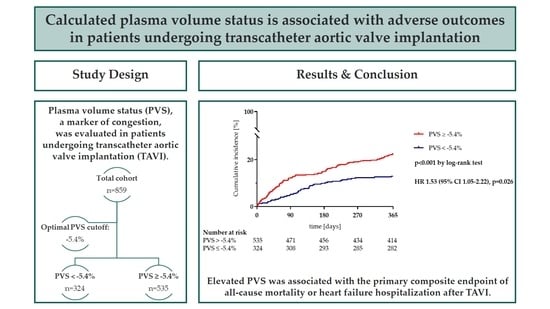
Calculated Plasma Volume Status Is Associated with Adverse Outcomes in Patients Undergoing Transcatheter Aortic Valve Implantation
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Baseline Characteristics
3.2. Periprocedural Complications
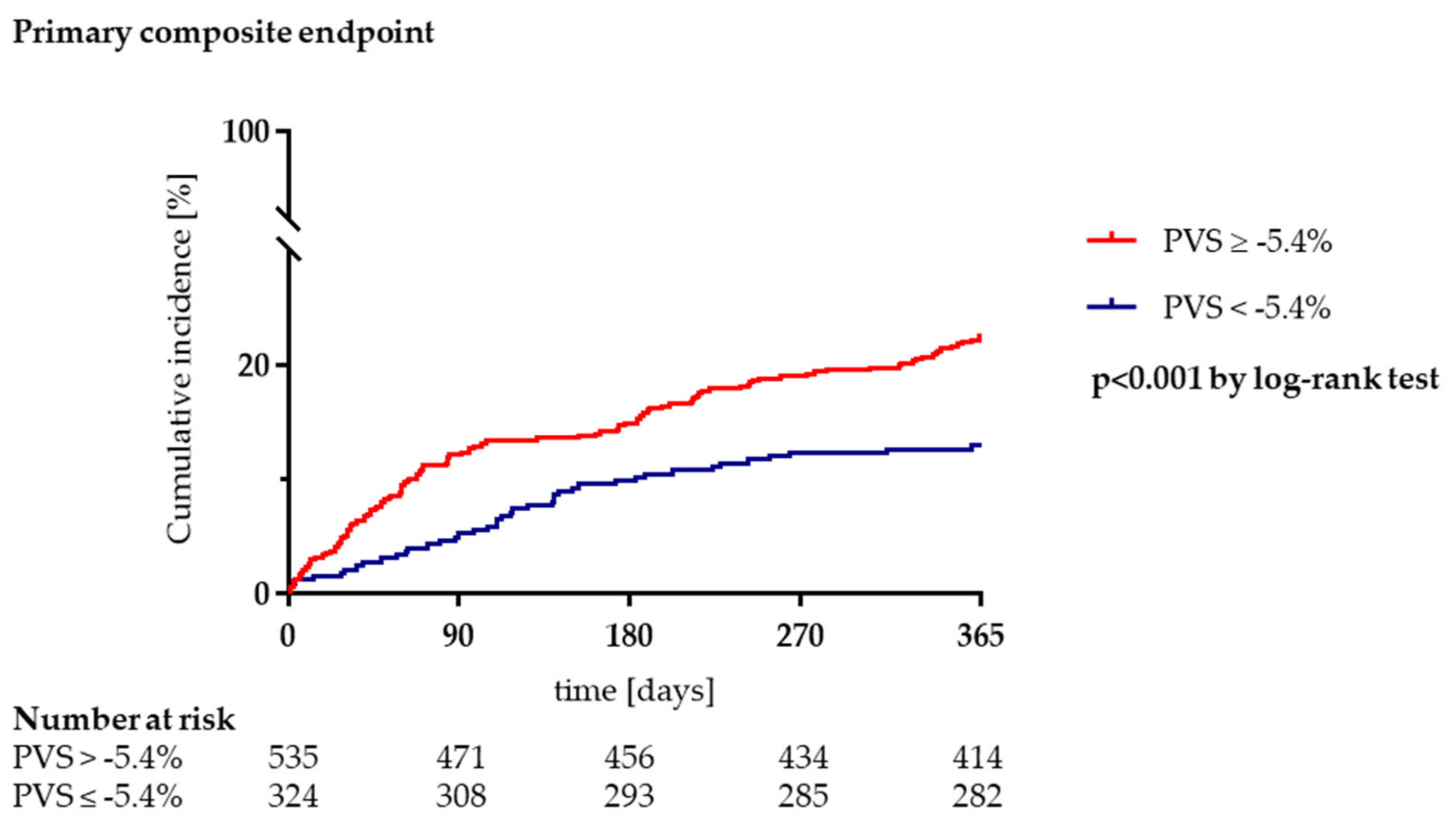
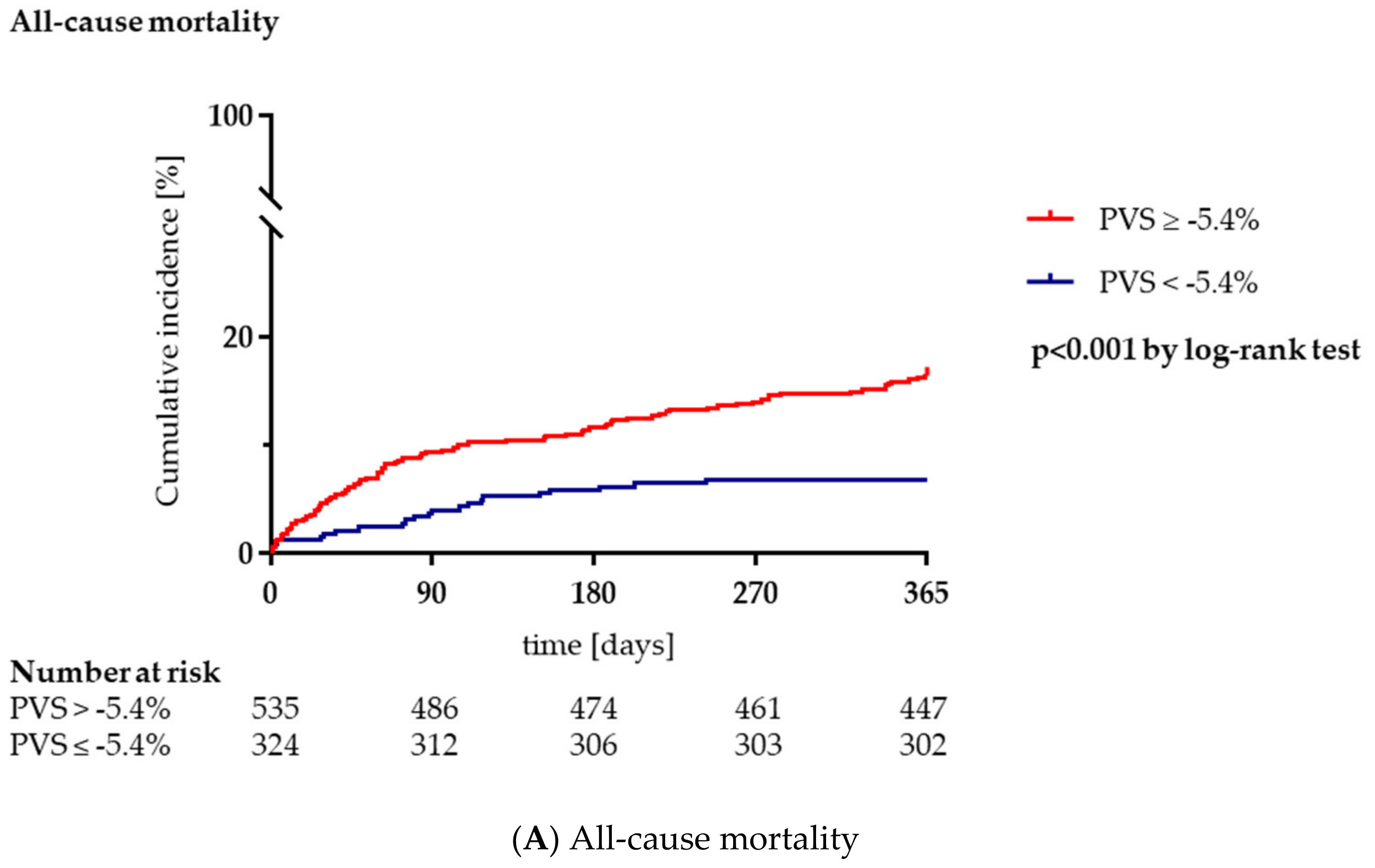
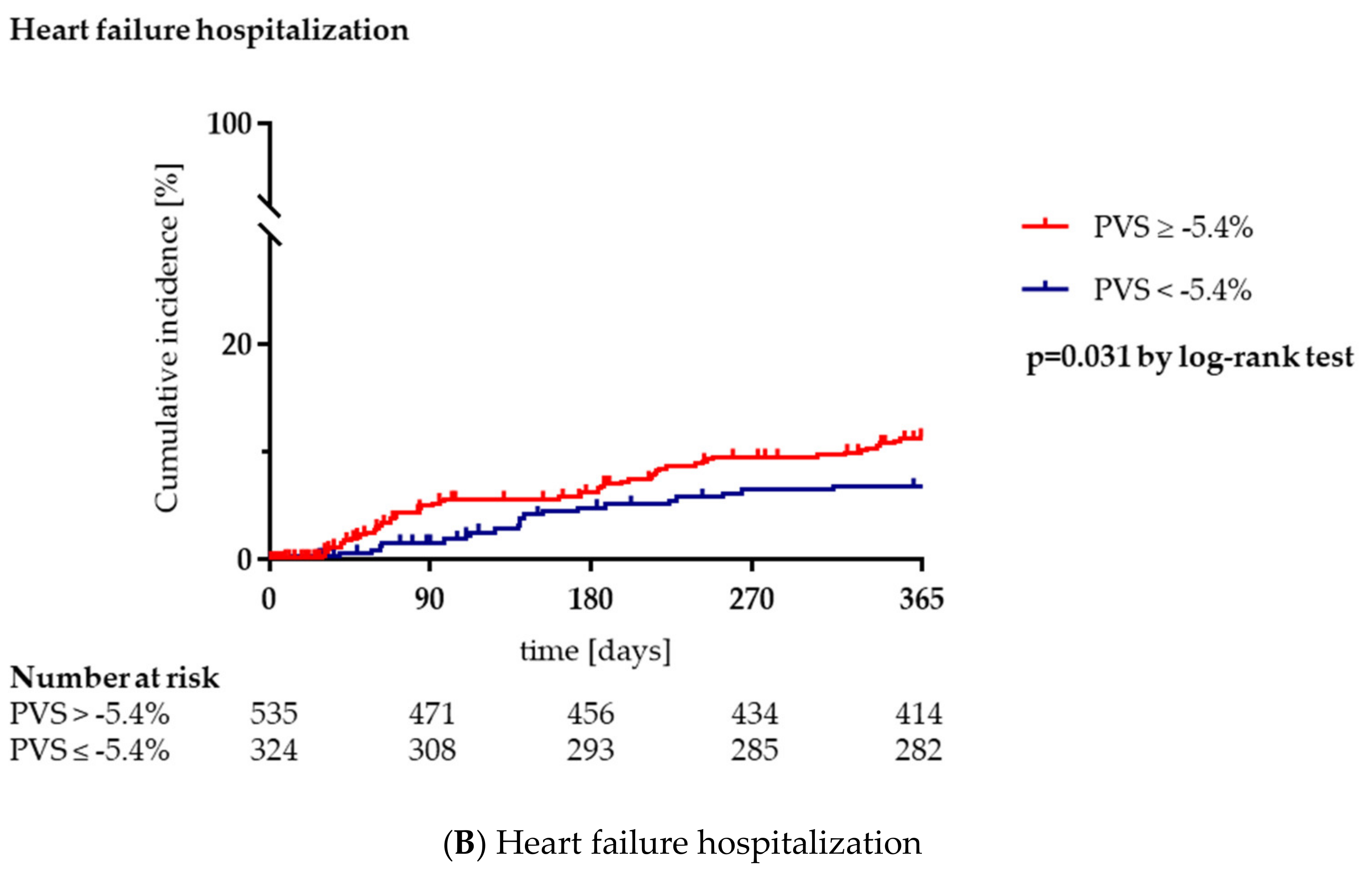
3.3. Clinical Outcome during Long-Term Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carroll, J.D.; Mack, M.J.; Vemulapalli, S.; Herrmann, H.C.; Gleason, T.G.; Hanzel, G.; Deeb, G.M.; Thourani, V.H.; Cohen, D.J.; Desai, N.; et al. STS-ACC TVT Registry of Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2020, 76, 2492–2516. [Google Scholar] [CrossRef]
- Writing Committee Members; Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., III; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 77, e25–e197. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, C.; Kammerlander, A.A.; Koschutnik, M.; Sinnhuber, L.; Forutan, N.; Eidenberger, A.; Donà, C.; Schartmueller, F.; Dannenberg, V.; Winter, M.; et al. Fluid overload in patients undergoing TAVR: What we can learn from the nephrologists. ESC Heart Fail. 2021, 8, 1408–1416. [Google Scholar] [CrossRef]
