) | 0.436 | 4 (8.5%) | 6 (54.5%) | 2 (40.0%) | 0 (0.0%) | 0.002 | 12 (21.8%) | 0 (0.0%) | 0.199 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, D.; Yang, H.-B.; Youn, J.; Kim, H.-Y. Clinical Outcomes of Pediatric Chronic Intestinal Pseudo-Obstruction. J. Clin. Med. 2021, 10, 2376. https://doi.org/10.3390/jcm10112376
Ko D, Yang H-B, Youn J, Kim H-Y. Clinical Outcomes of Pediatric Chronic Intestinal Pseudo-Obstruction. Journal of Clinical Medicine. 2021; 10(11):2376. https://doi.org/10.3390/jcm10112376
Chicago/Turabian StyleKo, Dayoung, Hee-Beom Yang, Joong Youn, and Hyun-Young Kim. 2021. "Clinical Outcomes of Pediatric Chronic Intestinal Pseudo-Obstruction" Journal of Clinical Medicine 10, no. 11: 2376. https://doi.org/10.3390/jcm10112376
APA StyleKo, D., Yang, H.-B., Youn, J., & Kim, H.-Y. (2021). Clinical Outcomes of Pediatric Chronic Intestinal Pseudo-Obstruction. Journal of Clinical Medicine, 10(11), 2376. https://doi.org/10.3390/jcm10112376