- Binanay, C.; Califf, R.M.; Hasselblad, V.; O’Connor, C.M.; Shah, M.R.; Sopko, G.; Stevenson, L.W.; Francis, G.S.; Leier, C.V.; Miller, L.W.; et al. Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness. JAMA 2005, 294, 1625–1633. [Google Scholar] [CrossRef] [Green Version]
- Miller, W.L.; Mullan, B.P. Volume Overload Profiles in Patients with Preserved and Reduced Ejection Fraction Chronic Heart Failure. JACC Heart Fail. 2016, 4, 453–459. [Google Scholar] [CrossRef]
- Gibson, J.G.; Seligman, A.M.; Peacock, W.C.; Aub, J.C.; Fine, J.; Evans, R.D. The distribution of red cells and plasma in large and minute vessels of the normal dog, determined by radioactive isotopes of iron and iodine 1. J. Clin. Investig. 1946, 25, 848–857. [Google Scholar] [CrossRef]
- Martens, P.; Nijst, P.; Dupont, M.; Mullens, W. The Optimal Plasma Volume Status in Heart Failure in Relation to Clinical Outcome. J. Card. Fail. 2019, 25, 240–248. [Google Scholar] [CrossRef]
- Ling, H.Z.; Flint, J.; Damgaard, M.; Bonfils, P.K.; Cheng, A.; Aggarwal, S.; Velmurugan, S.; Mendonca, M.; Rashid, M.; Kang, S.; et al. Calculated plasma volume status and prognosis in chronic heart failure. Eur. J. Heart Fail. 2015, 17, 35–43. [Google Scholar] [CrossRef]
- Hakim, R.M. Plasmapheresis. In Handbook of Dialysis, 3rd ed.; Daugirdas, J.T., Blake, P.G., Ing, T.S., Eds.; Lippincott, Williams and Wilkins: Philadelphia, PA, USA, 2001; p. 236. [Google Scholar]
- Longo, D. Table 218: Body fluids and other mass data. In Harrison’s Principles of Internal Medicine, 18th ed.; Longo, D.L., Fauci, A.S., Kasper, D.L., Hauser, S.L., Jameson, J.L., Loscalzo, J., Eds.; McGraw–Hill: New York, NY, USA, 2011; p. A-1. [Google Scholar]
- VARC-3 Writing Committee; Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; A Leipsic, J.; Blanke, P.; et al. Valve Academic Research Consortium 3: Updated endpoint definitions for aortic valve clinical research. Eur. Heart J. 2021, 42, 1825–1857. [Google Scholar] [CrossRef]
- Shahian, D.M.; Jacobs, J.P.; Badhwar, V.; Kurlansky, P.A.; Furnary, A.P.; Cleveland, J.C.; Lobdell, K.W.; Vassileva, C.; von Ballmoos, M.C.W.; Thourani, V.H.; et al. The Society of Thoracic Surgeons 2018 Adult Cardiac Surgery Risk Models: Part 1—Background, Design Considerations, and Model Development. Ann. Thorac. Surg. 2018, 105, 1411–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, S.M.; Feng, L.; He, X.; Xian, Y.; Jacobs, J.P.; Badhwar, V.; Kurlansky, P.A.; Furnary, A.P.; Cleveland, J.C.; Lobdell, K.W.; et al. The Society of Thoracic Surgeons 2018 Adult Cardiac Surgery Risk Models: Part 2—Statistical Methods and Results. Ann. Thorac. Surg. 2018, 105, 1419–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maznyczka, A.M.; Barakat, M.; Aldalati, O.; Eskandari, M.; Wollaston, A.; Tzalamouras, V.; Dworakowski, R.; Deshpande, R.; Monaghan, M.; Byrne, J.; et al. Calculated plasma volume status predicts outcomes after transcatheter aortic valve implantation. Open Heart 2020, 7, e001477. [Google Scholar] [CrossRef]
- Adlbrecht, C.; Piringer, F.; Resar, J.; Watzal, V.; Andreas, M.; Strouhal, A.; Hasan, W.; Geisler, D.; Weiss, G.; Grabenwöger, M.; et al. The impact of subclinical congestion on the outcome of patients undergoing transcatheter aortic valve implantation. Eur. J. Clin. Investig. 2020, 50, e13251. [Google Scholar] [CrossRef] [PubMed]
- Afilalo, J.; Lauck, S.; Kim, D.H.; Lefèvre, T.; Piazza, N.; Lachapelle, K.; Martucci, G.; Lamy, A.; Labinaz, M.; Peterson, M.D.; et al. Frailty in Older Adults Undergoing Aortic Valve Replacement. J. Am. Coll. Cardiol. 2017, 70, 689–700. [Google Scholar] [CrossRef]
- Shimura, T.; Yamamoto, M.; Yamaguchi, R.; Adachi, Y.; Sago, M.; Tsunaki, T.; Kagase, A.; Koyama, Y.; Otsuka, T.; Yashima, F.; et al. Calculated plasma volume status and outcomes in patients undergoing transcatheter aortic valve replacement. ESC Heart Fail. 2021, 8, 1990–2001. [Google Scholar] [CrossRef]
- Seoudy, H.; Al-Kassou, B.; Shamekhi, J.; Sugiura, A.; Frank, J.; Saad, M.; Bramlage, P.; Seoudy, A.K.; Puehler, T.; Lutter, G.; et al. Frailty in patients undergoing transcatheter aortic valve replacement: Prognostic value of the Geriatric Nutritional Risk Index. J. Cachex Sarcopenia Muscle 2021, 12, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Komajda, M.; Hanon, O.; Hochadel, M.; Lopez-Sendon, J.L.; Follath, F.; Ponikowski, P.; Harjola, V.-P.; Drexler, H.; Dickstein, K.; Tavazzi, L.; et al. Contemporary management of octogenarians hospitalized for heart failure in Europe: Euro Heart Failure Survey II. Eur. Heart J. 2008, 30, 478–486. [Google Scholar] [CrossRef] [Green Version]
- Grodin, J.L.; Philips, S.; Mullens, W.; Nijst, P.; Martens, P.; Fang, J.C.; Drazner, M.H.; Tang, W.W.; Pandey, A. Prognostic implications of plasma volume status estimates in heart failure with preserved ejection fraction: Insights from TOPCAT. Eur. J. Heart Fail. 2019, 21, 634–642. [Google Scholar] [CrossRef]
- Imamura, T.; Narang, N.; Combs, P.; Siddiqi, U.; Mirzai, S.; Stonebraker, C.; Bullard, H.; Simone, P.; Jeevanandam, V. Impact of plasma volume status on mortality following left ventricular assist device implantation. Artif. Organs 2021, 45, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Vitamin and Mineral Nutrition Information System; WHO/NMH/NHD/MNM/11.1; World Health Organization: Geneva, Switzerland, 2011; Available online: http://www.who.int/vmnis/indicators/haemoglobin.pdf (accessed on 18 July 2021).
- Nagao, K.; CURRENT AS Registry Investigators; Taniguchi, T.; Morimoto, T.; Shiomi, H.; Ando, K.; Kanamori, N.; Murata, K.; Kitai, T.; Kawase, Y.; et al. Anemia in Patients with Severe Aortic Stenosis. Sci. Rep. 2019, 9, 1924. [Google Scholar] [CrossRef] [Green Version]
- Androne, A.-S.; Katz, S.D.; Lund, L.; LaManca, J.; Hudaihed, A.; Hryniewicz, K.; Mancini, D.M. Hemodilution Is Common in Patients with Advanced Heart Failure. Circulation 2003, 107, 226–229. [Google Scholar] [CrossRef] [Green Version]
- Otto, J.; Plumb, J.; Clissold, E.; Kumar, S.B.; Wakeham, D.J.; Schmidt, W.; Grocott, M.; Richards, T.; Montgomery, H.E. Hemoglobin concentration, total hemoglobin mass and plasma volume in patients: Implications for anemia. Haematologica 2017, 102, 1477–1485. [Google Scholar] [CrossRef]
- Felker, G.M.; Anstrom, K.J.; Adams, K.F.; Ezekowitz, J.A.; Fiuzat, M.; Houston-Miller, N.; Januzzi, J.L.; Mark, D.B.; Piña, I.L.; Passmore, G.; et al. Effect of Natriuretic Peptide-Guided Therapy on Hospitalization or Cardiovascular Mortality in High-Risk Patients With Heart Failure and Reduced Ejection Fraction. JAMA 2017, 318, 713–720. [Google Scholar] [CrossRef]
- Seoudy, H.; Frank, J.; Neu, M.; Güßefeld, N.; Klaus, Y.; Freitag-Wolf, S.; Lambers, M.; Lutter, G.; Dempfle, A.; Rangrez, A.Y.; et al. Periprocedural Changes of NT-proBNP Are Associated with Survival After Transcatheter Aortic Valve Implantation. J. Am. Heart Assoc. 2019, 8, e010876. [Google Scholar] [CrossRef] [Green Version]
- Oury, C.; Nchimi, A.; Lancellotti, P.; Bergler-Klein, J. Can Blood Biomarkers Help Predicting Outcome in Transcatheter Aortic Valve Implantation? Front. Cardiovasc. Med. 2018, 5, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, P.Y.; Chao, C.-T.; Chan, D.-C.; Huang, J.-W.; Hung, K.-Y. Contributors, risk associates, and complications of frailty in patients with chronic kidney disease: A scoping review. Ther. Adv. Chronic Dis. 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Total (n = 859) | PVS < −5.4% (n = 324) | PVS ≥ −5.4% (n = 535) | p-Value | |
|---|---|---|---|---|
| Age (years) | 81.9 (78.7–85.8) | 81.0 (77.6–84.1) | 82.8 (79.5–86.9) | <0.001 |
| Female, n (%) | 452 (52.6) | 152 (46.9) | 300 (56.1) | 0.009 |
| BMI (kg/m2) | 26.2 (23.9–29.6) | 28.9 (25.9–32.0) | 25.0 (22.7–27.7) | <0.001 |
| Atrial fibrillation, n (%) | 373 (43.4) | 134 (41.4) | 239 (44.7) | 0.342 |
| CAD, n (%) | 550 (64.0) | 199 (61.4) | 351 (65.6) | 0.215 |
| COPD, n (%) | 94 (10.9) | 31 (9.6) | 63 (11.8) | 0.315 |
| CVD, n (%) | 150 (17.5) | 56 (17.3) | 94 (17.6) | 0.915 |
| Diabetes mellitus, n (%) | 258 (30.0) | 96 (29.6) | 162 (30.3) | 0.840 |
| Dyslipidemia, n (%) | 431 (50.2) | 177 (54.6) | 254 (47.5) | 0.042 |
| Hypertension, n (%) | 759 (88.4) | 286 (88.3) | 473 (88.4) | 0.951 |
| NYHA class III or IV, n (%) | 612 (71.2) | 219 (67.6) | 393 (73.5) | 0.067 |
| PAD, n (%) | 72 (8.4) | 26 (8.0) | 46 (8.6) | 0.769 |
| PAH, n (%) | 115 (13.4) | 46 (14.2) | 69 (12.9) | 0.588 |
| Prev. cardiac surgery, n (%) | 126 (14.7) | 54 (16.7) | 72 (13.5) | 0.198 |
| STS-Score (%) | 3.7 (2.4–5.5) | 2.9 (2.1–4.6) | 4.2 (2.6–6.3) | <0.001 |
| Hematocrit (%) | 0.36 (0.32–0.39) | 0.40 (0.38–0.42) | 0.34 (0.31–0.36) | <0.001 |
| Hemoglobin (g/dL) | 12.4 (11.1–13.4) | 13.6 (12.8–14.4) | 11.5 (10.4–12.4) | <0.001 |
| Anemia | ||||
| Mild | 253 (29.5) | 52 (16.0) | 201 (37.6) | <0.001 |
| Moderate | 173 (20.1) | 5 (1.5) | 168 (31.4) | <0.001 |
| Severe | 5 (0.6) | 0 (0) | 5 (0.9) | 0.081 |
| Non-Anemia | 428 (49.8) | 267 (82.4) | 161 (30.1) | <0.001 |
| eGFR (mL/min/1.73 m2) | 54 (40–67) | 59 (48–70) | 51 (37–63) | <0.001 |
| NT-proBNP (pg/mL) | 1461 (573–3462) | 986 (445–2287) | 2102 (704–4529) | <0.001 |
| LVEF | ||||
| ≥55%, n (%) | 555 (64.6) | 226 (69.8) | 329 (61.5) | 0.014 |
| 45–54%, n (%) | 162 (18.9) | 52 (16.0) | 110 (20.6) | 0.101 |
| 35–44%, n (%) | 72 (8.4) | 19 (5.9) | 53 (9.9) | 0.038 |
| <35%, n (%) | 70 (8.1) | 27 (8.3) | 43 (8.0) | 0.878 |
| AVA (cm2) | 0.8 (0.6–0.9) | 0.8 (0.6–0.9) | 0.8 (0.6–0.9) | 0.075 |
| MPG (mmHg) | 38 (29–50) | 38 (30–49) | 38 (29–50) | 0.838 |
| MR III-IV, n (%) | 72 (8.4) | 26 (8.0) | 46 (8.6) | 0.769 |
| TR III-IV, n (%) | 36 (4.2) | 14 (4.3) | 22 (4.1) | 0.882 |
| Calculated actual PV (mL) | 2907 (2621–3264) | 2951 (2693–3321) | 2883 (2566–3235) | 0.003 |
| Calculated ideal PV (mL) | 3000 (2613–3393) | 3315 (3000–3783) | 2800 (2457–3120) | <0.001 |
| PVS (%) | −2.6 (−8.6–4.1) | −10.4 (−14.0–−7.5) | 1.8 (−2.0–7.2) | <0.001 |
| Heart failure medication | ||||
| ACE-I/ARB | 714 (83.1) | 275 (84.9) | 439 (82.1) | 0.285 |
| Betablocker | 639 (74.4) | 235 (72.5) | 404 (75.5) | 0.332 |
| MRA | 108 (12.6) | 35 (10.8) | 73 (13.6) | 0.223 |
| Diuretics | 598 (69.6) | 211 (65.1) | 387 (72.3) | 0.026 |
| Total (n = 859) | PVS < −5.4% (n = 324) | PVS ≥ −5.4% (n = 535) | p-Value | |
|---|---|---|---|---|
| Valve type | 0.146 | |||
| Self-expanding, n (%) | 433 (50.4) | 153 (47.2) | 280 (52.3) | |
| Balloon-expandable, n (%) | 426 (49.6) | 171 (52.8) | 255 (47.7) | |
| Procedural duration (min) | 50 (40–63) | 49 (40–61) | 49 (40–65) | 0.302 |
| Contrast medium (mL) | 84 (70–105) | 85 (70–107) | 80 (68–103) | 0.219 |
| VARC-3 | ||||
| New permanent pacemaker, n (%) | 101 (11.8) | 43 (13.3) | 58 (10.8) | 0.284 |
| Myocardial infarction, n (%) | 3 (0.3) | 1 (0.3) | 2 (0.4) | >0.999 |
| AKIN stage 3/4, n (%) | 9 (1.0) | 2 (0.6) | 7 (1.3) | 0.496 |
| Type 3 (life-threatening) bleeding, n (%) | 21 (2.4) | 4 (1.2) | 17 (3.2) | 0.108 |
| Stroke with disability, n (%) | 3 (0.3) | 1 (0.3) | 2 (0.4) | >0.999 |
| Primary composite outcome, n (%) | 163 (19.0) | 42 (13.0) | 121 (22.6) | <0.001 |
| All-cause mortality, n (%) | 110 (12.8) | 22 (6.8) | 88 (16.4) | <0.001 |
| Heart failure hospitalization, n (%) | 77 (9.0) | 21 (6.5) | 56 (10.5) | 0.048 |
| Variable | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
|---|---|---|---|---|
| PVS ≥ −5.4% | 1.99 (1.39–2.84) | <0.001 | 1.53 (1.05–2.22) | 0.026 |
| Atrial fibrillation | 1.70 (1.25–2.31) | <0.001 | ||
| Age > median (81.9 years) | 1.27 (0.93–1.73) | 0.132 | ||
| BMI > median (26.2 kg/m2) | 0.66 (0.48–0.90) | 0.008 | ||
| STS score > median (3.7%) | 2.23 (1.61–3.08) | <0.001 | 1.67 (1.17–2.38) | 0.005 |
| COPD | 1.58 (1.03–2.42) | 0.036 | 1.54 (0.99–2.39) | 0.056 |
| NT-proBNP > median (1461 pg/mL) | 1.94 (1.41–2.67) | <0.001 | 1.48 (1.06–2.09) | 0.023 |
| eGFR < 60 mL/min/1.73 cm2 | 1.85 (1.29–2.65) | <0.001 | ||
| LVEF < 55% | 1.73 (1.27–2.35) | <0.001 | ||
| TR III-IV | 3.37 (2.04–5.57) | <0.001 | 2.87 (1.72–4.80) | <0.001 |
| MR III-IV | 2.36 (1.55–3.59) | <0.001 | ||
| PAH | 1.55 (1.04–2.30) | 0.030 | ||
| AKIN stage 3/4 | 10.10 (4.72–21.70) | <0.001 | 8.50 (3.91–18.51) | <0.001 |
| Myocardial infarction | 5.11 (1.27–20.60) | 0.022 | 6.12 (1.47–25.40) | 0.013 |
| Stroke with disability | 7.73 (1.92–31.20) | 0.004 | ||
| Type 3 (life-threatening) bleeding | 4.60 (2.55–8.28) | <0.001 | 6.34 (3.50–11.49) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seoudy, H.; Saad, M.; Salem, M.; Allouch, K.; Frank, J.; Puehler, T.; Salem, M.; Lutter, G.; Kuhn, C.; Frank, D. Calculated Plasma Volume Status Is Associated with Adverse Outcomes in Patients Undergoing Transcatheter Aortic Valve Implantation. J. Clin. Med. 2021, 10, 3333. https://doi.org/10.3390/jcm10153333
Seoudy H, Saad M, Salem M, Allouch K, Frank J, Puehler T, Salem M, Lutter G, Kuhn C, Frank D. Calculated Plasma Volume Status Is Associated with Adverse Outcomes in Patients Undergoing Transcatheter Aortic Valve Implantation. Journal of Clinical Medicine. 2021; 10(15):3333. https://doi.org/10.3390/jcm10153333
Chicago/Turabian StyleSeoudy, Hatim, Mohammed Saad, Mostafa Salem, Kassem Allouch, Johanne Frank, Thomas Puehler, Mohamed Salem, Georg Lutter, Christian Kuhn, and Derk Frank. 2021. "Calculated Plasma Volume Status Is Associated with Adverse Outcomes in Patients Undergoing Transcatheter Aortic Valve Implantation" Journal of Clinical Medicine 10, no. 15: 3333. https://doi.org/10.3390/jcm10153333
APA StyleSeoudy, H., Saad, M., Salem, M., Allouch, K., Frank, J., Puehler, T., Salem, M., Lutter, G., Kuhn, C., & Frank, D. (2021). Calculated Plasma Volume Status Is Associated with Adverse Outcomes in Patients Undergoing Transcatheter Aortic Valve Implantation. Journal of Clinical Medicine, 10(15), 3333. https://doi.org/10.3390/jcm10153333